Abstract
The therapeutic potential of vascular endothelial growth factor C (VEGF-C) and VEGF-D in skeletal muscle has been of considerable interest as these factors have both angiogenic and lymphangiogenic activities. Previous studies have mainly employed adenoviral gene delivery for short-term expression of VEGF-C and VEGF-D in pig, rabbit and mouse skeletal muscles. Here we have used the activated mature forms of VEGF-C and VEGF-D expressed via recombinant adeno-associated virus (rAAV), which provides stable, long-lasting transgene expression in various tissues including skeletal muscle. Mouse tibialis anterior muscle was transduced with rAAV encoding human or mouse VEGF-C or VEGF-D. Two weeks later, immunohistochemical analysis showed increased numbers of both blood and lymph vessels, and doppler ultrasound analysis indicated increased blood vessel perfusion. The lymphatic vessels further increased at the four-week time point were functional, as shown by FITC-lectin uptake and transport. Furthermore, receptor activation and arteriogenic activity were increased by an alanine substitution mutant of human VEGF-C (C137A) having an increased dimer stability and by a chimeric CAC growth factor that contained the VEGF receptor-binding domain flanked by VEGF-C propeptides, but only the latter promoted significantly more blood vessel perfusion when compared to the other growth factors studied. We conclude that long-term expression of VEGF-C and VEGF-D in skeletal muscle results in the generation of new functional blood and lymphatic vessels. The therapeutic value of intramuscular lymph vessels in draining tissue edema and lymphedema can now be evaluated using this model system.
Keywords: VEGF-C, VEGF-D, adeno-associated virus, angiogenesis, lymphangiogenesis, skeletal muscle
Introduction
Blood and lymphatic vessels integrate to form the circulatory system, which provides oxygen and nutrients to the tissues and removes carbon dioxide and waste metabolites. Endothelial cells (ECs) form a surface barrier between the intra-and extravascular spaces, and EC number determines the functional capacity of the vasculature in each tissue. Vascular endothelial growth factors (VEGFs) are considered to be the main mitogenic and survival factors for ECs, obligatory for embryonic angio- and lymphangiogenesis and capable of activating ECs in the adult.1–3 In mammals, the five known VEGFs are VEGF-A, B, C, D and placenta growth factor (PlGF). Of these, VEGF-C and VEGF-D activate primarily lymphatic ECs, which express VEGF receptor (VEGFR)-3.4,5 After biosynthesis, full-length (fl) VEGF-C and VEGF-D undergo proteolytic cleavage of their C- and N-terminal propeptide domains.6,7 This releases the mature growth factor (ΔNΔC, here short form - sf) with an increased affinity towards VEGFR-3 and towards VEGFR-2, which is expressed mainly in blood vessels. VEGFR-2 binding has been assumed to be responsible for the angiogenic properties of VEGF-C and VEGF-D in a number of experimental conditions.8–10 However, no systematic studies have addressed the capacity of the proteolytically activated, mature VEGF-C/D forms to stimulate angiogenesis vs. lymphangiogenesis in vivo.
The ability to stimulate both VEGFR-2 and VEGFR-3 in blood and lymphatic vessels has made VEGF-C and VEGF-D attractive candidates for therapeutic improvement in many types of vascular insufficiencies, including skeletal and cardiac muscle ischemia, lymphedema, and lymphatic vessel hypoplasia.11 Adenoviral gene transfer was effective in delivering vascular growth factors to pig myocardium,12 rabbit and mouse skeletal muscle,9,13 and to the periadventitial tissue of rabbit carotid arteries.14 Transgene expression was found to be robust, but transient, lasting only one to three weeks, yet it resulted in significant improvement of tissue perfusion and capillary size. However, declining levels of transgene expression lead to significantly decreased perfusion and to pruning of newly formed blood vessels.9,12 In contrast to adenovirus, recombinant adeno-associated virus (rAAV) is an effective vehicle for delivering transgenes into many types of tissues and organs including muscle, liver, and brain.15,16 While rAAV transgene expression levels are lower compared to adenovirus, they are permanent in the muscle.17–19 This feature of rAAV may provide a vessel maintenance function when vascular growth factors are expressed. These properties of the rAAV vectors prompted us to study the long-term angiogenic vs. lymphangiogenic properties of the full-length vs. short forms of VEGF-C, VEGF-D and, for comparison, VEGF-A or a chimeric CAC growth factor that contained the VEGF receptor-binding domain flanked by VEGF-C propeptides, all delivered via rAAV into skeletal muscle of mice. We addressed in particular the effects of the activated, mature short forms of VEGF-C and VEGF-D.
