Summary
During LCMV infection, CD8+ T cells expand greatly. Bystander activation has been thought to play a role because few cells score as LCMV specific in limiting dilution analysis. In contrast, we find that at least a quarter of the CD8+ cells secrete IFNγ specifically in response to LCMV peptides at the peak of the response. Moreover, by analyzing the expansion of adoptively transferred LCMV-specific, TCR-transgenic CD8+ T cells in congenic hosts, we have determined that most of the CD8+ cell expansion is virus specific. Analysis of the effect of the monospecific TCR-transgenic T cells on the host response to three LCMV epitopes suggests that CTL precursors compete for sites on the APC in an epitope-specific fashion and that this competition determines the specificity of the response.
Introduction
Specific immune responses evolve following exposure to foreign antigens. According to the clonal selection theory, an antigen will stimulate cells that bear receptors specific for the antigen, resulting in their proliferation and functional activation. Virus infection can result in an intense activation of the immune system, and especially of CD8+ T cells.
Although CD8+ T cells are normally present in the spleen and lymph nodes of mice in lower numbers than CD4+ T cells, during many virus infections they are disproportionately expanded and may outnumber CD4+ T cells (Buchmeier et al., 1980; Rubin et al., 1981; Cauda et al., 1987; Tripp et al., 1995; Callan et al., 1996). During lymphocytic choriomeningitis virus (LCMV) infection of B6 mice, CD8+ T cells increase about 5-fold (Razvi et al., 1995), and in humans there is a large increase in circulating CD8+ T cells during cytomegalovirus, Epstein-Barr virus, and varicella-zoster virus infections (Rubin et al., 1981; Cauda et al., 1987; Callan et al., 1996). As the total pool of CD8+ cells expands during the virus infection, the number of antigen-specific cytotoxic T lymphocytes (CTL) also rises (Lau et al., 1994; Selin et al., 1994; Razvi et al., 1995; Tripp et al., 1995) as judged by limiting dilution analysis (LDA). However, LDA indicates that only a small number of the CD8+ T cells (1 in 4000 to 1 in 50, depending on the virus) are actually specific for the infecting virus (Lau et al., 1994; Selin et al., 1994; Razvi et al., 1995; Tripp et al., 1995). The specificities of the remainder of the CD8+ T cells have not been determined, although it has been shown that during LCMV infection there is an increase in the number of alloreactive and antigen cross-reactive CTL, not all of which recognize LCMV antigens (Yang and Welsh, 1986; Nahill and Welsh, 1993). In addition to their expanded numbers, most CD8+ T cells during the acute immune response to LCMV show signs of activation. They are enlarged; have elevated surface expression of CD11a, CD11b, CD44, CD49d, and the interleukin-2 receptor; and show reduced expression of CD62L (Lynch et al., 1989; McFarland et al., 1992; Andersson et al., 1995).
Because of the low frequency of virus-specific CTL, the large numbers of apparently activated cells, and the appearance of alloreactive and cross-reactive CTL, it has been assumed that the bulk of the CD8+ cells have been activated in a bystander fashion involving cytokines (Yang and Welsh, 1986; Tough and Sprent, 1996). In support of this idea, it has been demonstrated that cytokines can drive antigen-independent activation of naive and memory phenotype T cells in vitro (Unutmaz et al., 1994). There is also evidence that CD8+ T cells of memory phenotype may be more prone to bystander activation than naive cells (Tough and Sprent, 1994; Tripp et al., 1995), which has led to the suggestion that this is a mechanism for the maintenance of CD8+ T cell memory (Beverley, 1996). It also has been suggested that the cytokines expressed during the antiviral immune response may act to amplify other coincidentally active T cell responses that are not virus specific (Strang and Rickinson, 1987). Similarly, it has been proposed that bystander activation may play a role in the initiation and maintenance of autoimmune diseases by expanding the numbers of autoreactive T cells and breaking anergic tolerance (Rott et al., 1995; Mueller and Jenkins, 1997).
In this study we have used LCMV infection of B6 mice as a model for studying CD8+ T cell proliferation during the immune response to virus infection. By analyzing the frequency of cells secreting interferon-γ (IFNγ) in response to LCMV antigens, we have correlated CTL activity with antigen-specific cell frequency and have found that the number of LCMV-specific CD8+ T cells was much higher than initially expected (at least 24% of CD8+ cells). By adoptively transferring T cell receptor (TCR)–transgenic CD8+ T cells specific for an epitope of LCMV into host mice and extrapolating their proliferation during LCMV infection to the proliferation of host cells, we have determined that at least half of the CD8+ T cells present are specific for LCMV. Although a significant expansion of non–virus-specific CD8+ T cells may still occur during LCMV infection, the immune response is much more narrowly focused on the virus than has previously been believed. These results are more in keeping with the predictions of the clonal selection hypothesis.
Results
CD8+ T Cells Expand Dramatically during LCMV Infection
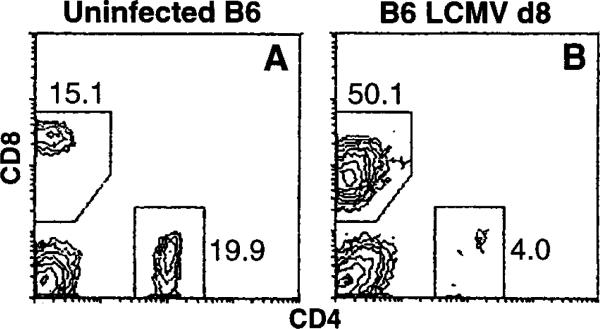
A group of three C57Bl/6 mice was intravenously infected with 105 plaque-forming units (pfu) of LCMV. On day 8 of infection, when the immune response to LCMV peaks (Buchmeier et al., 1980; our unpublished data), these mice and a group of three uninfected control mice were sacrificed and splenocytes were prepared. For the uninfected control mice there were 7.5 ± 0.5 × 107 cells per spleen (mean ± SEM); for the infected mice there were 1.5 ± 0.2 × 108 cells per spleen. These cells were analyzed for CD4+ and CD8+ cell content by two-color flow cytometry (Figure 1). The uninfected spleen cells contained 16% ± 1% CD8+ cells and 22% ± 3% CD4+ cells; infected spleens contained 50% ± 4% CD8+ cells and 5% ± 2% CD4+ cells. In absolute cell numbers the uninfected control mice contained an average of 1.2 ± 0.8 × 107 CD8+ cells and 1.7 ± 0.3 × 107 CD4+ cells. Infected spleens contained 8 ± 1 × 107 CD8+ cells and 8 ± 1 × 106 CD4+ cells—an approximately 6-fold increase in the number of CD8+ cells and a 2-fold decrease in the number of CD4+ cells. A similar increase in total cell number and CD8+ cell content was also observed in cells pooled from the mesenteric, brachial, axilliary, and inguinal lymph nodes of each mouse (data not shown).
