Abstract
Epileptiform neuronal activity during seizures is observed in many brain areas, but its origins following status epilepticus (SE) are unclear. We have used the Li-low dose pilocarpine rat model of temporal lobe epilepsy (TLE) to examine early development of epileptiform activity in the deep entorhinal cortex (EC). We show that during the 3 week latent period that follows SE, an increasing percentage of neurons in EC layer 5 respond to a single synaptic stimulus with polysynaptic burst depolarizations. This change is paralleled by a progressive depolarizing shift of the IPSP reversal potential in layer 5 neurons, apparently caused by upregulation of the Cl- inward transporter NKCC1 and concurrent downregulation of the Cl- outward transporter KCC2, both changes favoring intracellular Cl- accumulation. Inhibiting Cl- uptake in the latent period restored more negative GABAergic reversal potentials and eliminated polysynaptic bursts. The changes in the Cl- transporters were highly specific to the deep entorhinal cortex. They did not occur in layers 1-3, perirhinal cortex, subiculum or dentate gyrus during this period. We propose that the changes in Cl- homeostasis facilitate hyperexcitability in the deep entorhinal cortex leading to epileptiform discharge there, which subsequently affects downstream cortical regions.
Keywords: rat, Li-pilocarpine; GABA; NKCC1; KCC2; bumetanide
Introduction
Mesial temporal lobe epilepsy (TLE) is the most common type of epilepsy in adults (Engel, 1989), and frequently becomes resistant to drug therapy, leaving ultimately only a neurosurgery option to control seizures. TLE develops following a variety of insults, including brain injury and status epilepticus (SE). Precipitating insults are followed by a characteristic seizure-free “latent period” lasting months or even years in humans (Annegers et al., 1980; Weiss et al., 1986). The TLE in rats that arises several weeks after a lithium-pilocarpine induced SE reproduces most clinical and neuropathological features of human TLE, and presents a very useful animal model of the disease (Ormandy et al., 1989; Turski et al., 1991; Cavalheiro, 1995; Dube et al., 2000; Andre et al., 2007). Here we exploit this rat model of TLE for the study of early events in the latent period that cannot be accessed in human tissue. Even though initial causes may vary, the behavioral and histo-pathological hallmarks of TLE are remarkably similar in all etiologies. This has lead a number of investigators to hypothesize that there is a major common pathway downstream of initiating causes, probably the intense synchronous activity that is a signature of seizures (Du et al., 1995; Wu and Schwarcz, 1998; Schwarcz et al., 2000). This synchronous activity is usually seen in hippocampus and parahippocampal cortices, including the EC (Schwartzkroin and Knowles, 1984; Bartolomei et al., 2004). Over the longer term, specific mesial temporal lobe atrophy has been shown ipsilateral to the seizure focus (Bartolomei et al., 2005). Examination of surgically resected specimens has revealed cell loss and astrogliosis in EC (Yilmazer-Hanke et al., 2000).
Exactly where important early changes in neuronal physiology occur after the SE is unclear; however, neuroprotection experiments of hippocampal Cornu Ammonis (CA) regions vs. parahippocampal cortices suggest a key role of the parahippocampal cortices at the early steps of epileptogenesis (Andre et al., 2007). Moreover, we have observed in previous studies that the deep EC exhibits unusual high network excitation, in striking contrast to layers 2 and 3 (Gloveli et al., 1999; Egorov et al., 2003), suggesting that the deep EC may be particularly susceptible to the development of hyperexcitability triggered by status epilepticus. We demonstrate here the early development of greatly increased excitability in the deep EC, i.e. during the latent period post SE. Neurons in L5 begin to respond to normally subthreshold synaptic stimuli with polysynaptic burst discharge. Moreover, we show that, during this latent period, a large depolarizing shift of the GABAA receptor reversal potential occurs due to altered expression of the Cl- transporters NKCC1 (inward) and KCC2 (outward). These changes in transporter expression occur only in the deep EC, but not adjacent hippocampal and cortical areas, during this early stage of epilepsy development. The direct connection between alterations in Cl- transport activity and increased polysynaptic bursting is supported by the finding that block of NKCC1 by bumetanide largely restored IPSP reversal potential and greatly reduced polysynaptic burst discharge. Our results implicate ECL5 as an important locus in the development of TLE.
Materials and Methods
TLE induction
All procedures were approved by the Institutional Animal Care and Use Committee at the University of New Mexico Health Sciences Center and were in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals. Our protocol for the experiments here was a slight modification of a protocol published by Andre et al. (Andre et al., 2007). Twenty four hours prior to seizure induction, male Wistar rats, 2-3 months old, were administered s.c. 3 mmol/kg lithium chloride dissolved in isotonic NaCl saline. Pilocarpine was then administered s.c. (25 mg/kg in isotonic NaCl). Once generalized limbic convulsions began, subcutaneous needle EEG electrodes were placed to confirm the development of status epilepticus (SE), defined as the occurrence of continuous high amplitude EEG spiking (Ormandy et al., 1989). These needles were fashioned from 25 gauge hypodermic needles (Becton Dickinson), with the hub cut off and bent at 60 degree angles so they will stay in place. One needle was inserted subcutaneously in the scalp over the parietal cortex and the other, which served as the reference electrode, over the scapula. The EEG recordings, using a Grass Model 8 electroencephalograph (Astro-Med, West Warwick, RI), showed continuous high amplitude spiking for a 1 hour period following the onset of SE, in clear contrast to normal EEGs, recorded in a previous study with intracranial implanted electrodes (Peterson et al., 2005). Recording was then generally discontinued. Atropine sulfate (10 mg/kg s.c.) and Diazepam (4 mg/kg i.m.) were administered in order to improve survival, decrease anxiety and induce muscle relaxation. This dose of Diazepam did not stop seizures but induced muscle relaxation within one hour and rats were lying on their ventral side, not using their limbs. Nor did atropine stop seizures. 12 hours after SE onset the EEG was recorded again for 5-10 min, and all rats had intermittent or organized, i.e. bursts of EEG spiking. Those rats that showed organized EEG spiking at this time, such as bursts of spiking or continuous trains of spiking (~20% of cases) received an additional 2 mg/kg diazepam, i.m.. 5% dextrose in Ringers solution was administered s.c.12 hours following SE onset.
This protocol differs from the one of Andre et al. (Andre et al., 2007) in that we administered diazepam 1 hour after onset of SE instead of 2 hours. In addition, we applied atropine to reduce peripheral pilocarpine effects (Du et al., 1995). We therefore believe that our protocol should provide a milder insult to the animals.
The protocol of Andre et al. was shown to reliably induce epilepsy in 100% of adult rats (Dube et al., 2000; Andre et al., 2007). This is also true of an earlier protocol from the same lab that used a higher pilocarpine dose with lithium (Dube et al., 2000; Andre et al., 2007). In our lab a previous study using the Andre protocol (Andre et al., 2007) showed all rats developing spontaneous rank 3, 4 or 5 seizures after a latent period of 28.6 ± 2.43 (SEM) days (n=14; continuous 24 hour/day video recording, S. Peterson, unpublished results). Only 1 rat had a seizure by day 15. By day 21 4 rats had experienced a seizure, From fitting these latency data with a normal distribution it is expected that on average ~25% of rats have had a first seizure within 3 weeks of the pilocarpine/2 hr Diazepam treatment. However, we never observed seizures during observing and handling rats prior to sacrifice. The epileptic outcome for our mild protocol was tested 8 weeks post SE with video recordings of up to a total of 36 hours/rat, and, indeed, spontaneous seizures were observed in 10 out of 13 rats. Given the wide variability of durations of the latent phase in humans, we would surmise that with this mild protocol the outcome would reach a significantly higher percentage at longer times post SE. A total of 155 rats was used, 44 Li-control rats, 42 rats 2 weeks post SE, 47 rats 3 weeks post SE, and 22 rats post SE for video recording.
Slice preparation
All experiments were performed on acute horizontal brain slices containing temporal neocortex, entorhinal cortex, subiculum and the ventral hippocampus. Control, or rats 2 or 3 weeks post-seizure, were anesthetized deeply with a mixture of ketamine and xylazine (85 and 15 mg/ml, respectively) and decapitated. The brains were removed rapidly and placed in an oxygenated (95% O2/5% CO2) ice-cold cutting solution containing (in mM): 3 KCl, 1.25 NaH2PO4, 6 MgSO4, 26 NaHCO3, 0.2 CaCl2, 10 glucose, 220 sucrose, and 0.43 ketamine. Slices (350 μm) were cut in ice-cold cutting solution with a vibratome (Dosaka DTK-1000) and transferred from there into Artificial Cerebro-Spinal Fluid (ACSF, in mM: 126 NaCl, 3 KCl, 1.25 NaH2PO4, 1 MgSO4, 26 NaHCO3, 2 CaCl2, and 10 glucose) equilibrated with 95%O2/5%CO2 at 34°C temperature for recovery. After 1 hr ACSF was changed again, and the slices were held at room temperature until used for recording.
Electrophysiology
Individual slices were transferred to a recording chamber mounted on a fixed-stage microscope (Zeiss Axioskop, Jena, Germany) and superfused with warmed (34°C), oxygenated ACSF at 2 ml/min. Intracellular recordings were made from EC layer 5 neurons with borosilicate glass sharp microelectrodes (80-150 MΩ) filled with either 1 M K2SO4, or 0.5 M potassium acetate/0.5 M KCl for lower electrode resistance, or 2 M CsSO4/0.1 M KCl for improved space clamp. In the absence of Cl- transport blockers all intracellular solutions gave identical results for the GABAAergic PSP reversal potential that were therefore pooled (see also: (Misgeld et al., 1986)). During Cl- transport block by bumetanide it was found that sharp microelectrodes filled with 0.5 M potassium acetate/0.5 M KCl-filled sharp microelectrode did shift EIPSP in depolarizing direction by ~20 mV. Therefore all final recordings/data for bumetanide effects on GABAergic PSP reversal potential were obtained with microelectrodes filled with 1 M K2SO4 or 2 M CsSO4/0.1 M KCl.
Neurons were impaled in the medial entorhinal cortex just above the angular bundle, up to ¼ of cortex thickness, using a Nanostepper micropositioner (Scientific Precision Instruments, Oppenheim, Germany). Recordings were made using an Axoclamp 2B amplifier and digitized and recorded using an Axon Instruments Digidata 1322A (Molecular Devices, Union City, CA). Neurons with a RMP negative to −68 mV were tested with hyperpolarizing pulses to ~ −85 mV for the presence of Ih indicative of pyramidal neurons (Egorov et al., 2002; Egorov et al., 2003). By this criterion 75% of the recorded population was pyramidal with the remainder presumably multipolar neurons. In a study by Hamam et al. even 85% of neurons impaled with a sharp electrode in L5 showed a prominent long apical dendrite typical for pyramidal neurons (Hamam et al., 2000). In contrast to our studies this study reported presence of Ih also in non-pyramidal neurons (Hamam et al., 2000). Concentric bipolar stimulating electrodes (200 μm diameter, FHC Inc., ME, USA) were placed in layer 5 of EC, ~150-250 μm from the recording electrode. Stimuli (70-100 μs, 100-800 nA) were controlled by a Master 8 programmable pulse generator connected to a stimulus isolation unit (A.M.P.I., Jerusalem, Israel). Full input-output relations for the EPSP and the IPSP were recorded, and stimulation intensities giving 70-90% of maximal response were used. Where indicated stimulation intensity was reduced to elicit 5-10% of maximal response.