Materials and Methods
Expanded Materials and Methods section containing detailed description of cell survival assay and analysis of protein expression, rAAV vector preparation, protein purification, isothermal titration calorimetry, VEGFR-2 and VEGFR-3 ELISA assays, immunohistochemistry, Doppler ultrasound measurements of perfusion in the transduced muscles and FITC-lectin microlymphography are provided as supplemental material.
Muscle transduction by the rAAV vectors
Six to seven week-old female FVB/NJ, ICR and C57BL/6J mice (three to four per group) were anesthetized with xylazine (Rompun, Bayer)-ketamine (Ketalar, Pfizer), and 5 × 1010 rAAV particles (in 30 μl volume) were injected into each tibialis anterior (t.a.) muscle. All mouse experiments were approved by the Provincial State Office of Southern Finland and carried out in accordance with institutional guidelines.
Fluorescent angiography of blood vessels via cardiac perfusion
Blood vessels were directly visualized by cardiac perfusion with the lipophilic carbocyanine dye 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI)20. After perfusion, the tibialis anterior muscle was isolated and 120 μm sections were analysed by confocal microscopy using the excitation wavelength of 543 nm.
Statistical analysis
We evaluated statistical significance by analysis of variance using the Dunnett (2-sided) test as a post-hoc test, with P<0.05 regarded as significant. Where pairwise comparisons between experimental groups were made, the Tukey test was used. Where indicated, Student’s t-test was also used. The results are presented as mean values ±SD, unless otherwise indicated. All experiments were reproduced at least three times.
Results
Expression and analysis of recombinant VEGF-C and VEGF-D
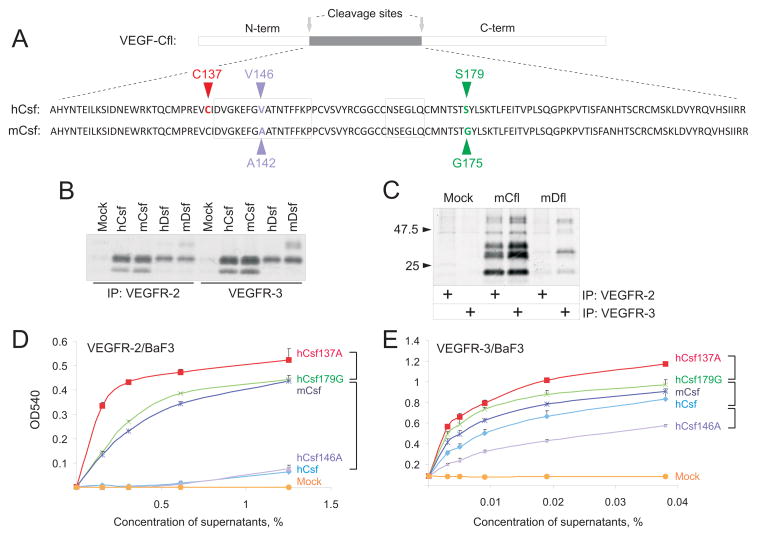
The regions encoding the proteolytically processed mature forms of human and mouse VEGF-C and VEGF-D, denoted VEGF-Csf and VEGF-Dsf, were cloned downstream of a signal sequence placed under the CMV promoter in the rAAV8 vector. Figure 1A shows the amino acid sequences of human and mouse VEGF-Csf. The residues that differ between the mouse and human sequences are marked in blue and green (alignment of sequences from seven animal species is shown in Online Figure I). Two residues (valine-146 and serine-179) in hVEGF-Csf were substituted to alanine (V146A) and glycine (S179G), respectively, that are found in the mVEGF-Csf protein. Alternatively, the cysteine-137 residue in hVEGF-Csf (marked red) that is not conserved in VEGF-A, VEGF-B or PlGF, was substituted to alanine.