Figure 1. LCMV Infection of C57Bl/6 Mice Induces Massive Expansion of CD8+ T Cells.
Representative CD4 versus CD8 flow cytometry profiles of splenocytes from uninfected (A) or day 8 LCMV-infected B6 mice (B). The numbers beside the gating boxes indicates the percentage of live-gated cells. d8, day 8.
Antigen-Specific Lytic Activity and Frequency of IFNγ Secretion by Primary Antiviral CD8+ T Cells Are Proportional to One Another
The B6 CTL response to LCMV is dominated by three Db-restricted epitopes: gp33, gp276, and np396 (Gairin et al., 1995). The data presented in Figure 2 show that the responses to gp33 and np396 are stronger than the response to gp276. Pooled spleen cells from pairs of mice were prepared from uninfected and day 8 LCMV-infected mice for use as effector cells in an 8 hr 51Cr-release assay against peptide-coated EL4 target cells (Figure 2A) and in an 18 hr IFNγ ELISPOT assay against peptide-coated EL4 cells (Figure 2B). Cells from the un-infected mice displayed no lytic activity, and the data were omitted from Figure 2A for the sake of clarity. Cells from the infected mice were highly lytic for gp33- and np396-coated EL4 cells and less active against gp276-coated targets. Lytic units calculated at 30% lysis were 5-fold lower for gp276 than for gp33 and np396.
Figure 2. Antigen-Specific Cytolytic Activity Is Proportional to the Frequency of IFNγ-Secreting Cells.
(A) Erythrocyte-depleted spleen cells were prepared from a pair of day 8 LCMV-infected B6 mice and were used to determine primary ex vivo CTL activity in an 8 hr 51Cr-release assay against EL4 target cells uncoated or coated with the indicated LCMV peptides.
(B) The same pool of effector cells was used in an ELISPOT assay to determine the frequency of cells secreting IFNγ in response to LCMV epitope peptides. The number of CD8+ cells was determined by FACS analysis. Error bars indicate the standard deviation of triplicate samples. Three additional experiments yielded similar results. d8, day 8.
(C) The titration of spots per well versus number of LCMV day 8 splenocytes per well is shown for ELISPOT data from a separate experiment. Splenocytes were stimulated in triplicate samples with either control EL4 cells (open circles) or with gp33-coated EL4 cells (filled squares). Stimulation with gp276- or np396-coated EL4 cells resulted in similarly linear responses (data not shown).
These data correlate with the data from the ELISPOT assay of the frequency of cells secreting IFNγ in response to each of the three LCMV epitopes: 10.6% ± 1.7% of CD8+ cells secreted IFNγ in response to gp33, 10.0% ± 1.4% in response to np396, and 2.9% ± 1.2% in response to gp276. The ELISPOT response to EL4 alone was 0.5% ± 0.3% of CD8+ cells, and fewer than 0.2% ± 0.04% of the CD8+ cells from uninfected B6 mice secreted IFNγ in this assay with any peptide. When splenocytes from day 8 LCMV-infected mice were stimulated overnight with LCMV peptide-coated EL4 cells in the presence of Brefeldin A and then analyzed by fluorescence-activated cell sorting (FACS), greater than 90% of the cells staining positive for IFNγ were also CD8+ (data not shown). For this reason, and because the IFNγ secretion is revealed only in the presence of the major histocompatibility (MHC) class I–binding peptides, we have presented these ELISPOT data as the percentage of CD8+ cells secreting IFNγ. Also, because a plot of the number of spots per well versus the number of splenocytes per well is linear (Figure 2C), the assay measures only antigen-specific cells and not cytokine-induced bystanders. From data of this type we conclude that the relative frequency of CD8+ T cells specific for each LCMV epitope can be determined by titration of their ability to lyse peptide coated target cells.
The aggregate number of cells secreting IFNγ in response to the known LCMV epitopes (gp33 + gp276 + np396) is 24% ± 4% of CD8+ T cells in the experiment shown in Figure 2B. To estimate the efficiency of the ELISPOT assay, we performed control experiments with cloned anti-LCMV CTL. We were able to detect 24%–100% of the CTL in ELISPOT assays, depending on the particular clone and the time during its restimulation cycle at which it was tested (data not shown). This suggests that, although 24% of splenic CD8+ T cells from LCMV-infected mice were detected by ELISPOT, the true number of anti-LCMV CTL could be substantially greater.
TCR-Transgenic CD8+ T Cells of Irrelevant Specificity Do Not Expand Significantly during Virus Infections
OT-1 mice are transgenic for a TCR that recognizes ovalbumin 257–264 (OVA257-264) in the context of H-2Kb (Hogquist et al., 1994). To see if we could emulate the bystander proliferation of non–virus-specific CD8+ T cells during infection, we transferred 107 purified OT-1 CD8+ T cells into age- and sex-matched Thy1 congenic B6.PL mice (PL/OT-1 chimeras). Mice were infected the next day with LCMV, VVflu-np (a recombinant vaccinia virus [VV] expressing the H-2Kd-restricted influenza nucleoprotein epitope, NP147—155 [Yewdell et al., 1985]), or VVova (which expresses the full-length ovalbumin protein [Bacik et al., 1994]). On day 6 of VV infection and day 8 of LCMV infection, spleen cells were analyzed for donor (CD8+Thy1.2+) cell content (Figure 3). As expected, there were no CD8+Thy1.2+ cells in uninfected control B6.PL mice (Figure 3A), whereas 7 days after transfer of OT-1 cells, 3% of the CD8+ cells in uninfected PL/OT-1 mice were of donor origin (Figure 3B). In the VVflu-np–infected mice, 1.3% of the CD8+ cells were donor cells (Figure 3C), and in LCMV-infected mice, 0.8% of the CD8+ cells were OT-1 cells (Figure 3D). The lower representation of the OT-1 cells in the LCMV-infected chimeras probably reflects a greater dilution of these cells by the larger overall expansion of CD8+ cells that occurs during LCMV infection than occurs during VV infection. In the VVova-infected mice, 27% of the CD8+ cells were Thy1.2+ (Figure 3E), confirming that the donor cells were capable of proliferating in the host mice. Not only did the OT-1 cells fail to proliferate significantly during VVflu-np or LCMV infection; there also was no ex vivo anti-OVA257-264 cytolytic activity following VVflu-np or LCMV infections, although such activity was readily apparent following VVova infection (data not shown).
Figure 3. Adoptively Transferred TCR-Transgenic CD8+ T Cells Do Not Expand Nonspecifically during VV or LCMV Infection.