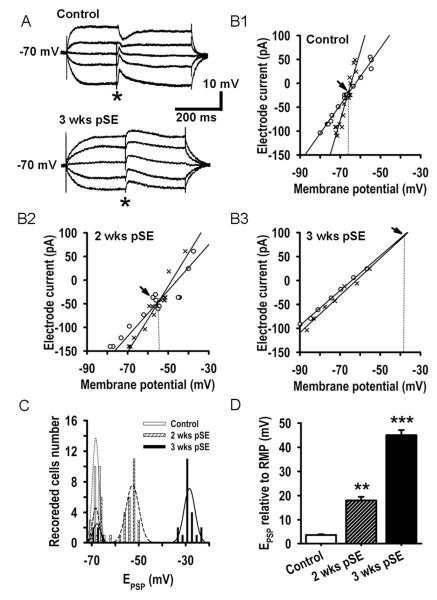
Figure 2A shows the protocol used to determine the reversal potential for GABAA receptor-mediated, postsynaptic potentials. Monosynaptic PSPs were elicited in the presence of glutamate receptor blockers CNQX (10 μM) and APV (50 μM). Stimulation intensity was adjusted to give approximately 80% of maximal evoked PSP amplitude. Prior to the field stimulus, the membrane voltage (Vm) was changed by either a negative or a positive DC current pulse (500 ms, ≤400 pA). After Vm reached steady state, the PSP was evoked (*). I-V relationships of both the steady membrane potential (Fig. 2B, o’s) and peak voltage of the PSP (Fig. 2B, ×’s), were plotted and the linear regressions calculated using SigmaPlot, giving directly the membrane conductance values. By measuring the I-Vs in the potential range of linear membrane behavior (−80 to −50 mV) we avoided errors in the slope conductance due to voltage-dependent activation of delayed rectifier or other rectifying currents. Reversal potential was determined by the intersection point of the regression lines. Typical PSP traces are shown for both a control and a test animal at 3 weeks. CNQX, DL-2-Amino-5-phosphonopentanoic acid, picrotoxin, bumetanide, pilocarpine, and atropine were obtained from Sigma, St. Luis, MO. Diazepam was from Hospira Inc., Lake Forest, IL. Stock solutions, 1000-fold concentrated, were stored in the freezer, and were diluted prior to use in ACSF. For stock solutions, CNQX and picrotoxin were dissolved in DMSO, bumetanide in ethanol. Neither DMSO (1:1000) nor ethanol (1:1000) had any effect on PSPs, I-V relations, or burst responses.
Figure 2. Positive shift of the GABAAergic PSP reversal potential 2 and 3 weeks after SE.
A: Monosynaptic GABAAergic PSPs evoked at 5 different membrane potentials in EC L5 neuron from a control (upper traces) and from a rat 3 weeks after SE (lower traces) recorded with a sharp microelectrode. Membrane potential was altered by current application (500 ms pulses) through the microelectrode. Asterisks denote time of presynaptic stimulus. Recordings were performed in the presence of the AMPA/kainate glutamate receptor antagonist CNQX (10 μM) and the NMDA receptor antagonist APV (50 μM). B1,2,3: I-V relationship of prestimulus membrane potential (o) and the absolute value of the GABAAergic PSP (×) measured 15 ms after stimulation vs. electrode current. Data were obtained using the protocol of part A, fitted with linear regressions. Intersection of membrane potential and PSP plots occurs at the PSP reversal potential (EPSP, arrows). C: Histograms showing the number of EC L5 neurons displaying a GABAAergic reversal potential within a 2 mV range at the 3 time points examined. Envelops (dotted curves) are best Gaussian fits to the 5 obvious populations. In neurons from control rats, the EPSP Gaussian fit has a median value of ~ −69 mV. Neurons from rats both 2 and 3 weeks after SE broke into 2 classes, no EPSP shift, and significant shift. At 2 weeks 77% of the neurons had a shifted mean of ~ −54 and at 3 weeks 81% displayed a mean shift to ~ −28 mV. The non-shifting neurons comprised 23 and 19% of the populations respectively. Their EPSP distribution was indistinguishable from the control group. N=24, 34 and 26 recorded cells for control and 2 and 3 week groups. D: Mean±SEM of GABAAergic EPSP relative to resting membrane potential (RMP) for the shifted populations 2 (n=26) and 3 weeks after SE (n=21) demonstrate a progressive depolarizing shift of the reversal potential in comparison with control (n=24, ** p<0.01, *** p<0.001).
Immunoblotting
For immunoblotting studies, layer 5-6 of medial EC (=tissue adjacent to the angular bundle and up to ~25% of cortical thickness; the angular bundle is well visible in transillumination) were dissected from the 350 μm thick slices, snap-frozen on dry ice and stored at −80°C till processed. For preparation of membrane fractions the tissues were homogenized in buffer containing 250 mM sucrose, 10 mM Tris-HCl, 10 mM HEPES, 1 mM EDTA and protease inhibitors (pH 7.2) followed by differential centrifugation as described previously (Payne et al., 1996). The purified membrane fraction (50μg) was then processed for SDS-polyacrylamide gel electrophoresis (7%) and immunoblotting with anti-KCC2 (1:1000; NeuroMab clone N1/12, UC Davis) and anti-actin (1:500; Sigma) antibodies respectively. Antigen-antibody complex was detected by enhanced chemiluminescence (ECL-plus from Amersham, Arlington Heights, IL) and x-ray films were quantified using NIH ImageJ software.
Fluorescence in-situ hybridization (FISH)
When preparing brain slices for FISH, one brain hemisphere was quick-frozen in a beaker of isopentane equilibrated in dry ice/ethanol slurry and stored at −80°C. Horizontal brain sections (16 μm) were prepared using a cryostat and arranged on slides (Superfrost Plus, VWR), air dried and stored frozen at −80°C until processed.
NKCC1 (clone ID 4824556, accession number BC033003) and KCC2 (clone ID 6838880, accession number BC054808) plasmids were obtained from Open Biosystems (Huntsville, AL). The NKCC1 cDNA sequence was inserted into a pBluescriptR vector between the SalI/XhoI and BamHI restriction sites. KCC2 cDNA sequence was inserted into a pYX-Asc vector between the EcoRI and NotI sites. The plasmids were then sequenced to confirm the NKCC1 and KCC2 sequences (DNA Research Services, University of New Mexico, Health Sciences Center). EcoR1 and Not1 were both used to generate the antisense strand of different lengths, and Kpn1 for the sense strand (New England Biolabs Inc., Ipswich, MA). The antisense strand for KCC2 was generated using AscI, and PacI for the sense strand. The restriction digestion reaction was incubated for 2 hours at 37° C.
Single-labeled FISH for NKCC1a,b and KCC2a,b was performed as previously described (Guzowski et al., 1999). Briefly, digoxigenin-labeled KCC2 and NKCC1 whole length antisense riboprobes were generated from linearized cDNA using a commercially available transcription kit (Maxiscript; Ambion, Austin, TX) and premixed RNA digoxigenin labeled nucleotides using T3 polymerase (Roche Molecular Biochemicals, Palo Alto, CA). Hybridization of both NKCC1 and KCC2 digoxigenin-labeled riboprobes was done overnight at 56°C on separate slides (1ng/μl). Then, anti-digoxigenin horseradish peroxidase conjugate (HRP; 1:200; Roche Molecular Biochemicals, Palo Alto, CA) was incubated overnight at 4°C. TSA-cyanine-3 (Cy3) (1:50; PerkinElmer, Waltham, MA) was used to detect the HRP conjugate. Nuclei were counterstained with 4′, 6′-diamidino-2-phenylindole (DAPI) (1:500; Invitrogen, Carlsbad, CA). Images were acquired with Nikon TE2000U epifluorescence microscope (20X objective) equipped with a spinning disk confocal system (Spinning Disk Confocal Imager CARV, Atto Bioscience, Rockville, MD) and CoolSNAP-Hq CCD Camera (Roper Scientific, Tucson, AZ). The images were further analyzed using MetaMorph software (Universal Imaging) and quantified using NIH ImageJ software. Signals were corrected for background (obtained with ROIs covering clearly negative areas in the image), and ratios against nuclear stains were calculated. Where indicated these data were normalized relative to data for control rats.
Immunohistochemistry
Slices were fixed overnight with 4% paraformaldehyde in 100 mM phosphate buffered saline (PBS), cryoprotected in 15% and 30% sucrose in PBS and then frozen in Optimal Cutting Temperature (OCT) compound (Sakura Finetek, Torrance, CA). Immunohistochemistry was performed at room temperature for 24 hours on 300 μm floating sections with anti-KCC2 (NeuroMab clone N1/12, UC Davis) or NKCC1 antibodies (rabbit polyclonal, Chemicon, Temecula, CA; results were in agreement with results obtained with mouse NKCC monoclonal antibody MAb T4, kindly provided by Dr. C. Lytle, UC Riverside). Slices were mounted on glass slides and images were taken with a Biorad two-photon microscope and 40x objective 75 μm below the slice surface. NeuroTrace fluorescent Stain (Molecular Probes, Eugene, OR) was used for counterstaining neurons in 60 μm sections prepared from slices using a cryostat, and images were taken with an epifluorescence microscope with CCD camera and a 40x objective. For immuno-staining with NKCC1 antibody tissue sections were pretreated in 1% SDS and 8% 2-mercaptoethanol for 5 minutes before blocking (Sung et al., 2000). In many attempts without this pretreatment we failed to obtain clear NKCC signals with the MAb T4 antibody. All sections were blocked with 10% normal goat serum in PBS-T (PBS containing 0.2% Triton-X-100) and incubated at 4°C overnight with the respective primary antibodies (1:200). After extensive washes in PBS-T, tissue sections were incubated in Cy3-conjugated goat anti-mouse or anti-rabbit IgG (1:200, Jackson Immuno Research, West Grove, PA). Controls received identical treatment without primary antibody and were always negative. Sections were extensively rinsed in PBS-T and cover-slipped for viewing. Hoechst 33342 DNA staining (10 μm; Invitrogen, Carlsbad, CA) was used for counterstaining cell nuclei.