Figure 1. Biochemical characterisation of recombinant growth factors.
A, Alignment of the human (h) and mouse (m) VEGF-Csf (Csf) sequences. The amino acid residue differences (V146/A142, S179/G175) are indicated in blue and green and the mutated residue (C137) in red. B, Soluble VEGFR-2 and VEGFR-3 precipitation of human and mouse factors followed by SDS-PAGE analysis under reducing conditions. Empty plasmid was used as the mock control. C, Coprecipitation of mouse full-length (VEGF-Cfl) and VEGF-Dfl with VEGFR-2 or VEGFR-3. D, MTT cell survival assay with VEGFR-2/BaF3 cells for the VEGF-Csf native and mutant molecules produced in transfected 293T cells. Mouse and human VEGF-Csf are denoted by mCsf and hCsf, respectively. V146A, S179G and C137A mutants of hVEGF-Csf are denoted as hCsf146A, hCsf179G, and hCsf137A, respectively. E, The same assay as in D, but utilizing VEGFR-3/BaF3 cells. In D and E, statistically significant (P<0.05) differences between the activities of certain proteins over range of studied concentrations are indicated by square brackets.
Production and receptor binding of the vector-encoded growth factors was analyzed from the medium of transfected 293T cells, precipitated with VEGFR-2-Ig or VEGFR-3-Ig (Figure 1B). The full-length cDNAs were also expressed and their products migrated as full-length, partially-processed and fully processed polypeptides (Figure 1C), as described previously.6,7 Note that the VEGF-Dsf and VEGF-Dfl bands appear relatively weaker when compared to the corresponding VEGF-C bands. This may reflect their significantly lower affinity to the receptors used for precipitation.2
VEGF-C137A provides increased dimer stability and receptor activity
Prior to the in vivo experiments, recombinant proteins were assayed for stimulation of receptor dimerization-mediated cell proliferation using mouse BaF3 pro-B cells expressing either VEGFR-2-Epo or VEGFR-3-Epo chimeric receptor (see supplemental Materials and Methods for details). Dilutions of transfected 293T cell media containing similar amounts of the growth factors were used. While the full-length growth factors were processed to several forms in the conditioned medium, the short forms had increased activity (see asterisks and brackets in Online Figure II). Overall, the results also indicated that VEGF-D is less active than VEGF-C in stimulating VEGFR-3 and especially VEGFR-2 activation (Online Figure II). These results are consistent with previously published data on VEGFR-2/VEGF-D interaction2,5,21 and with the data reported for phosphorylation of VEGFR-2 stably expressed by porcine aortic endothelial cells.22
Of all the mutant human VEGF-C:s, the strongest stimulation of BaF3 cell proliferation was obtained with hVEGF-Csf C137A (Figure 1D and E). Compared to the parental growth factor (hVEGF-Csf), this protein was particularly active towards VEGFR-2/BaF3 cells (Figure 1D). The G175 residue in the mouse sequence also seemed to provide increased activity over the human S179 (hVEGF-Csf S179G vs. hVEGF-Csf in Figure 1D), whereas replacement of V146 of hVEGF-Csf to A (corresponding to A142 in mouse VEGF-C) resulted in unaltered or diminished activity (Figure 1D, E).