Flow cytometry profiles of pooled spleen cells from pairs of B6.PL control mice (A) and B6.PL host mice that received 107 purified OT-1 CD8+ T cells and were injected the next day with PBS (B), VVflu-np (C), LCMV (D), or VVova (E). Splenocytes were analyzed on day 6 (A–C and E) or day 8 (D) of infection. The numbers beside the gating boxes indicate the number of donor CD8+ cells as a percentage of total CD8+ cells. A repetition of the experiment yielded similar results.
TCR-Transgenic gp33-Specific CD8+ T Cells Expand Dramatically during LCMV Infection
As an independent method of tracking the number of LCMV-specific CD8+ cells at the peak of the immune response to LCMV, we adoptively transferred unstimulated, LCMV-specific TCR-transgenic T cells into host mice and infected them with LCMV. Similar methods have been used to follow the in vivo behavior of both CD4+ and CD8+ T cells (Zimmerman et al., 1996; Kedl and Mescher, 1997; Pape et al., 1997). P14 mice are transgenic for a TCR that recognizes LCMV gp33/Db (Pircher et al., 1990). We transferred 105 P14 spleen cells containing 30% CD8+ T cells into Thy1 congenic B6.PL host mice. These PL/P14 chimeras and B6.PL control mice (three per group) were infected with LCMV. Spleen cells were prepared and analyzed by flow cytometry for T cell types and donor cell content on day 8 of infection. The expansion of CD8+ cells for both the B6.PL and PL/P14 mice (Figure 4) was identical to that observed in B6 mice (Figure 1). In control, uninfected B6.PL mice that received 105 P14 spleen cells, 0.2% of CD8+ cells were of donor origin 9 days after transfer (equivalent to day 8 of LCMV infection; data not shown). However, in the LCMV-infected PL/P14 chimeras the donor cells were greatly expanded, reaching 23% of the CD8+ cell pool in these mice (Figure 4D). Although the total CD8+ T cell expansion appeared similar in the two sets of infected mice, we wondered whether the artificially high precursor frequency of gp33-specific CTL had substantially altered the overall response of the chimeras to the virus.
Figure 4. GP33-Specific TCR-Transgenic CD8+ T Cells Expand Dramatically during LCMV Infection.
Control B6.PL mice (A and C) or B6.PL mice that had received 105 P14 spleen cells (~3 × 104 transgenic CD8+ cells) 1 day earlier (B and D) were infected with LCMV. After 8 days spleens were removed and assayed for CD4 versus CD8 staining (A and B) and donor (CD8+Thy1.2+) CTL content (C and D). The numbers beside the gating boxes in (A) and (B) indicate the percentage of live gated cells; in (C) and (D) the numbers indicate the number of Thy1.2+ donor cells as a percentage of CD8+ cells. Results are from representative individual mice from groups of three. In uninfected chime-ric mice the donor cells were less than 0.5% of CD8+ cells (data not shown). d8, day 8.
Adoptive Transfer of P14 Splenocytes Does Not Significantly Alter the Overall CTL Response to LCMV
To assess the effect of donor P14 cells on the host response to LCMV, we studied B6.PL mice and PL/P14 chimeras that had received 103, 105, or 106 P14 spleen cells. One day after cell transfer the mice were infected with LCMV. Spleen cells were prepared on day 8 of infection, used as effector cells in a primary CTL assay against peptide-pulsed EL4 target cells (Figure 5), and analyzed by FACS. Uninfected chimeras that received 105 P14 cells did not respond to LCMV peptides. The CTL responses of the infected PL/P14 chimeras to each of the three LCMV epitopes were essentially identical to those of the infected B6.PL mice, although following transfer of 106 P14 cells the response to gp276 was reduced (Figure 5C). In this experiment, the donor CD8+ cells expanded to 1.5%, 26%, and 28% of CD8+ cells in the chimeras that received 103, 105, and 106 P14 cells, respectively.
Figure 5. Adoptive Transfer of a Limited Number of gp33-Specific CD8+ T Cells Does Not Significantly Alter the Overall Response to gp33, gp276, or np396 during the Immune Response to LCMV.
Primary CTL activity of splenocytes from uninfected B6.PL mice that received 105 P14 cells (open circles) and day 8 LCMV-infected mice (filled symbols) that received no P14 cells (squares), 103 P14 cells (diamonds), 105 P14 cells (triangles), or 106 P14 cells (crosses) 1 day prior to infection. Lysis was determined on day 8 of infection by 51Cr-release assay using EL4 targets without additional peptide (A) and EL4 pulsed with gp33 (B), gp276 (C), or np396 (D) peptides. Data are presented as the average specific lysis of targets by effectors from three individual mice per group, with standard deviation indicated by error bars (some of which fall within the symbols). Two repetitions of the experiment yielded similar results.
As another way of looking at the effect of transferring gp33-specific T cells into naive hosts, we compared the responses to the three epitopes of LCMV in IFNγ ELISPOT assays. In individual LCMV-infected B6 and B6.PL mice, the ratio of np396-stimulated IFNγ-secreting cells to gp33-stimulated IFNγ-secreting cells varied from 0.7 to 1.7, and the ratio of gp276- to gp33-stimulated IFNγ-secreting cells from 0.1 to 0.3 (data not shown). In an additional experiment, groups of three PL/P14 mice received 102, 103, 104, 105, or 106 P14 spleen cells and were infected with LCMV the next day. The np396 to gp33 IFNγ-secreting ratios were 0.76 ± 0.02, 1.0 ± 0.5, 1.5 ± 0.4, 1.3 ± 0.3, and 1.6 ± 0.5, respectively. The gp276 to gp33 IFNγ ratios were 0.09 ± 0.03, 0.13 ± 0.04, 0.16 ± 0.05, 0.11 ± 0.04, and 0.09 ± 0.04. Therefore, addition of 30±3 × 105 unstimulated gp33-specific CD8+ T cells does not alter the choice or hierarchy of epitopes targeted in the anti-LCMV CTL response.
The FACS data from the PL/P14 mice that received 105 P14 cells indicated that at least 23%–26% of the CD8+ cells were gp33 specific. By comparing the lysis of np396- and gp276-coated targets to gp33-specific lysis, we calculated that another 23%–26% of the CD8+ cells were np396 specific and that 5% of the CD8+ cells were gp276 specific. Therefore, a total of 54% of the CD8+ cells were LCMV specific on day 8 of infection.