Statistics
Means ± SEM are given where not otherwise indicated. Statistical comparisons between groups of data were performed with SigmaPlot software using a two-tailed unpaired, or paired, where appropriate, Student’s t-test to test the null hypothesis. Values of probability of P < 0.05 are considered to be statistically significant.
Results
Development of polysynaptic burst responses in EC L5 neurons post SE
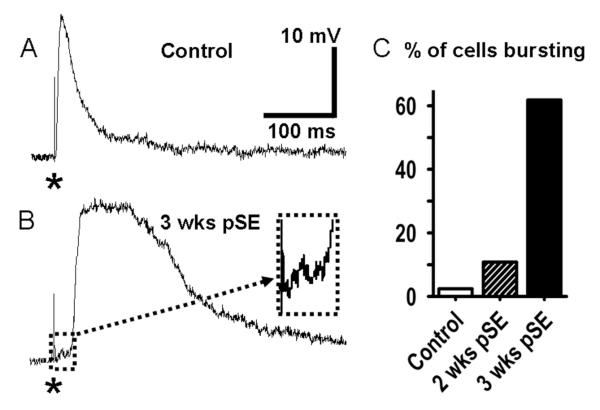
In slices from control rats, single presynaptic stimulation to EC L5 elicited a monosynaptic, fast EPSP of up to 20mV in amplitude in L5 neurons at resting membrane potential (RMP) of ~ −70 mV (Fig. 1 A), followed by a fast inhibitory postsynaptic potential (IPSP) whose amplitude varied with the membrane holding potential. In strong contrast, recordings made 3 weeks after SE showed polysynaptic epileptiform activity in response to a single synaptic stimulation, even when stimulation intensity was greatly reduced from values used for the control preparations (Fig. 1 B). The polysynaptic burst responses showed little change with variation of the postsynaptic membrane potential by DC injection in a range from −95 to −60 mV, except in firing of action potentials for the more depolarized voltages. In the example shown, the slight delay of the polysynaptic depolarization with weak stimulation allowed the initial EPSP to become clearly evident; and to be seen as only 1-2mV in amplitude (inset, cell hyperpolarized by negative DC to prevent firing). These weak stimuli reliably elicited polysynaptic burst responses in 10.8% of the neurons tested at 2 weeks (n=37), and in 61.8% (n=34) at 3 weeks (Fig. 1C). For these determinations, a polysynaptic burst was defined as a post-synaptic response lasting more than 150 ms at half amplitude, elicited from −75 mV < RMP < −68 mV. In control slices such responses were seen only in 1 neuron out of 42 recorded neurons.
Figure 1. Increased polysynaptic burst excitation in EC L5 neurons post SE.
A: Postsynaptic response in EC-L5 neuron from untreated rat to single presynaptic stimulus (*, 500 nA, 70 μs ). B: Polysynaptic burst response in L5 neuron 3 weeks after SE to a much smaller stimulus (*, 100 nA, 70 μs, see also Figs. 10 & 11). The primary evoked PSP (box) is shown enlarged in the inset (30ms × 5mV). For this recording the cell was hyperpolarized by negative DC to prevent firing. C: Bar chart showing percent of EC-L5 neurons responding to a single stimulation with a polysynaptic depolarization. A single stimulus evokes a polysynaptic response in only 2.4% of EC-L5 cells from control rats (n=42), but in 10.8% and in 61.8% of cells from rats 2 (n=37) and 3 weeks after SE (n=34), respectively.
GABAAergic PSPs become depolarizing, post SE
Drawing on existing observations of important changes in EIPSP and Cl- transporter systems in a subpopulation of subicular neurons with long established TLE (Cohen et al., 2002; de Guzman et al., 2006; Palma et al., 2006; Huberfeld et al., 2007; Munoz et al., 2007; Sen et al., 2007), we looked for ongoing changes in GABAergic transmission at the very early stages of interest here that might promote burst activity. Figure 2A illustrates monosynaptic GABAAergic PSPs, during block of glutamate receptors by CNQX (10 μM) and APV (50 μM), that were elicited by field stimulation in EC L5 while cells were held at different steady membrane potentials (see Methods). The PSP reversal potential (E(PSP)) was determined by plotting the I-V relationships of both the steady membrane potential (Fig. 2B, o’s) and peak voltage of the PSP (Fig. 2B, ×’s), and calculating the linear regressions. The crossover point of membrane potential and PSP linear regressions is the reversal potential (Fig. 2B, arrows). I-V plots for representative neurons from control rats and from rats 2 and 3 weeks post SE illustrate a progressive shift of E(PSP) from −68.3±2.1 mV in control to −52.6±1.8 mV 2 weeks and −34.8±1.4 mV 3 weeks post SE, respectively (Fig. 2, B1-3). When the data were plotted as histograms, we observed two classes of cells in the slices from the treated rats, at both 2 and 3 weeks post SE (Fig. 2C, n=84). In one class, the mean E(PSP) was similar to controls, i.e. <5 mV positive to RMP (23.5% and 19.2% of the total cells analyzed at 2 and 3 weeks, respectively). The other class showed strong shifts (76.5% and 80.8% of recorded neurons at 2 and 3 weeks, respectively). Both classes of cells were observed not only in slices from the same rat, but also within single slices. Pre-sacrifice spontaneous seizures, expected in up to ~25% of rats at 3 weeks post SE (see Methods), could have further shifted E(PSP) in neurons from these rats, thus establishing a subpopulation with more strongly shifted E(PSP). The histogram does not show any sign of such a distinct subpopulation of neurons, indicating that spontaneous seizures did either not occur or did not significantly further shift E(PSP).
The bar graph of population data (Fig. 2D) summarizes the mean PSP reversal potential shift over time, excluding that part of the population where no change occurred (E(PSP) = 3.6±0.5 mV positive to RMP of −71.9±1.46 mV in control neurons, n=24; 2 weeks after SE E(PSP) = 18.0±3.6 mV positive to RMP of −70.9±2.8 mV, t56=8.3, P=0.006, n=26; three weeks after SE E(PSP) = 45.1±6.2 mV positive to RMP of −72.9±2.8 mV, t48=9.81, P=0.0008, n=21). At the 3 week measurement point, 81% and 75% of neurons in the shifting and non-shifting population, respectively, were pyramidal and 19% and 25%, respectively, multipolar neurons. These distributions are not significantly different from the overall distribution of these two cell types in EC L5 (Egorov et al., 2002; Egorov et al., 2003). It is notable that the positive shift at 3 weeks brings the E(PSP) very close to the level of spike initiation. Thus, post SE GABA release may not effectively limit repetitive firing, and might possibly even excite neurons.
The slightly depolarizing IPSP in control neurons, relative to resting membrane potential, may seem puzzling for a condition where apparently only Cl- outward transport is expressed (see below). The Cl- reversal potential should be negative to the resting membrane potential (or equal to it when Cl- outward transport becomes active only after Cl- influx). However, the GABAA-channels conduct bicarbonate to some extent, about 1/6 of the anion conductance, making the GABA-PSP reversal potential lie between ECl- and EHCO3- (Bormann et al., 1987). Intracellular energy metabolism generates carbon dioxide that reversibly reacts with water, catalyzed by carbonic anhydrase, to form bicarbonate. This process creates a strong outward gradient for HCO3- with a reversal potential as positive as −10 mV (Ben-Ari et al., 2007). This should shift the PSP reversal to a value more positive than ECl in normal animals (Staley et al., 1995). Also, it is possible that limited expression of Cl- inward transport could produce a slightly depolarizing PSP reversal potential (see below).
In addition to the reversal potential change, the membrane slope conductance, and the conductance increase during the PSP progressively declined post SE (cf. Fig. 2, B1-3). Resting conductance fell from 5.9±0.7 nS in control to 4.8±0.7 (t12=3.66, P=0.032) and 3.6±0.4 nS (t13= 3.79, P=0.026) 2 and 3 weeks after SE, respectively, probably due to downregulation of leak ion channels (Bernard et al., 2004; Gorter et al., 2006). Similarly, the peak GABA-activated conductance fell from 8.5±0.69 nS in control to 2.1±0.4.3 (t12=13.12, P=0.0018) and 0.24±0.07 nS (t13= 6.16, P=0.0005) 2 and 3 weeks after SE, respectively. This decrease was confirmed with larger GABAergic PSPs elicited by stimulation intensity close to maximum of the input-output relation, and repeated 3 times at 100 Hz, giving a larger undisturbed PSP after the third stimulation for all three groups.
Increase of NKCC1 mRNA after SE
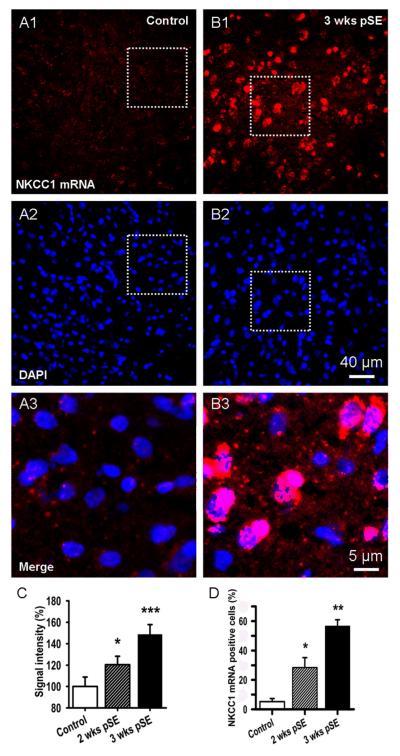
The strongly depolarizing GABAergic PSP post SE, requires active Cl- inward transport. However it is known that there is little expression of NKCC1 in adult neurons (Delpire, 2000; Wang et al., 2002). We examined whether NKCC1 mRNA expression is re-established in adult EC L5 neurons during the post SE latent period, recapitulating an early developmental stage (Yamada et al., 2004; Ben-Ari et al., 2007). We found that NKCC1 mRNA fluorescence was very low to absent in EC L5 in control rats (Fig. 3A), in agreement with previous studies. In contrast, at 2 and especially 3 weeks after Li-pilocarpine induced SE, we observed clear NKCC1 staining (Fig. 3B). At 2 weeks post SE, NKCC1 mRNA fluorescence had increased to 237±33.4 % of control and to 452±33.5 % at 3 weeks (Fig. 3B, E, n=100 ROIs, see Methods, 4 rats in each group, t38=2.15, P=0.037 and t49=3.54, P=0.0009, respectively). These percentages are somewhat arbitrary given the low expression in controls, but indicate a very large increase in expression. Nucleus counter-staining with DAPI demonstrated no change in cell numbers over the analysis period (12.2±2.7, 12.8±3.4 and 12.5±3.1 nuclei per 500 μm2 for control and 2 and 3 week post SE, respectively, t68=−0.49, P=0.62 and t68=−2.38, P=0.24, respectively, n=100 ROIs, 4 rats in each group; Fig. 3 A2, B2). Overlay of DAPI and NKCC1 mRNA images showed a primarily perinuclear location of the labeled mRNA (Fig. 3 B2). Analysis of NKCC1 positive cells relative to all cells in the fields, showed a labeling of 5.1±2.1 % of the cells in control tissue which increased to 28.5±6.7 % and 56.7±4.3 % at 2 and 3 weeks post SE, respectively (Fig. 3D, n=100 ROIs, 4 rats in each group, t38=3.27, P=0.042 and t49=2.71, P=0.0096, respectively).
Figure 3. mRNA for NKCC1, a Cl- inward transporter, progressively increases post SE in EC L5.
A, B: Fluorescence in-situ-hybridization shows up-regulation of NKCC1 mRNA 3 weeks after pilocarpine insult in comparison with control (red signal, A1, B1). DAPI nuclear stain indicated no change in cell numbers (blue signal A2, B2). The lower panels, A3 & B3 show magnified, merged views of the areas outlined in A1,2 & B1,2 and illustrate perinuclear localization of NKCC1 mRNA (A3, B3). C: Bar graph showing increase in NKCC1 mRNA post SE. Data were quantified by forming the ratio of the total NKCC1 signal to total fluorescence of the nuclear stain in a given ROI. These ratios were then normalized to the values from control rat (mean±SEM, n~100 ROIs for 4 rats in each group, * p<0.01, *** p<0.001). D: Mean number of NKCC1 mRNA-positive cells, expressed as % of nuclei number determined from DAPI staining (Mean±SEM, n~300 cells for 4 rats in each group, * p<0.05, ** p<0.01).
Increase of NKCC1 protein
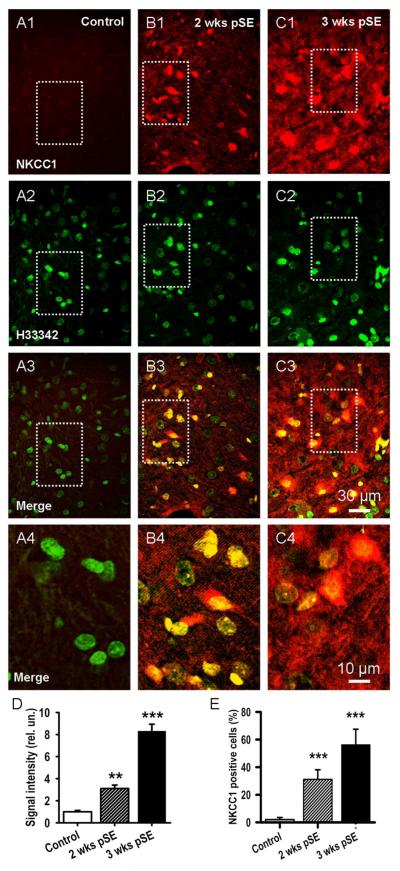
To analyze protein level changes of NKCC1 that occurred during the latent period we performed fluorescence immunohistochemistry. These data were obtained with the Chemicon NKCC1 antibody, as shown in Fig. 4, and demonstrate a progressive increase of NKCC1 protein from 2 to 3 weeks after SE, whereas in slices from control rats, there was very little specific signal (Fig. 4 A-C). Similar results were obtained with the MabT4 NKCC antibody. Superposition of stained nucleus images (Hoechst 33342) onto NKCC1 images revealed, particularly at 3 weeks post SE, a preferential perinuclear and somatic location of NKCC1, suggesting significantly increased neuronal protein synthesis (Fig. 4 B3, C3 and, at higher magnification, Bb4, C4). Again, the number of Hoechst stained nuclei did not show significant differences between the three rat groups (85.3±5.3 in control vs. 97.7±3.4 two weeks, and 86.0±4.9 three weeks post insult, 4 rats, t20=−1.33, P=0.20 and t18=−2.35, P=0.81, respectively, n=100 ROIs, 4 rats in each group). Fig. 4 D shows that average NKCC1 fluorescence intensities from cell bodies were some 350% and 860%, of control background fluorescence at 2 and 3 weeks respectively (n=100 ROIs, 4 rats in each group, t64=5.32, P=0.0006 and t51=4.56, P=0.0008, respectively). In the neuropil average NKCC1 fluorescence intensities were 123±21 % and 222±45 % of control background fluorescence at 2 and 3 weeks respectively, suggesting a delayed increase of NKCC1 protein in dendrites (n=100 ROIs, 4 rats in each group, t32=4.25, P=0.025 and t36=5.31, P=0.008, respectively). In terms of individual cells, 31.2±6.9 % and 56.3±11.2 % of cell bodies were clearly positive for NKCC1 2 and 3 weeks post SE, respectively, whereas none was evident in control (Fig. 4 E, n=100 ROIs, 4 rats in each group, t42=11.24, P=0.0009 and t37=7.12, P=0.0007, respectively).
Figure 4. NKCC1 protein is also upregulated.
A, B, C: Fluorescence immunohistochemistry demonstrates progressive increase in expression of NKCC1 protein from 2 to 3 weeks after SE in EC L5 (red signal, A1, B1, C1). Hoechst 33342 nuclei counterstain shows stable cell number (A2, B2 and C2, green signal). Merged views of the upper panel sets (A3, B3, C3) illustrate the pervasive increase in expression. The boxed regions of these panels are shown enlarged below to better illustrate perinuclear and somatic location of NKCC1 (A4, B4, C4). D: Bar graph showing increase in NKCC1 protein post SE. Data were quantified by forming the ratio of the total NKCC1 signal to total fluorescence of the nuclear stain in a given ROI. These ratios were then normalized to the values from control rat (mean±SEM, 3 rats, ** p<0.01, *** p<0,001). E: Mean number of NKCC1 positive cells, expressed as % of nuclei number (H33342, mean±SEM, 3 rats, *** p<0.01).
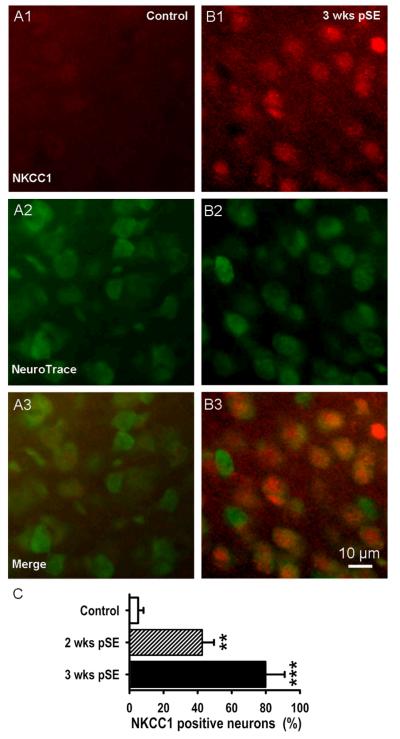
In Fig. 5 neuron-specific NeuroTrace counterstaining was used and showed that 77.1±8.1 % of neurons had become NKCC1 positive 3 weeks after insult (control tissue: 3.0±1.3 %, n=3 rats in each group, t21=3.78, P=0.008 and t33=4.23, P=0.001, respectively). The higher percentage of NKCC1 positive neurons than of NKCC1 positive cells suggests that NKCC1 is mostly upregulated in neurons. NeuroTrace positive cell number was also stable over the experimental period (35.4±7.2 in control vs. 37.1±6.4 two weeks, and 36.6±6.7 three weeks post insult, 3 rats in each group, t12=−0.51, P=0.6 and t16=−0.30, P=0.8, respectively), indicating that the stable H33342 cell count was not the result of compensating neuron loss and glial cell increase. The gradual increase in NKCC1 expression and the percentage of positive neurons is well suited to explain the concurrent progressive depolarizing shift in GABAA-PSP reversal potential.
Figure 5. EC L5 neurons increase expression of NKCC1 protein post SE.
A, B: Fluorescence immunohistochemistry demonstrates strong expression of NKCC1 protein 3 weeks after SE in EC L5 (red signal, A1, B1). Neuron-specific NeuroTrace counterstain shows stable cell number (A2, B2, green signal). Overlay views illustrate perinuclear and somatic location of NKCC1 (A3, B3). Here, neurons throughout a 60 μm thick tissue block are shown. C: Mean of NKCC1 positive neurons, in % of NeuroTrace positive cells, progressively increases 2 and 3 weeks after pilocarpine-evoked SE in EC L5 (mean±SEM, 3 rats, *** p<0.01).
Progressive decrease of KCC2 mRNA
The demonstrated upregulation of NKCC1 suggests active Cl- uptake. Normal Cl- extrusion by the neuronal KCl cotransporter KCC2 (Ben-Ari et al., 2007) would compete with, and more or less short-circuit this Cl- uptake. If KCC2 expression is maintained it would limit the effectiveness of Cl- uptake and waste metabolic energy. To see if this is the case we studied expression of KCC2 mRNA.
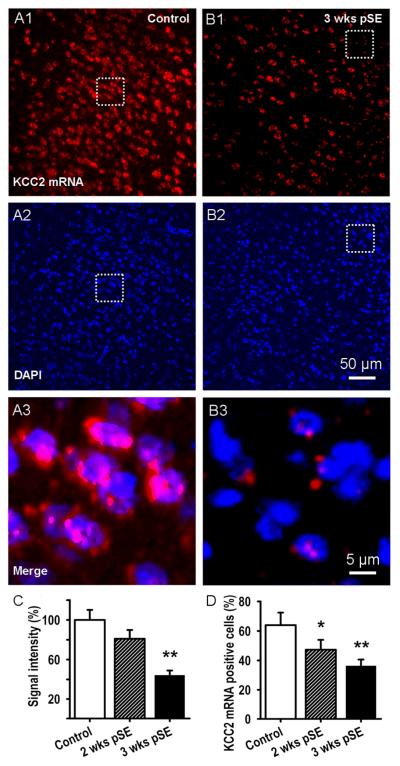
As above, we assessed the expression levels of KCC2 mRNA in L5 neurons by fluorescence in situ hybridization. Fig. 6 shows that KCC2 mRNA was expressed abundantly in control animals (A1) while 3 weeks after SE, expression of KCC2 mRNA was strongly diminished (B1). Staining of cell nuclei with DAPI did not show significant differences between the 3 time points (11.9±2.9, 12.3±3.8 and 12.1±3.2 nuclei per 500 μm2 respectively, t24=−0.36, P=0.17 and t24=−0.41, P=0.24, respectively, Fig. 6: A2, B2). The merged fluorescence images (Fig. 6: A3, B3) show that KCC2 mRNA is localized in the nuclear and perinuclear regions of the cells.