Donor P14 and Host T Cells Are Functionally Active in PL/P14 Chimeric Mice
To exclude the possibility that the TCR-transgenic donor cells were proliferating but not functional within the infected chimeras, control B6, B6.PL, and PL/P14 mice that had received 104 P14 cells 1 day earlier were infected with LCMV, and, 8 days later, spleen cells were prepared and treated with rabbit complement alone, anti-Thy1.1 and complement, or anti-Thy1.2 and complement and used as effectors in a 51Cr-release assay (Figure 6). By flow cytometry, 13% of the CD8+ cells in the infected chimeras were of donor origin prior to antibody treatment, and donor cells were reduced to fewer than 0.5% of the CD8+ cells by treatment with anti-Thy1.2 and complement. As expected, the cytolytic activity of B6 splenocytes (Figure 6A) was unaffected by anti-Thy1.1 treatment (Figure 6D) but was ablated by treatment with anti-Thy1.2 (Figure 6G). Conversely, B6.PL activity (Figure 6B) was abolished by anti-Thy1.1 treatment (Figure 6E) and unaffected by anti-Thy1.2 treatment (Figure 6H). The overall pattern of CTL activity of the PL/P14 chimeras (Figure 6C) was similar to the activity of the B6 and B6.PL mice (Figures 6A and 6B). Following treatment with anti-Thy1.1 and complement, all activity against gp276 and np396 was lost, while activity against gp33, the target of the donor cells, was reduced approximately 50% (Figure 6F). CTL activity of the splenocytes from the chimeras against gp276 and np396 was unaffected by treatment with anti-Thy1.2 and complement, while activity against gp33 was reduced approximately 50%. It thus appears that host CTL develop normally against gp276 and np396 in the chimeras and that the CTL activity against gp33 is a 50:50 composite of host-derived and donor P14-derived CTL.
Figure 6. Both Adoptively Transferred P14 and Host CD8+ T Cells Are Functionally Active in LCMV-Infected PL/P14 Chimeras.
A group of three B6.PL mice received 104 P14 spleen cells. The next day the PL/P14 chimeras (C, F, and I) and groups of three B6 (A, D, and G) and B6.PL (B, E, and H) mice were infected with LCMV. On day 8 of infection, splenocytes pooled for each group were prepared and treated with rabbit complement alone (A–C), with anti-Thy1.1 (anti-host) plus complement (D–F), or with anti-Thy1.2 (anti-donor) plus complement (G–I), and the ability of the surviving cells to lyse uncoated EL4 targets and EL4 cells coated with the indicated LCMV peptides was determined in a 51Cr-release assay.
Since half of the CTL activity against gp33 is contained in the donor cells and they make up 13% of the CD8+ cells, we infer that 26% of the CD8+ cells are gp33 specific. Therefore, by correlating effector cell numbers with the peptide-specific CTL activity, we propose that another 26% of the CD8+ cells are np396 specific and 5% of the CD8+ cells are gp276-specific, for a total of 57% of the CD8+ cells.
Discussion
The Extent of Antigen-Specific versus Bystander Expansion
Following Armstrong LCMV infection of B6 mice, there was a large increase in the number of splenocytes and in the representation of CD8+ cells within the population (Figures 1 and 4). It has been determined by LDA that 0.5%–2% of these CD8+ cells are specific for LCMV (Lau et al., 1994; Razvi et al., 1995) and, by inference, that the bulk of the proliferating cells are bystanders responding to cytokines produced during the immune response (Yang et al., 1989; Tough et al., 1996).
We have found that the primary cytotoxic activity directed against LCMV epitopes and the frequency of CD8+ cells that secrete IFNγ in response to these epitopes correlate well with each other (Figure 2). Although we cannot show strictly that the same cells have both activities, they both are clearly antigen-specific responses of the CD8+ T cells to viral epitopes. At the peak of the immune response to LCMV, when CD8+ T cells constitute half of the spleen (Figures 1 and 4), we found in ELISPOT assays that a total of 24% of the CD8+ T cells secrete IFNγ in response to the three principle H-2b–restricted epitopes of LCMV: gp33, gp276, and np396 (Figure 2). Therefore, since the ELISPOT assay may not be 100% efficient, at least a quarter of the CD8+ T cells present are responding specifically to the virus. In light of the earlier published reports indicating much lower CTL precursor frequencies, we became interested in the nature of bystander activation and its relation to virus specific CTL proliferation.
To see whether we could induce bystander proliferation of CD8+ T cells of known but irrelevant specificity, we adoptively transferred TCR-transgenic OT-1 cells into Thy1 congenic host mice, which we then infected with vaccina virus or LCMV. The OT-1 CD8+ T cells are specific for Kb/OVA257-264 and do not have measurable cytotoxic, cytokine, or proliferative responses to any of the three LCMV epitopes in vitro, nor do they respond to virus-infected cells (data not shown). We found that the representation of the donor cells was reduced in adoptively transferred chimeras infected with LCMV or VVflu-np, a recombinant virus that expresses an irrelevant influenza epitope, and was greatly increased after infection with VVova, which expresses ovalbumin (Figure 3). In agreement with our results, it has also been reported that H-Y–specific CD8+ T cells do not become activated when adoptively transferred into hosts responding to LCMV (Zarozinski and Welsh, 1997) and that vaccina virus infection of mice transgenic for an LCMV gp33-specific TCR does not lead to substantial activation of the CD8+ cells bearing the transgenic receptor (Ehl et al., 1997).
Since the number of nonspecific CD8+ T cells did not appear to be significantly expanded in mice responding to virus, we next sought to determine the degree to which virus-specific CTL would proliferate in chimeric mice that had received various numbers of gp33-specific P14 cells (PL/P14 chimeras). When chimeras that received 105 P14 spleen cells (~3 × 104 gp33-specific CD8+ cells) were infected with LCMV, the number of donor cells was greatly increased by day 8 of infection, to about 24.5% of total CD8+ cells. This occurred without significant alteration to the total expansion of CD8+ cells or the overall CD8:CD4 ratio from that observed in LCMV-infected B6 or B6.PL mice (Figures 1 and 4). Moreover, the proliferation of the donor-derived gp33-specific cells did not alter the targeting of the other two epitopes in CTL assays (Figure 5). Since the cytolytic activity is proportional to the frequency of the antigen-specific cells (Figure 2), we can deduce that the number of host-derived np396-specific cells must be similar to the number of gp33-specific cells and that there must be, in addition, approximately one fifth as many host-derived gp276-specific cells. Therefore, the total number of CD8+ T cells in these mice responding to the three LCMV epitopes on day 8 of infection must be at least 54% of the total CD8+ cells. It also has been reported that half of the CD8+ T cells express cytoplasmic granules containing serine proteinase-1 (MTSP-1) at the peak of LCMV infection (Kramer et al., 1989).