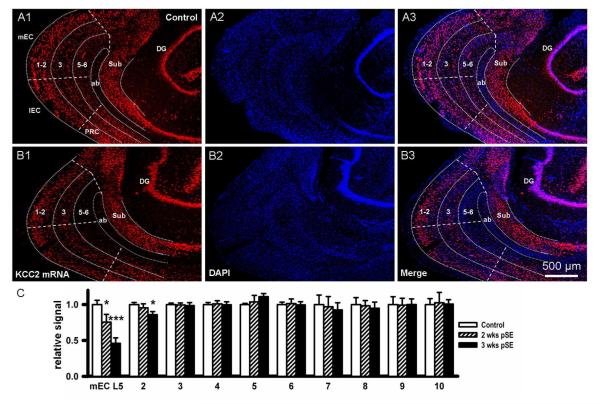
Figure 6. mRNA for KCC2, a neuronal Cl- outward transporter, progressively decreases post SE in EC L5.
A, B: KCC2 mRNA expression detected by fluorescence in-situ-hybridization shows down-regulation of KCC2 mRNA 3 weeks after pilocarpine induced SE in comparison with control (A1, B1, red signal). DAPI nuclear stain indicated no change in cell numbers (A2 & B2: blue signal). Merged magnified views of the areas outlined in A1,2 & B1,2 illustrate perinuclear localization of KCC2 mRNA (A3 & B3). C: Bar graph showing decrease in KCC2 mRNA post SE. Data were quantified by forming the ratio of the total KCC2 signal to total fluorescence of the nuclear stain in a given ROI. These ratios were then normalized to the values from control rat (mean±SEM, n~100 ROIs for 4 rats in each group, ** p<0.01). D: Mean number of KCC2 positive cells, expressed as % of nuclei number (DAPI, mean±SEM, n~300 cells for 4 rats in each group, * p<0.05 and ** p<0.01, respectively).
KCC2 mRNA fluorescence, normalized to control, showed a progressive decrease of KCC2 mRNA expression 2 and 3 weeks after SE to 81±8.3 and 44±7.8% of control level, respectively (Fig. 6 C, n~100 ROIs, see Methods, 4 rats in each group, t62=1.95, P=0.08 and t62=2.34, P=0.0095, respectively). Similarly, the proportion of EC L5 cells expressing KCC2 mRNA (at least 2 red spots around the nucleus as in Fig. 6 A3 & B3), relative to the number of nuclei, decreased from 63.9±8.5% of EC L5 cells in control animals to 47.3±6.7 and 36.3±4.3% of total cell number 2 and 3 weeks after SE, respectively (Fig. 6 D, mean±SEM, n~300 cells for 4 rats in each group, t34=3.14, P=0.045 and t28=4.21, P=0.007, respectively).
Progressive decrease of KCC2 protein
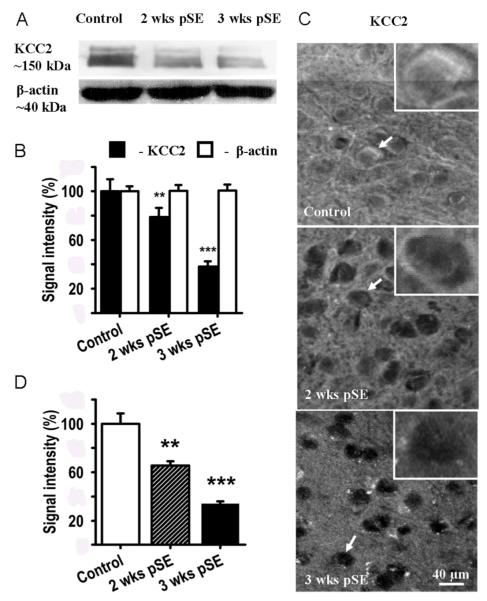
We also examined KCC2 protein levels in EC L5 from control and post SE rats using Western blot and single cell analysis. The results, shown in Fig. 7 A, B, illustrate a significant reduction of KCC2 protein 2 and 3 weeks after SE to 78.8±7.5 and 38.1±4.3% of control levels, respectively (mean±SEM, n=5 rats, t12=2.17, P=0.01 and t18=4.6, P=0.0006, respectively). Using fluorescence immunohistochemistry we also studied the distribution of KCC2 protein and its loss after treatment (Fig. 7 C). In control rats KCC2 immunoreactivity was detected as densely packed perisomatic fluorescence (Fig. 7 C, top panel, and inset) on top of a diffuse neuropil signal. Two and 3 weeks post SE, KCC2 immunofluorescence had gradually decreased in the neuronal cell bodies (Fig. 7 C, D) with a weak discrete fluorescence remaining in the perisomatic areas of the EC L5 neurons (Fig. 7 C, middle and lower panel, and insets). Somatic immunofluorescence for KCC2 decreased to 66±3.5 and 34±1.9% of control level 2 and 3 week after SE, respectively (Fig. 7 D, mean±SEM, n=100 ROIs from 4 rats, t34=7.85, P=0.007 and t41=6.39, P=0.00006, respectively).
Figure 7. KCC2 protein also decreases post SE.
A: Western blot KCC2 bands (~150 kDa) show significant gradual down-regulation of KCC2 in an EC L5 region of rats 2 (2 wks pSE) and 3 weeks (3 wks pSE) after pilocarpine induced SE in comparison with control. β-actin (~ 40 kDa) served as a loading control. B: Average optical densities, extracted from blots as in A, in % of control (mean±SEM, n=5, ** p<0.01, *** p<0.001). These data are in excellent agreement with the mRNA and IPSP reversal potential data. C: Immunohistochemistry shows a gradual decrease of KCC2 protein 2 and 3 weeks after pilocarpine induced SE, most prominent in somata. The white outlined boxes in each panel show a magnified view of the cells identified by white arrows in each panel. D: Bar graph showing decrease in average immunohistochemistry KCC2 signals post SE, normalized to control.(mean±SEM, n~100 ROIs for 4 rats in each group, ** p<0.01, *** p<0.001).
In the neuropil, the KCC2 signal decrease was more delayed. Here, the KCC2 signal decreased to 96±4.1% and 60±4.4%, at 2 and 3 weeks respectively. Roughly estimating a 1:1 ratio for soma versus neuropil volume, total KCC2 immunofluorescence changes are in good agreement with the Western Blot data. To establish that neurons were not dying and replaced by glia, the neuron-specific fluorescent NeuroTrace Stain was used for counterstaining. These stainings showed stable number of neurons (157.6±6.5 in control vs. 154.6±21.3 three weeks post insult, 3 rats, t26=2.34, P=0.44) and that the KCC2 signal was highly specific for neurons (> 95% of KCC2 positive cells were NeuroTrace positive). Interestingly, in the high-dose pilocarpine model, there is also evidence for an early, functional downregulation of KCC2 in the downstream dentate gyrus that compromises the gatekeeper function of this structure for spread of epileptiform activity from the EC into the hippocampus (Pathak et al., 2007). Concurrent with the compromise in this gatekeeper function, there is evidence for epileptiform EEG activity in vivo (El-Hassar et al., 2007).
Early changes in NKCC1 and KCC2 are specific to EC L5
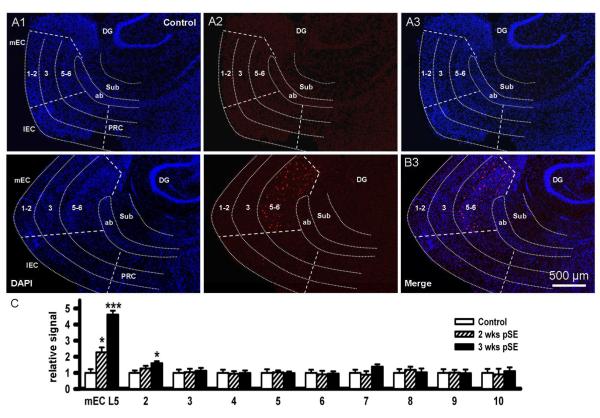
The high amplitude EEG spiking that occurs during SE might well be sufficient to trigger the progressive changes in NKCC1 and KCC2 expression seen in EC L5 in other cortical areas, including the hippocampus and subiculum. We therefore extended our analysis to include the superficial layers of the EC, as well as subiculum, dentate gyrus, and perirhinal cortex. Fig. 8 A,B show in a low magnification overview, the expression of NKCC1 mRNA in the deep EC 3 weeks after SE, while superficial layers, subiculum, dentate gyrus and perirhinal cortex show no expression. The plot of mean values of fluorescence from these areas (Fig. 8C, from higher magnificiation images, and normalized to controls) demonstrates this highly specific expression of NKCC1 mRNA in the deep medial EC, and, to a much weaker extent, in the deep lateral EC (columns 2), while all other regions tested negative (columns 3-10).
Figure 8. mRNA for NKCC1 does not increase in other cortical regions.
A, B: Fluorescence in-situ-hybridization studies for NKCC1 mRNA showing the brain region encompassing medial (mEC) and lateral EC (lEC), angular bundle (ab), subiculum (Sub), dentate gyrus (DG), and perirhinal cortex (PRC): A, control slice; B, slice taken 3 weeks post SE. Left panels show DAPI staining of the region overlayed with rough outlines of the component areas (A1, B1). Middle panels show NKCC1 signal. In control, there is little to none while 3 weeks after SE signal is apparent in mid EC L5-6 but nowhere else (A2, B2). Images are merged in the right hand panels (A3, B3, for high power images see Fig. 3). C: Bar graph showing increase in NKCC1 mRNA only in deep EC post SE (mEC L5, t54=2.15, P=0.037 and t54=3.54, P=0.0009, respectively) and lateral EC L5 labed # 2 (t54=1.02, P=0.12 and t54=1.28, P=0.047, respectively), but no significant changes occurred in mEC L3 (# 3, t54=0.93, P=0.24 and t49=0.42, P=0.34, respectively), lEC (# 4, t54=0.27, P=0.86 and t54=0.24, P=0.094, respectively), mEC L1-2 (# 5, t54=−1.25, P=0.32 and t54=0.34, P=0.23, respectively), lEC L1-2 (# 6, t54=1.24, P=0.078 and t54=0.52, P=0.27, respectively), subiculum (# 7, t54=1.54, P=0.49 and t54=1.67, P=0.063, respectively), dentate gyrus (# 8, t54=0.45, P=0.098 and t54=−1.35, P=0.26, respectively), deep perirhinal cortex (# 9, t54=0.53, P=0.47 and t54=2.01, P=0.48, respectively) and PRC L1-3 (# 10 t54=−1.36, P=0.81 and t54=0.28, P=0.81, respectively; data extracted from higher magnificiation images).