In a group of PL/P14 chimeric mice that initially received 104 P14 spleen cells (approximately 3000 transgenic CD8+ T cells), the donor cells increased to 13% of the CD8+ cells by day 8 of infection. Since the total anti-gp33 activity that develops during the immune response is the same regardless of the number of donor cells transferred into the host (Figure 5), the total number of anti-gp33 CTL (host + donor) must also be the same. Following antibody plus complement depletion of host or donor CTL from the day 8 spleen cells of PL/P14 chimeras that received 104 P14 (Figure 6), a comparison of the reduction in gp33-specific cytolytic activity shows that about 50% of the CTL activity was derived from Thy1.1+ host cells and 50% of the activity was derived from Thy1.2+ donor cells. The total number of gp33-specific CTL, therefore, was 2 × 13%, or 26%. This implies that another 26% of the cells must be np396 specific and that 5% of the CD8+ cells are gp276 specific. Thus, 57% of the CD8+ cells are LCMV specific, a figure in close agreement with the results obtained after transfer of 105 P14 cells (Figure 5).
Although we estimate on the basis of our adoptive transfer studies that about half of the CD8+ T cells on day 8 of infection are specific for LCMV, only a quarter of CD8+ cells were detected as secreting IFNγ in response to the three LCMV peptides in our ELISPOT assays. It is possible that only half of the anti-LCMV CD8+ cells are able to secrete IFNγ in response to viral antigens or that the ELISPOT assay is only 50% efficient at detecting IFNγ-secreting cells.
If the non–LCMV-specific CD8+ T cells of the naive repertoire remain largely within the spleen during LCMV infection, then, together with the half of the CD8+ cells that are LCMV specific, they constitute 63% of the CD8+ cells within the spleen on day 8 of infection. This means that the upper limit of bystander-expanded CD8+ cells is 37%, or about 2.5 times the original number of splenic CD8+ cells. It has been reported that the proliferation of low-affinity CD8+ T cells can be stimulated by much lower peptide densities than are required for functional responses such as in vivo protection, cytotoxicity, or IFNγ secretion (Speiser et al., 1992; Kageyama et al., 1995; Valitutti et al., 1996). Thus, it may be that some of the CD8+ cells in the day 8 infected mice that we cannot account for are, in fact, low-affinity anti-LCMV CD8+ T cells rather than cells expanding as a result of bystander effects. This would further reduce the significance of bystander-induced proliferation during LCMV infection.
Indirect evidence for the expansion of similarly large numbers of antiviral CD8+ T cells can be found in the expansion of particular TCR Vβ–bearing CD8+ populations during certain infections (Cose et al., 1997). In some individuals responding to Epstein-Barr virus infection (Callan et al., 1996) or to human immunodeficiency virus infection (Pantaleo et al., 1994), a transient, oligoclonal expansion of CD8+ T cells expressing particular TCR Vβ chains to as much as 40% of CD8+ T cells has been observed. Because these expansions parallel the rise and fall of circulating virus in the blood, because they are oligoclonal, and because different TCR Vβ are expanded in different individuals, the expansions do not appear to be superantigen driven. In one case, virus-specific CTL activity could also be found in the expanded cell population (Pantaleo et al., 1994), suggesting that the cells were antigen specific. The significance of these expansions is unclear because they are seen in only some individuals, but it may be that in the usual response to infection there is an equally large expansion of a polyclonal repertoire of antiviral CD8+ T cells. Our data support this model and suggest that large expansions of antiviral CD8+ T cells may be a common feature of antiviral immune responses.
An Estimate of Precursor Frequency
The precursor frequency of CTL prior to antigen exposure has been determined for some antigens by LDA; for LCMV it has been estimated to be 1 in 560,000 (Selin et al., 1994). Because the gp33-specific CTL response of PL/P14 chimeras that receive 104 P14 spleen cells (~3000 CD8+ cells) is evenly divided between host- and donor-derived cells, we believe that there are approximately 3000 gp33-specific CTL precursors in naive B6.PL mouse. Since we estimate that there are approximately 3 × 107 CD8+ T cells in a naive mouse, the precursor frequency of gp33-specific CTL is about 10–4. We presume that the frequency of np396- and gp276-specific precursors is roughly similar. By day 8 of LCMV infection we estimate that the total number of CD8+ T cells rises to about 1.5 × 108 (E. A. B., unpublished data); 55%, or 8.3 × 107, are LCMV specific. This would necessitate about 15 divisions over the 8 days of infection, or 1 division every 13 hr. While rapid, this rate of proliferation is well below the 7 hr dividing time that has been observed for B cells in germinal centers (Liu et al., 1991). Since some of the P14 donor cells are probably lost during the adoptive transfer, this is likely to be an overestimate of the CTL precursor frequency. There is also a delay following infection of the mice as the viral proteins are generated and subsequently processed and presented by antigen-presenting cells (APC) to T cells, so the time during which the T cells expand will be shorter than 8 days. For these reasons the real rate of T cell division will be greater than our estimates.
Epitope-Specific Competition for the APC
Since the TCR-transgenic donor cells make up a smaller fraction of the total CD8+ cells in the LCMV-infected chimeras when fewer P14 cells are initially transferred, it is clear that precursor frequency plays a large role in deciding which clones respond to a given epitope in the response to the virus. Thus, as greater numbers of P14 cells are transferred they come to dominate the anti-gp33 response. However, since we can vary the precursor frequency of gp33-specific CD8+ T cells by more than 100-fold without affecting the number of gp33-specific, np396-specific, or gp276-specific CTL that ultimately develop, precursor frequency does not dictate epitope dominance in this system. Rather, it must be that antigen presentation is limiting in the priming and expansion of the CD8+ T cells.
When 103–105 P14 spleen cells (representing 3 × 102–3 × 104 CD8+ cells) are transferred into naive B6.PL hosts, there is no effect on the overall CTL response to the three LCMV epitopes. At all of these doses, the level of host CTL response to np396 and gp276 is unchanged from the response of control B6.PL mice. The total host-plus-donor T cell response to gp33 also remains constant, but the composition of this response changes. For example, at 103 cells transferred, only 1.5%–1.7% of the CD8+ T cells are donor derived, and these make up a small fraction of the total CTL activity against gp33. At 104 cells transferred, donor-derived cells make up 13%–16% of total CD8+ cells and account for about 50% of the activity against gp33. At 105 cells transferred, donor CD8+ cells make up 23%–26% of total CD8+ cells and dominate the anti-gp33 activity to the point of suppressing host T cells of this specificity. It is noteworthy that, as we increase the number of donor cells transferred from 104 to 105, the contribution of gp33-specific donor CD8+ cells to the response does not increase 10-fold. Thus, an excess of gp33-specific precursors holds the host and donor precursor T cells of the same specificity in check while, at the same time, the host response to the other two epitopes is unaffected. From this observation we conclude that there is an epitope-specific regulation of the response.