The same specificity was observed in the corresponding studies of KCC2 expression. The overview in Fig. 9 A,B shows downregulation of KCC2 mRNA 3 weeks after SE specifically in the deep EC, particularly in the medial part. The quantitative analysis of mean fluorescence demonstrates ongoing downregulation of KCC2 mRNA in the deep mEC by 56±7.8 % (3 weeks post SE, t62=2.34, P=0.0095) and in the deep lateral EC by 14±4.5 % (3 weeks post SE, t62= 2.41, P=0.039), whereas superficial layers of the EC, as well as subiculum, dentate gyrus, and perirhinal cortex do not show any significant changes (Fig. 9C).
Figure 9. mRNA for KCC2 decreases only in deep EC.
A, B: Fluorescence in-situ-hybridization studies for KCC2 mRNA showing the brain region encompassing medial (mEC) and lateral EC (lEC), angular bundle (ab), subiculum (Sub), dentate gyrus (DG), and perirhinal cortex (PRC): A, control slice; B, slice taken 3 weeks post SE. Left panels show KCC2 staining of the region, overlayed with rough outlines of the component areas (A1, A2). In control, there is ubiquitous expression while 3 weeks after SE signal has disappeared in EC L5-6, but nowhere else. Middle panels show DAPI staining of the region (A2, B2). Images are merged in the right hand panels (A3, B3, for high power images see Fig. 6). C: Bar graph showing decrease in KCC2 mRNA only in deep EC post SE (mEC L5, t62=1.95, P=0.082 and t62=2.34, P=0.0095, respectively), and lateral EC L5 labed # 2 (t62=1.86, P=0.093 and t62=2.41, P=0.039, respectively), but no significant changes occurred in mEC L3 (# 3, t62=0.34, P=0.32 and t62=−1.22, P=0.51, respectively), lEC (# 4, t62=−0.25, P=0.17 and t62=0.24, P=0.36, respectively), mEC L1-2 (# 5, t62=1.06, P=0.85 and t62=0.43, P=0.62, respectively), lEC L1-2 (# 6, t62=−0.45, P=0.68 and t62=0.15, P=0.39, respectively), subiculum (# 7, t62=−1.01, P=0.098 and t62=1.84, P=0.065, respectively), dentate gyrus (# 8, t62=1.05, P=0.77 and t62=−2.03, P=0.49, respectively), deep perirhinal cortex (# 9, t62=1.33, P=0.28 and t62=−1.52, P=0.53, respectively) and PRC L1-3 (# 10, t62=1.12, P=0.085 and t62=0.36, P=0.14, respectively; data extracted from higher magnificiation images).
A further important outcome of the extended analysis is that there is no significant early neuronal loss in L3 following the “low dose pilocarpine - Diazepam 1 hour after SE onset” protocol used here, as opposed to “Diazepam administration >2 hour after SE onset” protocols (Du et al., 1995; Kobayashi et al., 2003). First, the absence of significant changes of KCC2 mRNA in EC L3 indicates that L3 pyramidal neurons have not died up to 3 weeks post SE in our rats. Second, neuron survival was confirmed in NeuroTrace stainings that showed stable neuron counts in L3 (114±14.2, 112±18.1 and 116±15 neurons in Control and 2 and 3 weeks after SE, respectively, t10= −0.18, P= 0.86 and t 10 = −0.77, P= 0.46, respectively, 3 rats).
Cl- transport block inhibited burst activity
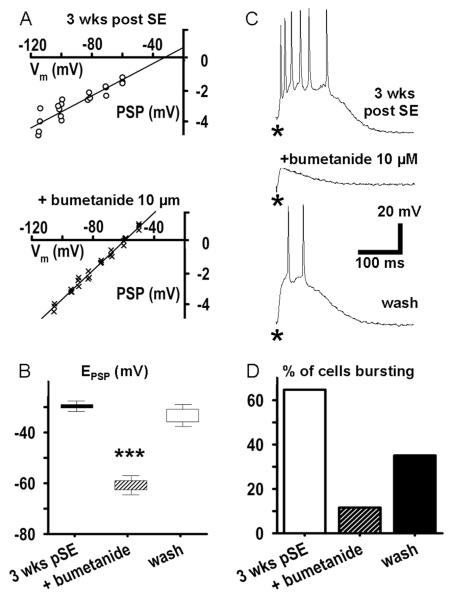
To explore the functional role of NKCC1 upregulation for the shift in EIPSP and the increase in polysynaptic burst responses post insult we employed bumetanide, a diuretic that acts via blockade of Cl- transporters, and is more effective in blocking NKCC vs. KCC transporters (Russell, 2000; Sung et al., 2000; Hannaert et al., 2002; Beck et al., 2003). Here its lack of absolute specificity is compensated by 1) the demonstrated strong down-regulation of KCC2 and 2) the fact that only Cl- inward transport can shift ECl positive to RMP. Block of residual KCC2 activity will not prevent a strong hyperpolarizing shift of ECl during block of NKCC1. Fig. 10 A shows plots of PSP amplitude, 3 weeks post SE, against membrane voltage, demonstrating that the depolarizing shift in E(PSP) disappeared after a 20 min superfusion with bumetanide (10 μM). This corresponds to a passive redistribution of Cl- across the membrane. Fig. 10 B shows an average PSP reversal potential relative to RMP (~ −72mV) for EC L5 neurons 3 weeks post SE of more than +40 mV in normal recording condition, reduced to only +10 mV in the presence of bumetanide (absolute voltage average E(PSP) was −29.7±2.3 mV in control conditions, −60.9±4.1 mV in the presence of bumetanide (t24=2.26, P=0.00009), and −34.2±3.4 mV during washout of bumetanide, n=5, t24=1.52, P=0.0057 vs. bumetanide).
Figure 10. The NKCC1 inhibitor bumetanide partially restores E(PSP), and suppresses polysynaptic excitation.
A: Plots of PSP amplitude against membrane potential before (top, open circles) and after a 20 min exposure to bumetanide (bottom, crosses) of an EC L5 neuron from a rat 3 weeks after SE demonstrate a hyperpolarizing shift of PSP reversal potential. Data were obtained from protocols shown in Fig. 2A. The E(PSP) shifts from −29.7 mV to −60.9 mV. Measurements were made during block of EPSPs by CNQX (10 μM) and APV (50 μM). B: Population data showing that the positive-shifted GABAA-PSP reversal potential is strongly and reversibly repolarized by bumetanide (mean±SEM, n=3, *** p<0.001). C: Top record shows that the polysynaptic response to a single stimulus (100 nA) observed 3 weeks post SE is blocked by bumetanide (middle trace) but shows recovery with 30 minutes washout of the drug (bottom trace). D: Population data showing that bumetanide decreases the % of EC-L5 neurons 3 weeks post SE exhibiting a polysynaptic depolarization in response to a single stimulation from 64.8% to 11.6% (n=14). This number recovered to 35.1% after a 30 min washout of bumetanide for 30 minutes.
If disinhibition, or even excitation, secondary to the depolarizing shift of E(PSP) is crucial for the occurrence of polysynaptic burst responses in the post SE latent period, then restoring the reversal potential by bumetanide should also block polysynaptic burst responses. Figure 10 C demonstrates that, indeed, perfusion with bumetanide (10 μM) effectively inhibited polysynaptic responses to single shock stimulation. After recording the strong polysynaptic burst response shown in the top panel of Fig. 10 C, bumetanide superfusion was begun, and by 20 min, burst discharge was strongly attenuated (middle trace). With washout of bumetanide for 30 minutes the strong polysynaptic response was restored (lower trace). On average, bumetanide reduced the number of neurons with a polysynaptic response from 64.8% (9 neurons out of a total of 14) to 11.6% (2/14) of cells from rats 3 weeks after pilocarpine insult. Washout of bumetanide for 30 min restored the number of neurons responding to 35.1% (5/14, Fig. 10 D). The response duration at half-maximal amplitude decreased in bumetanide by 36±9% of the control response (t13=13.8, P=0.0000000004), and recovered to 75±11 % during washout (t13=−5.07, P=0.0002 vs. bumetanide).
GABAAergic excitation has minor effect on bursts
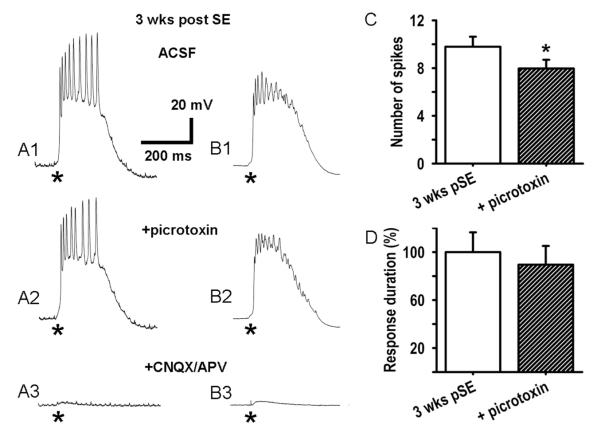
The depolarizing shift in PSP reversal potential would lead to both, disinhibition and direct GABAergic excitation of L5 neurons. We examined the role of direct excitation in burst responses by blocking GABAAergic excitation with picrotoxin. Unlike bumetanide, picrotoxin (100 μM) had only modest effects on polysynaptic burst discharge 3 weeks post SE (Fig. 11 A1, A2; in picrotoxin we observed also spontaneous bursts; only evoked bursts recorded >5s after a spontaneous burst were analyzed; these bursts were apparently not affected by a preceding spontaneous burst). In the population of cells studied, picrotoxin reduced the number of spikes in polysynaptic burst responses from 9.8±0.8 to 7.9±0.7 at 3 weeks post SE (Fig. 11C, n=8 neurons, t24=2.26, P=0.000089). When ten of such responses from the same cell were averaged, it was found as in Fig. 11 B1, B2, that picrotoxin reduced action potential amplitudes in the averaged burst responses by 10.5±3.4%, suggesting an increased variability in the timing of action potentials after stimulation (n=8, p=0.094). Thus, the presence of GABAergic excitation apparently increased timing precision of firing in the network, probably due to an overall increase in excitability, rather than direct excitation, as GABAergic transmission may be delayed due to being downstream of interneuron excitation. By contrast to the modest effect of picrotoxin alone, addition of CNQX (10 μM) plus APV (50 μM) to the ACSF/picrotoxin saline almost completely blocked the synaptic responses (−95.9±0.26%, t7=−10.05, P=0.00002), demonstrating that the bursts were mediated primarily by polysynaptic glutamatergic transmission (Fig. 11 A3, B3; in CNQX and APV, with no picrotoxin, monosynaptic PSPs were evoked, as shown in Fig. 2A). There was a trend toward reduction of burst duration in picrotoxin which did not reach statistical significance (Fig. 11D).
Figure 11. Picrotoxin has a minor effect on polysynaptic bursting.