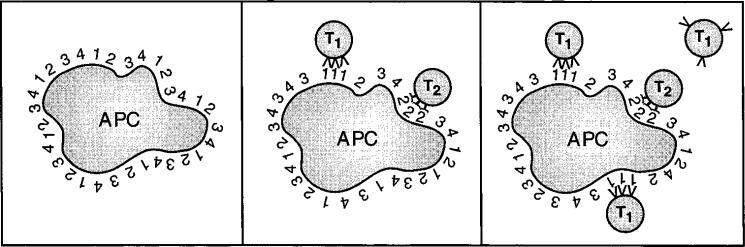
The APC that present the LCMV peptides to CTL precursors are either LCMV-infected themselves or have phagocytosed other infected cells and processed their proteins for presentation by MHC class I (Bevan, 1995). Since it is inconceivable that separate APC present each of the three LCMV epitopes, we must conclude that CD8+ T cells compete for epitopes on the same APC. Figure 7 illustrates this notion by supposing that, following viral infection, an APC presents a set of viral epitopes on its surface. Due to competition with self peptides that bind MHC class I, the number of copies of each epitope expressed per APC is likely to be 103 or fewer (Rammensee et al., 1993). CD8+ T cells specific for any epitope will take up space on the APC surface, but in addition to this epitope-nonspecific competition, we propose that T cells occupying the APC will sequester their own target epitopes in a specific manner (Figure 7, middle). An excess of CD8+ T cells specific for epitope 1 will sequester this epitope, reducing the available density from 103 per APC to a point where other T cells of the same specificity cannot recognize the APC (Figure 7, right). At the same time, the APC still presents other epitopes at 103 available copies per cell, so CD8+ T cells specific for these epitopes can continue to bind the APC and become activated.
Figure 7. Competition for MHC Class I–Peptide Complexes during CTL Priming.
(Left) A virus-infected APC displays a large number of MHC class I–peptide complexes, indicated by the different numbers on the surface of the cell.
(Center) When CTL precursors specific for epitopes 1 and 2 (T1 and T2) interact with the APC, they aggregate their respective target MHC–peptide complexes at the zones of contact.
(Right) When enough T1 interact with the APC, they reduce the available MHC–peptide 1 complexes to the point where other T1 cells cannot be primed, although cells of other specificities (T2) can still interact productively with the APC.
What is the nature of the sequestration of MHC class I peptide epitopes on the APC? It may be that the TCR and its ligand aggregate at the zone of contact of the two cells. This aggregation of MHC molecules could lead to their endocytosis by the APC. In this way the class I–peptide complexes would be turned over just as TCR complexes are turned over during T cell activation (Valitutti et al., 1995). Even without TCR-induced endocytosis of MHC class I, however, it is clear that TCR engagement may hide epitopes in a specific way. Whatever the mechanism, some degree of concentration or aggregation of specific class I epitopes on the APC into the zone of contact with the T cell would be required to explain the epitope-specific regulation of the response.
This model has important implications for our understanding of how the epitope profile of a CD8+ T cell response is controlled and suggests that the nature of T cell priming works to diversify the epitopes targeted by CTL responses.
Experimental Procedures
Mice
C57Bl/6 (B6) mice were purchased from Taconic Farms (Germantown, PA). Thy1 congenic B6.PL-Thy1a/Cy (B6.PL) mice were purchased from Jackson Laboratories (Bar Harbor, ME), and anti-Db/LCMV GP-1 (33–41) TCR-transgenic P14 mice on a B6 background (Pircher et al., 1990) were purchased from Jackson Laboratories and then bred in the University of Washington specific pathogen–free animal facilities. Anti-Kb/chicken OVA257-264 TCR-transgenic OT-1 mice on a B6 background have been described elsewhere (Hogquist et al., 1994) and were bred in our specific pathogen–free animal facilities. All mice used in these studies were 1- to 4-month-old females.
Cell Lines
EL4 (ATCC TIB-41) are a B6-derived (H-2b), MHC class II– thymoma cell line and were maintained in RP10 (RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum, 2 mM l-glutamine, and antibiotics).
Viruses
The Armstrong 53b strain of LCMV was originally obtained from Peter J. Southern (University of Minnesota, Minneapolis, MN) and was grown on BHK-21 cells (ATCC CCL-10) and titered on Vero Cl008 cells (ATCC CRL-1586) as described (Ahmed et al., 1984). Mice were infected by intravenous injection of 1 × 105 pfu of LCMV. Recombinant VV were obtained from Jonathan Yewdell (National Institutes of Health, Bethesda, MD). VVova expresses a full-length cDNA for OVA (Bacik et al., 1994), and VVflu-np expresses a partial influenza nucleoprotein cDNA (Yewdell et al., 1985). VV were grown on HeLa S3 cells and titered on Vero cells according to standard protocols (Mackett et al., 1985). Mice were infected by intravenous injection of 5 × 106 pfu of VV.
Peptides
The H-2Db-binding LCMV peptides LCMV gp33 (KAVYNFATC), gp276 (SGVENPGGYCL), and np396 (FQPQNGQFI) and the Kb-binding OVA257-264 (SIINFEKL) were synthesized using an Applied Biosystems Synergy (Foster City, CA) peptide synthesizer. Peptide concentrations were determined using the BCA assay (Pierce Chemical, Rockford, IL). The gp33 and gp276 peptides were dissolved in acidified RPMI-1640 with 1 mM 2-mercaptoethanol to prevent cysteine dimer formation.
Antibodies and Flow Cytometry
For flow cytometry we used directly conjugated anti-CD4–fluorescein isothiocyanate (FITC), anti-CD8-phycoerythrin, anti-Thy1.1-FITC, and anti-TCR Vα2-FITC (Pharmingen, San Diego, CA) and anti-Thy1.2-biotin and streptavidin-Tricolor (CalTag, South San Francisco, CA). All staining also included 2% normal mouse serum and the anti-FcγRII antibody 24G2 (Pharmingen) to reduce nonspecific and Fc receptor–mediated binding. Analysis was done on a Becton-Dickenson FACScan with Lysis-II software (Becton-Dickinson, Mountain View, CA).
The anti-IFNγ antibody R4-6A2 (ATCC HB-170) was protein G purified from tissue culture supernatants. Biotinylated XMG-1.2, which recognizes a different epitope of murine IFNγ, was purchased from Pharmingen. The 19E12 monoclonal antibody, specific for Thy1.1, and the 30H12 anti-Thy1.2 monoclonal antibody were produced as ascites.