A: Block of GABAA receptors by picrotoxin (100 μM) decreased action potential firing during the polysynaptic burst response by a small but significant amount (A1, A2; stimulus 100 nA, 70 μs delivered at *). Subsequent additional block of ionotropic glutamate receptors by CNQX (10 μM) and APV (50 μM) nearly eliminated all postsynaptic responses (A3). B: Averages of 10 bursts from the same cell reveal a shorter burst duration in picrotoxin. Decreased spike amplitudes in these averages indicate reduced precision in the firing pattern during the burst in picrotoxin. C: Population data showing that picrotoxin reduces the mean number of spikes in polysynaptic burst responses from 9.8±0.8 to 7.9±0.7 spikes per response (mean±SEM, n=8 cells, 5 rats, *p<0.05). D: In picrotoxin there is a trend towards a 10.5% decrease of the duration of polysynaptic bursts (mean±SEM, n=8, p=0.094).
Discussion
The principal findings of this study are that in the latent period post SE, prior to occurrence of behavioral seizures, there is a strong increase in neuronal excitability in the deep medial EC, i.e. a progressive increase in the percentage of EC L5 neurons that respond to weak synaptic stimulation with polysynaptic burst discharge. Natural activity patterns in the EC become therefore more and more likely to trigger a spontaneous seizure that by definition will end the latent period. Our findings suggest that after SE, TLE develops primarily in the deep EC. To the best of our knowledge there has been no other study demonstrating epileptiform activity in response to synaptic stimulation in any other brain region during the latent period. This regional specificity is corroborated by the specificity of changes in NKCC1 and KCC2 expression for the deep EC which would lead to disinhibition. The several other cortical and hippocampal regions examined did not show any changes, although latent period interictal activity has been shown in-vivo for CA1 in the high-dose pilocarpine model of TLE (El-Hassar et al., 2007). It is very important that the changes in L5 neuron excitability we have reported occurred without appreciable loss of EC L3 pyramidal neuron loss. A high degree of loss of L3 pyramidal neurons has been shown to be typical of the pathology of late TLE (Du et al., 1993; Du et al., 1995), and can occur within 24 hours of prolonged SE (Du et al., 1995), but these findings are not relevant here since appreciable loss does not occur.
Our protocol for generating SE may be considered to be somewhat weaker than that of Andre et al (Andre et al., 2007) in that Diazepam is administered 1 hr after SE onset rather than 2 hr, plus administration of atropine. Even though neither Diazepam nor atropine stop SE immediately at either time point, we found that this alteration facilitated recovery of rats and produced brain slices with better viability. Rightly or wrongly, we also reasoned that a weaker initial insult might produce more gradually developing cellular or network changes and therefore be easier to analyze. When tested by video monitoring, our protocol produced demonstrable seizure activity at 8 weeks post SE in 10 of 13 rats observed (see Methods). We surmise that the percentage might have gone higher had resources been available for more extended observations. In any event, the observed cellular changes will facilitate epileptiform activity, and will most likely be triggered also by the stronger Li-pilocarpine SE protocols.
The change in EC L5 excitability is concurrent with, and we propose aided by, a strong and progressive depolarizing shift in the GABAAergic PSP reversal potential which occurs in about 80% of EC L5 neurons. This percentage is in excellent agreement with the upregulation of the Cl- inward transporter NKCC1. The concurrent downregulation of the Cl- outward transporter KCC2 in most EC L5 neurons will facilitate net Cl- accumulation by NKCC1, but will not bring by itself ECl positive to resting membrane potential. The ongoing changes in NKCC1 and KCC2 are also in excellent agreement with the strongly shifted E(PSP) at 3 weeks post SE, even though determination of the latter is less precise, thus adding measurement variability, due to the extrapolation of PSP amplitudes and the reduced difference in slopes.
Weaker shifts in the GABAergic PSP reversal potential have been observed at a much later stage of TLE disease progression; i.e., in late chronic epilepsy in subicular neurons, apparently due to much weaker changes in NKCC1 expression (Cohen et al., 2002; de Guzman et al., 2006; Palma et al., 2006; Huberfeld et al., 2007; Munoz et al., 2007; Sen et al., 2007). The differences in region, stage of disease progression, level of interictal and ictal activity, and expression levels suggest fundamental differences in the signaling network that initiate and further control changed expression levels of NKCC1 and KCC2 in the latent period vs. late chronic TLE.
Normal reversal potentials for GABA-PSP’s in EC L5 neurons, i.e. ~Vrest, have been found in two distinct SE-TLE models during chronic epilepsy (Fountain et al., 1998; de Guzman et al., 2006), suggesting that the very large shifts in GABAA E(PSP) we report may largely recover once chronic epilepsy has been established. There are also other possible explanations: first, recordings may have been obtained from the lateral EC, where we found only small changes in NKCC1 and KCC2. Furthermore, different TLE models were used. De Guzman et al. (2006) studied the high dose pilocarpine rat model of TLE that has been shown to differ significantly from the Li-pilocarpine rat model in the underlying signal transduction pathways that are activated during SE (Ormandy et al., 1989), and also by differences in L3 neuronal loss (above). This could make a difference in the E(PSP) shift as well as whether it recovers in chronic epilepsy or not. We are planning to investigate some of these important issues in future studies.
The inference that the strong depolarizing shift in the GABAAergic PSP is the primary cause for the observed change in deep EC excitability is supported by the effects of bumetanide, a blocker of the Cl- inward transporter NKCC1. Bumetanide restored a more hyperpolarized PSP reversal potential and largely eliminated epileptiform burst responses to stimulation. These findings demonstrate the necessity for a strongly depolarized E(PSP) for the occurrence of the epileptiform burst responses in this phase post SE, but do not exclude the possibility that other concurrent changes, e.g. the observed reduction in the input conductance of L5 neurons, are required, in addition. The effects of bumetanide further demonstrate that, 3 weeks after SE, inhibition can still be very effective despite the observed decrease in the GABA-PSP conductance that may be due to downregulation of GABAAR genes or loss of inhibitory synapses and interneurons (Bernard et al., 2004; Gorter et al., 2006; Kumar and Buckmaster, 2006). The GABA-PSP has been already slightly depolarizing in control neurons, and, 3 weeks post SE, bumetanide did not fully restore it to that level. Thus, inhibition would be always “shunting”, as opposed to “hyperpolarizing”. One should keep in mind, however, that for preventing triggering of AP firing by excitatory currents the most important parameter appears to be the amplitude of inhibitory currents at membrane potentials close to the firing threshold. This current amplitude gradually varies with a shift in E(PSP) according to the driving force for Cl- close to the firing threshold (~ −45 mV). Hence 3 weeks after SE and in the presence of bumetanide (E(PSP) ≈ −60 mV) the driving force at −45mV is ~15 mV, as opposed to ~26 mV in control rats, and thus reduced by ~ 42%. In consequence Cl- currents in this critical membrane potential region are attenuated by both, a decrease in driving force and in GABA-PSP conductance, but, in the presence of bumetanide, are still quite effective to suppress epileptiform activity. This gives hope that largely restoring the Cl--gradient may have therapeutic benefits in-vivo.
We believe that our findings uncover key elements operative in the early development of TLE and emphasize the importance of the deep entorhinal cortex in the origin and progression of changes that lead to the disorder. As such they may suggest useful starting points for blocking the development of TLE following high risk insults.
Acknowledgements
This work was supported by National Institutes of Health Grant RR15636. We thank Dr. C. Lytle for a gift of monoclonal NKCC antibody, Drs. Fernando Valenzuela and Honglian Shi for helpful discussions and supplying molecular biology materials and Drs. Francisco J. Alvarez-Leefmans, Patric K. Stanton and Rafael Gutierrez for helpful discussions, suggestions, and editorial help. The FISH work was supported by NIH grant AA015614 (to FV). Jennifer Sanderson was supported by funds from NIH training grant #AA014127 and NRSA pre-doctoral fellowship #AA016880.