IFNγ ELISPOT Assays
Cells secreting IFNγ in an antigen-specific manner were detected using a standard ELISPOT assay (Miyahira et al., 1995). In brief, EL4 target cells were incubated in phosphate-buffered saline (PBS) with or without 1 μM peptide as indicated, washed several times, and added at 105 per well to graded numbers of erythrocyte-depleted effector cells in 96-well Multiscreen-HA plates (Millipore, Bedford MA) that had been precoated with protein G–purified R4–6A2. After 20–24 hr, cells were removed; the plates were extensively washed; and the plates were developed by incubation with XMG-1.2-biotin, followed by streptavidin–horseradish peroxidase and diaminobenzi-dine (Sigma, St. Louis, MO).
Primary Ex Vivo Chromium Release Assays
Target cells were prepared by incubation for 1–2 hr with or without peptide in the presence of sodium 51Cr-chromate, washed three times in PBS and resuspended in RP10. For the assay, 104 target cells were added to 96-well round-bottom plates along with different numbers of erythrocyte-depleted effector cells in a total volume of 200 μl. After 8 hr, 100 μl of supernatant was removed and counted in a Wallac 1470 Wizard γ-counter (Wallac Oy, Turku, Finland). Specific lysis was calculated as ([experimental release – spontaneous release]/[maximum release – spontaneous release]) × 100%. Spontaneous release was determined for target cells in medium alone, and maximum release was determined by incubating target cells in 1% Triton X-100. Spontaneous release was typically 10%–20%. Lytic units were calculated as the number of effector cells required to achieve 30% lysis of target cells.
In some experiments donor or host effector T cells were depleted by incubating spleen cell suspensions with medium alone, with 5 μg/ml anti-Thy1.1 antibody (19E12), or with 10 μg/ml anti-Thy1.2 (30H12) on ice for 30 min followed by addition of rabbit complement (Low-Tox M, Cedarlane, Westbury, NY) and incubation for 30 min at 37°C.
Acknowledgments
We thank Marianne Zollman and Ethan Ojala for technical assistance. We thank Stefan Martin, Brad Cookson, Jacqueline Kirchner, Ananda Goldrath, and Laurel Lenz for their comments on the manuscript.
References
- Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MBA. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984;160:521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson EC, Christensen JP, Scheynius A, Marker O, Thomsen AR. Lymphocytic choriomeningitis virus infection is associated with long-standing perturbation of LFA-1 expression on CD8+ T cells. Scand J Immunol. 1995;42:110–118. doi: 10.1111/j.1365-3083.1995.tb03633.x. [DOI] [PubMed] [Google Scholar]
- Bacik I, Cox JH, Anderson R, Yewdell JW, Bennink JR. TAP (transporter associated with antigen processing)-independent presentation of endogenously synthesized peptides is enhanced by endoplasmic reticulum insertion sequences located at the amino- but not carboxyl-terminus of the peptide. J Immunol. 1994;152:381–387. [PubMed] [Google Scholar]
- Bevan MJ. Antigen presentation to cytotoxic T lymphocytes in vivo. J Exp Med. 1995;182:639–641. doi: 10.1084/jem.182.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverley PCL. Generation of T-cell memory. Current Opinion in Immunology. 1996;8:327–330. doi: 10.1016/s0952-7915(96)80120-8. [DOI] [PubMed] [Google Scholar]
- Buchmeier MJ, Welsh RM, Dutko FJ, Oldstone MBA. The virology and immunobiology of lymphocytic choriomeningitis virus infection. Adv Immunol. 1980;30:275–331. doi: 10.1016/s0065-2776(08)60197-2. [DOI] [PubMed] [Google Scholar]
- Callan MF, Steven N, Krausa P, Wilson JD, Moss PA, Gillespie GM, Bell JI, Rickinson AB, McMichael AJ. Large clonal expansions of CD8+ T cells in acute infectious mononucleosis. Nat Med. 1996;2:906–911. doi: 10.1038/nm0896-906. [DOI] [PubMed] [Google Scholar]
- Cauda R, Prasthofer EF, Tilden AB, Whitley RJ, Grossi CE. T-cell imbalances and NK activity in varicella-zoster virus infections. Viral Immunol. 1987;1:145–152. doi: 10.1089/vim.1987.1.145. [DOI] [PubMed] [Google Scholar]
- Cose SC, Jones CM, Wallace ME, Heath WR, Carbone FR. Antigen-specific CD8+ T cell subset distribution in lymph nodes draining the site of herpes simplex virus infection. Eur. J. Immunol. 1997;27:2310–2316. doi: 10.1002/eji.1830270927. [DOI] [PubMed] [Google Scholar]
- Ehl S, Hombach J, Aichele P, Hengartner H, Zinkernagel RM. Bystander activation of cytotoxic T cells: studies on the mechanism and evaluation of in vivo significance in a transgenic mouse model. J Exp Med. 1997;185:1241–1251. doi: 10.1084/jem.185.7.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gairin JE, Mazarguil H, Hudrisier D, Oldstone MB. Optimal lymphocytic choriomeningitis virus sequences restricted by H-2Db major histocompatibility complex class I molecules and presented to cytotoxic T lymphocytes. J Virol. 1995;69:2297–2305. doi: 10.1128/jvi.69.4.2297-2305.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Kageyama S, Tsomides TJ, Sykulev Y, Eisen HN. Variations in the number of peptide-MHC class I complexes required to activate cytotoxic T cell responses. J Immunol. 1995;154:567–576. [PubMed] [Google Scholar]
- Kedl RM, Mescher MF. Migration and activation of antigen-specific CD8+ T cells upon in vivo stimulation with allogeneic tumor. J Immunol. 1997;159:650–663. [PubMed] [Google Scholar]
- Kramer MD, Fruth U, Simon HG, Simon MM. Expression of cytoplasmic granules with T cell-associated serine proteinase-1 activity in Ly-2+(CD8+) T lymphocytes responding to lymphocytic choriomeningitis virus in vivo. Eur J Immunol. 1989;19:151–156. doi: 10.1002/eji.1830190124. [DOI] [PubMed] [Google Scholar]
- Lau LL, Jamieson BD, Somasundaram T, Ahmed R. Cytotoxic T-cell memory without antigen. Nature. 1994;369:648–652. [PubMed] [Google Scholar]
- Liu YJ, Zhang J, Lane PJ, Chan EY, MacLennan IC. Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur J Immunol. 1991;21:2951–2962. doi: 10.1002/eji.1830211209. [DOI] [PubMed] [Google Scholar]
- Lynch F, Doherty PC, Ceredig R. Phenotypic and functional analysis of the cellular response in regional lymphoid tissue during an acute virus infection. J Immunol. 1989;142:3592–3598. [PubMed] [Google Scholar]