Abbreviations
- ACSF
Artificial Cerebro-Spinal Fluid
- CA
Cornu Ammonis
- DAPI
4′, 6′-diamidino-2-phenylindole
- EC
entorhinal cortex
- E(PSP)
PSP reversal potential
- FISH
fluorescence in-situ hybridization
- IPSP
inhibitory postsynaptic potential
- PBS
phosphate buffered saline
- PBS-T
PBS containing 0.2% Triton-X-100
- PSP
postsynaptic potential
- RMP
resting membrane potential
- SE
status epilepticus
- TLE
temporal lobe epilepsy
References
- Andre V, Dube C, Francois J, Leroy C, Rigoulot MA, Roch C, Namer IJ, Nehlig A. Pathogenesis and pharmacology of epilepsy in the lithium-pilocarpine model. Epilepsia. 2007;48(Suppl 5):41–47. doi: 10.1111/j.1528-1167.2007.01288.x. [DOI] [PubMed] [Google Scholar]
- Annegers JF, Grabow JD, Groover RV, Laws ER, Jr., Elveback LR, Kurland LT. Seizures after head trauma: a population study. Neurology. 1980;30:683–689. doi: 10.1212/wnl.30.7.683. [DOI] [PubMed] [Google Scholar]
- Bartolomei F, Khalil M, Wendling F, Sontheimer A, Regis J, Ranjeva JP, Guye M, Chauvel P. Entorhinal cortex involvement in human mesial temporal lobe epilepsy: an electrophysiologic and volumetric study. Epilepsia. 2005;46:677–687. doi: 10.1111/j.1528-1167.2005.43804.x. [DOI] [PubMed] [Google Scholar]
- Bartolomei F, Wendling F, Regis J, Gavaret M, Guye M, Chauvel P. Pre-ictal synchronicity in limbic networks of mesial temporal lobe epilepsy. Epilepsy Res. 2004;61:89–104. doi: 10.1016/j.eplepsyres.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Beck J, Lenart B, Kintner DB, Sun D. Na-K-Cl cotransporter contributes to glutamate-mediated excitotoxicity. J Neurosci. 2003;23:5061–5068. doi: 10.1523/JNEUROSCI.23-12-05061.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev. 2007;87:1215–1284. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- Bernard C, Anderson A, Becker A, Poolos NP, Beck H, Johnston D. Acquired dendritic channelopathy in temporal lobe epilepsy. Science. 2004;305:532–535. doi: 10.1126/science.1097065. [DOI] [PubMed] [Google Scholar]
- Bormann J, Hamill OP, Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalheiro EA. The pilocarpine model of epilepsy. Ital J Neurol Sci. 1995;16:33–37. doi: 10.1007/BF02229072. [DOI] [PubMed] [Google Scholar]
- Cohen I, Navarro V, Clemenceau S, Baulac M, Miles R. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science. 2002;298:1418–1421. doi: 10.1126/science.1076510. [DOI] [PubMed] [Google Scholar]
- de Guzman P, Inaba Y, Biagini G, Baldelli E, Mollinari C, Merlo D, Avoli M. Subiculum network excitability is increased in a rodent model of temporal lobe epilepsy. Hippocampus. 2006;16:843–860. doi: 10.1002/hipo.20215. [DOI] [PubMed] [Google Scholar]
- Delpire E. Cation-Chloride Cotransporters in Neuronal Communication. News Physiol Sci. 2000;15:309–312. doi: 10.1152/physiologyonline.2000.15.6.309. [DOI] [PubMed] [Google Scholar]
- Du F, Eid T, Lothman EW, Kohler C, Schwarcz R. Preferential neuronal loss in layer III of the medial entorhinal cortex in rat models of temporal lobe epilepsy. J Neurosci. 1995;15:6301–6313. doi: 10.1523/JNEUROSCI.15-10-06301.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du F, Whetsell WO, Abou KB, Blumenkopf B, Lothman EW, Schwarcz R. Preferential neuronal loss in layer III of the entorhinal cortex in patients with temporal lobe epilepsy. Epilepsy Res. 1993;16:223–233. doi: 10.1016/0920-1211(93)90083-j. [DOI] [PubMed] [Google Scholar]
- Dube C, Marescaux C, Nehlig A. A metabolic and neuropathological approach to the understanding of plastic changes that occur in the immature and adult rat brain during lithium-pilocarpine-induced epileptogenesis. Epilepsia. 2000;41(Suppl 6):S36–43. doi: 10.1111/j.1528-1157.2000.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Egorov AV, Angelova PR, Heinemann U, Müller W. Ca2+-independent muscarinic excitation of rat medial entorhinal cortex layer V neurons. Eur J Neurosci. 2003;18:3343–3351. doi: 10.1111/j.1460-9568.2003.03050.x. [DOI] [PubMed] [Google Scholar]
- Egorov AV, Heinemann U, Müller W. Differential excitability and voltage-dependent Ca2+ signalling in two types of medial entorhinal cortex layer V neurons. Eur J Neurosci. 2002;16:1305–1312. doi: 10.1046/j.1460-9568.2002.02197.x. [DOI] [PubMed] [Google Scholar]
- El-Hassar L, Milh M, Wendling F, Ferrand N, Esclapez M, Bernard C. Cell domain-dependent changes in the glutamatergic and GABAergic drives during epileptogenesis in the rat CA1 region. J Physiol. 2007;578:193–211. doi: 10.1113/jphysiol.2006.119297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J. Clinical evidence for the progressive nature of epilepsy. Epilepsy Res. (Supplement) 1989;12:9–20. Engel, J., (1989) Epilepsy Res. (Supple) 12:9-20. [PubMed] [Google Scholar]
- Fountain NB, Bear J, Bertram EH, 3rd, Lothman EW. Responses of deep entorhinal cortex are epileptiform in an electrogenic rat model of chronic temporal lobe epilepsy. J Neurophysiol. 1998;80:230–240. doi: 10.1152/jn.1998.80.1.230. [DOI] [PubMed] [Google Scholar]
- Gloveli T, Egorov AV, Schmitz D, Heinemann U, Müller W. Carbachol-induced changes in excitability and [Ca2+]i signalling in projection cells of medial entorhinal cortex layers II and III. Eur. J. Neurosci. 1999;11:3626–3636. doi: 10.1046/j.1460-9568.1999.00785.x. [DOI] [PubMed] [Google Scholar]
- Gorter JA, van Vliet EA, Aronica E, Breit T, Rauwerda H, da Silva F.H. Lopes, Wadman WJ. Potential new antiepileptogenic targets indicated by microarray analysis in a rat model for temporal lobe epilepsy. J Neurosci. 2006;26:11083–11110. doi: 10.1523/JNEUROSCI.2766-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- Hamam BN, Kennedy TE, Alonso A, Amaral DG. Morphological and electrophysiological characteristics of layer V neurons of the rat medial entorhinal cortex. J Comp Neurol. 2000;418:457–472. [PubMed] [Google Scholar]
- Hannaert P, Alvarez-Guerra M, Pirot D, Nazaret C, Garay RP. Rat NKCC2/NKCC1 cotransporter selectivity for loop diuretic drugs. Naunyn Schmiedebergs Arch Pharmacol. 2002;365:193–199. doi: 10.1007/s00210-001-0521-y. [DOI] [PubMed] [Google Scholar]
- Huberfeld G, Wittner L, Clemenceau S, Baulac M, Kaila K, Miles R, Rivera C. Perturbed chloride homeostasis and GABAergic signaling in human temporal lobe epilepsy. J Neurosci. 2007;27:9866–9873. doi: 10.1523/JNEUROSCI.2761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Wen X, Buckmaster PS. Reduced inhibition and increased output of layer II neurons in the medial entorhinal cortex in a model of temporal lobe epilepsy. J Neurosci. 2003;23:8471–8479. doi: 10.1523/JNEUROSCI.23-24-08471.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SS, Buckmaster PS. Hyperexcitability, interneurons, and loss of GABAergic synapses in entorhinal cortex in a model of temporal lobe epilepsy. J Neurosci. 2006;26:4613–4623. doi: 10.1523/JNEUROSCI.0064-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misgeld U, Deisz RA, Dodt HU, Lux HD. The role of chloride transport in postsynaptic inhibition of hippocampal neurons. Science. 1986;232:1413–1415. doi: 10.1126/science.2424084. [DOI] [PubMed] [Google Scholar]
- Munoz A, Mendez P, DeFelipe J, Alvarez-Leefmans FJ. Cation-chloride cotransporters and GABA-ergic innervation in the human epileptic hippocampus. Epilepsia. 2007;48:663–673. doi: 10.1111/j.1528-1167.2007.00986.x. [DOI] [PubMed] [Google Scholar]
- Ormandy GC, Jope RS, Snead OC., 3rd Anticonvulsant actions of MK-801 on the lithium-pilocarpine model of status epilepticus in rats. Exp Neurol. 1989;106:172–180. doi: 10.1016/0014-4886(89)90091-5. [DOI] [PubMed] [Google Scholar]
- Palma E, Amici M, Sobrero F, Spinelli G, Di Angelantonio S, Ragozzino D, Mascia A, Scoppetta C, Esposito V, Miledi R, Eusebi F. Anomalous levels of Cl- transporters in the hippocampal subiculum from temporal lobe epilepsy patients make GABA excitatory. Proc Natl Acad Sci U S A. 2006;103:8465–8468. doi: 10.1073/pnas.0602979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak HR, Weissinger F, Terunuma M, Carlson GC, Hsu FC, Moss SJ, Coulter DA. Disrupted dentate granule cell chloride regulation enhances synaptic excitability during development of temporal lobe epilepsy. J Neurosci. 2007;27:14012–14022. doi: 10.1523/JNEUROSCI.4390-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JA, Stevenson TJ, Donaldson LF. Molecular characterization of a putative K-Cl cotransporter in rat brain. A neuronal-specific isoform. J Biol Chem. 1996;271:16245–16252. doi: 10.1074/jbc.271.27.16245. [DOI] [PubMed] [Google Scholar]
- Peterson SL, Purvis RS, Griffith JW. Comparison of neuroprotective effects induced by alpha-phenyl-N-tert-butyl nitrone (PBN) and N-tert-butyl-alpha-(2 sulfophenyl) nitrone (S-PBN) in lithium-pilocarpine status epilepticus. Neurotoxicology. 2005;26:969–979. doi: 10.1016/j.neuro.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Russell JM. Sodium-potassium-chloride cotransport. Physiol Rev. 2000;80:211–276. doi: 10.1152/physrev.2000.80.1.211. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Eid T, Du F. Neurons in layer III of the entorhinal cortex. A role in epileptogenesis and epilepsy? Ann N Y Acad Sci. 2000;911:328–342. doi: 10.1111/j.1749-6632.2000.tb06735.x. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin PA, Knowles WD. Intracellular study of human epileptic cortex: in vitro maintenance of epileptiform activity? Science. 1984;223:709–712. doi: 10.1126/science.6695179. [DOI] [PubMed] [Google Scholar]
- Sen A, Martinian L, Nikolic M, Walker MC, Thom M, Sisodiya SM. Increased NKCC1 expression in refractory human epilepsy. Epilepsy Res. 2007;74:220–227. doi: 10.1016/j.eplepsyres.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Staley KJ, Soldo BL, Proctor WR. Ionic mechanisms of neuronal excitation by inhibitory GABAA receptors. Science. 1995;269:977–981. doi: 10.1126/science.7638623. [DOI] [PubMed] [Google Scholar]
- Sung KW, Kirby M, McDonald MP, Lovinger DM, Delpire E. Abnormal GABAA receptor-mediated currents in dorsal root ganglion neurons isolated from Na-K-2Cl cotransporter null mice. J Neurosci. 2000;20:7531–7538. doi: 10.1523/JNEUROSCI.20-20-07531.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turski L, Diedrichs S, Klockgether T, Schwarz M, Turski WA, Sontag KH, Bortolotto ZA, Calderazzo-Filho LS, Cavalheiro EA. Paradoxical anticonvulsant activity of the gamma-aminobutyrate antagonist bicuculline methiodide in the rat striatum. Synapse. 1991;7:14–20. doi: 10.1002/syn.890070103. [DOI] [PubMed] [Google Scholar]
- Wang C, Shimizu-Okabe C, Watanabe K, Okabe A, Matsuzaki H, Ogawa T, Mori N, Fukuda A, Sato K. Developmental changes in KCC1, KCC2, and NKCC1 mRNA expressions in the rat brain. Brain Res Dev Brain Res. 2002;139:59–66. doi: 10.1016/s0165-3806(02)00536-9. [DOI] [PubMed] [Google Scholar]
- Weiss GH, Salazar AM, Vance SC, Grafman JH, Jabbari B. Predicting posttraumatic epilepsy in penetrating head injury. Arch Neurol. 1986;43:771–773. doi: 10.1001/archneur.1986.00520080019013. [DOI] [PubMed] [Google Scholar]
- Wu HQ, Schwarcz R. Focal microinjection of gamma-acetylenic GABA into the rat entorhinal cortex: behavioral and electroencephalographic abnormalities and preferential neuron loss in layer III. Exp Neurol. 1998;153:203–213. doi: 10.1006/exnr.1998.6908. [DOI] [PubMed] [Google Scholar]
- Yamada J, Okabe A, Toyoda H, Kilb W, Luhmann HJ, Fukuda A. Cl-uptake promoting depolarizing GABA actions in immature rat neocortical neurones is mediated by NKCC1. J Physiol. 2004;557:829–841. doi: 10.1113/jphysiol.2004.062471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmazer-Hanke DM, Wolf HK, Schramm J, Elger CE, Wiestler OD, Blumcke I. Subregional pathology of the amygdala complex and entorhinal region in surgical specimens from patients with pharmacoresistant temporal lobe epilepsy. J Neuropathol Exp Neurol. 2000;59:907–920. doi: 10.1093/jnen/59.10.907. [DOI] [PubMed] [Google Scholar]