- Mackett M, Smith GL, Moss B. The construction and characterization of vaccinia virus recombinants expressing foreign genes. In: Glover DM, editor. DNA cloning: a practical approach. IRL; Oxford: 1985. pp. 191–211. [Google Scholar]
- McFarland HI, Nahill SR, Maciaszek JW, Welsh RM. CD11b (Mac-1): a marker for CD8+ cytotoxic T cell activation and memory in virus infection. J Immunol. 1992;149:1326–1333. [PubMed] [Google Scholar]
- Miyahira Y, Murata K, Rodriguez D, Rodriguez JR, Esteban M, Rodriguez MM, Zarala F. Quantification of antigen specific CD8+ T cells using an ELISPOT assay. J. Immunol. Meth. 1995;181:45–54. doi: 10.1016/0022-1759(94)00327-s. [DOI] [PubMed] [Google Scholar]
- Mueller DL, Jenkins MK. Autoimmunity: when self-tolerance breaks down. Curr Biol. 1997;7:255–257. doi: 10.1016/s0960-9822(06)00115-1. [DOI] [PubMed] [Google Scholar]
- Nahill SR, Welsh RM. High frequency of cross-reactive cytotoxic T lymphocytes elicited during the virus-induced polyclonal cytotoxic T lymphocyte response. J Exp Med. 1993;177:317–327. doi: 10.1084/jem.177.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleo G, Demarest JF, Soudeyns H, Graziosi C, Denis F, Adelsberger JW, Borrow P, Saag MS, Shaw GM, Sekaly RP. Major expansion of CD8+ T cells with a predominant V beta usage during the primary immune response to HIV. Nature. 1994;370:463–467. doi: 10.1038/370463a0. et, a. l. [DOI] [PubMed] [Google Scholar]
- Pape KA, Kearney ER, Khoruts A, Mondino A, Merica R, Chen ZM, Ingulli E, White J, Johnson JG, Jenkins MK. Use of adoptive transfer of T-cell-antigen-receptor-transgenic T cell for the study of T-cell activation in vivo. Immunol Rev. 1997;156:67–78. doi: 10.1111/j.1600-065x.1997.tb00959.x. [DOI] [PubMed] [Google Scholar]
- Pircher H, Moskophidis D, Rohrer U, Burki K, Hengartner H, Zinkernagel RM. Viral escape by selection of cytotoxic T cell-resistant virus variants in vivo. Nature. 1990;346:629–633. doi: 10.1038/346629a0. [DOI] [PubMed] [Google Scholar]
- Rammensee HG, Falk K, Rotzschke O. Peptides naturally presented by MHC class I molecules. Annu Rev Immunol. 1993;11:213–244. doi: 10.1146/annurev.iy.11.040193.001241. [DOI] [PubMed] [Google Scholar]
- Razvi ES, Welsh RM, McFarland HI. In vivo state of antiviral CTL precursors. Characterization of a cycling cell population containing CTL precursors in immune mice. J Immunol. 1995;154:620–632. [PubMed] [Google Scholar]
- Rott O, Mignon GK, Fleischer B, Charreire J, Cash E. Superantigens induce primary T cell responses to soluble autoantigens by a non-V beta-specific mechanism of bystander activation. Cell Immunol. 1995;161:158–165. doi: 10.1006/cimm.1995.1022. [DOI] [PubMed] [Google Scholar]
- Rubin RH, Carney WP, Schooley RT, Colvin RB, Burton RC, Hoffman RA, Hansen WP, Cosimi AB, Russell PS, Hirsch MS. The effect of infection on T lymphocyte subpopulations: a preliminary report. Int J Immunopharmacol. 1981;3:307–312. doi: 10.1016/0192-0561(81)90024-2. [DOI] [PubMed] [Google Scholar]
- Selin LK, Nahill SR, Welsh RM. Cross-reactivities in memory cytotoxic T lymphocyte recognition of heterologous viruses. J Exp Med. 1994;179:1933–1943. doi: 10.1084/jem.179.6.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speiser DE, Kyburz D, Stubi U, Hengartner H, Zinkernagel RM. Discrepancy between in vitro measurable and in vivo virus neutralizing cytotoxic T cell reactivities: Low T cell receptor specificity and avidity sufficient for in vitro proliferation or cytotoxicity to peptide-coated target cells but not for in vivo protection. J Immunol. 1992;149:972–980. [PubMed] [Google Scholar]
- Strang G, Rickinson AB. In vitro expansion of Epstein-Barr virus-specific HLA-restricted cytotoxic T cells direct from the blood of infectious mononucleosis patients. Immunology. 1987;62:647–654. [PMC free article] [PubMed] [Google Scholar]
- Tough DF, Sprent J. Turnover of naive- and memory-phenotype T cells. J Exp Med. 1994;179:1127–1135. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tough DF, Sprent J. Viruses and T cell turnover: evidence for bystander proliferation. Immunol. Rev. 1996;150:129–142. doi: 10.1111/j.1600-065x.1996.tb00699.x. [DOI] [PubMed] [Google Scholar]
- Tough DF, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 1996;272:1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- Tripp RA, Hou S, McMickle A, Houston J, Doherty PC. Recruitment and proliferation of CD8+ T cells in respiratory virus infections. J Immunol. 1995;154:6013–6021. [PubMed] [Google Scholar]
- Unutmaz D, Pileri P, Abrignani S. Antigen-independent activation of naive and memory resting T cells by a cytokine combination. J Exp Med. 1994;180:1159–1164. doi: 10.1084/jem.180.3.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valitutti S, Muller S, Cella M, Padovan E, Lanzavecchia A. Serial triggering of many T-cell receptors by a few peptide-MHC complexes. Nature. 1995;375:148–151. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- Valitutti S, Muller S, Dessing M, Lanzavecchia A. Different responses are elicited in cytotoxic T lymphocytes by different levels of T cell receptor occupancy. J Exp Med. 1996;183:1917–1921. doi: 10.1084/jem.183.4.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Welsh RM. Induction of alloreactive cytotoxic T cells by acute virus infection of mice. J Immunol. 1986;136:1186–1193. [PubMed] [Google Scholar]
- Yang HY, Dundon PL, Nahill SR, Welsh RM. Virus-induced polyclonal cytotoxic T lymphocyte stimulation. J Immunol. 1989;142:1710–1718. [PubMed] [Google Scholar]
- Yewdell JW, Bennink JR, Smith GL, Moss B. Influenza A virus nucleoprotein is a major target antigen for cross-reactive anti-influenza A virus cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1985;82:1785–1789. doi: 10.1073/pnas.82.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarozinski CC, Welsh RM. Minimal bystander activation of CD8 T cells during the virus-induced polyclonal T cell response. J Exp Med. 1997;185:1629–1639. doi: 10.1084/jem.185.9.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman C, Brduscha RK, Blaser C, Zinkernagel RM, Pircher H. Visualization, characterization, and turnover of CD8+ memory T cells in virus-infected hosts. J Exp Med. 1996;183:1367–1375. doi: 10.1084/jem.183.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]