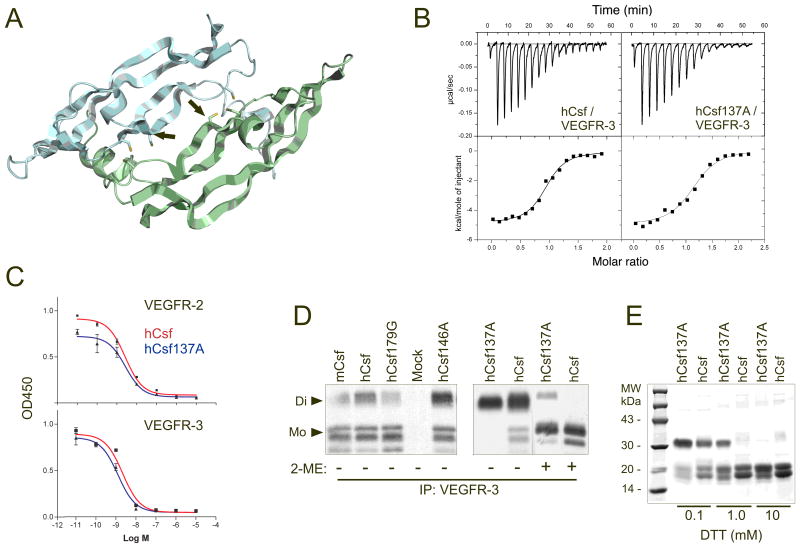
According to a model based on the structures of the other VEGF family proteins23–25, we predicted that the location of the C137 residue is in the dimer interface of hVEGF-Csf (Figure 2A). Despite its increased activity in BaF3 cell proliferation assays, isothermal titration calorimetry indicated that hVEGF-Csf C137A binds VEGFR-3 with an affinity similar to that of the wild-type protein (Kd 280 ± 30 nM and Kd 310 ± 50 nM, respectively; Figure 2B). Further analysis employed an ELISA-based competition assay, where plates were coated with VEGF165 (for VEGFR-2 binding) or hVEGF-Csf (for VEGFR-3) and then incubated with VEGFR-2-Ig or VEGFR-3-Ig proteins preincubated with dilutions of a blocking hVEGF-Csf or its C137A mutant. The amounts of receptor fusion proteins bound to the plates were then quantified by using HRP-conjugated antibodies. The results indicated that wild-type and mutant hVEGF-Csf do not differ significantly in binding to VEGFR-2 (EC50 2.6 ± 0.3 and 2.7 ± 0.6 nM, respectively) or VEGFR-3 (EC50 2.2 ± 0.6 and 1.3 ± 0.3 nM, respectively; P>0.05 for both comparisons, Student’s t-test) (Figure 2C). However, a difference was obtained in non-reducing conditions where hVEGF-Csf C137A migrated as a dimer, while the other two hVEGF-Csf mutants and the wild-type protein also contained some monomers. When treated with the reducing agents 2-ME or DTT (Figure 2D,E), hVEGF-Csf C137A retained a dimeric structure in conditions in which the native hVEGF-Csf protein was monomeric (1 mM DTT). Thus, the increased activity of the hVEGF-Csf C137A mutant protein in the cellular bioassay probably resulted from an increased stability of its dimeric structure.
Figure 2. Biochemical properties of wild-type versus mutant VEGF-Csf and VEGF-Dsf.
A, A 3-D computer model (SWISS-MODEL45) shows the putative location of the C137A mutation in the VEGF-Csf structure. The mutant residue (shown by red in Figure 1A) is marked with arrows. The two antiparallel polypeptide chains of hVEGF-Csf homodimer are shown in grey and green. The hVEGF-Csf model is based on the known crystal structure of VEGF amino terminal residues 8-10923 and other VEGF family proteins24,25. B, Analysis of the VEGFR-3 binding affinities of human VEGF-Csf and its C137A mutant by isothermal titration calorimetry. The proteins were produced as described in Materials and Methods. C, ELISA-based competitive binding assay with VEGF-Csf and its C137A mutant. D, VEGFR-3 co-precipitation of [35S]-labelled human VEGF-Csf mutants or wild type proteins, which were obtained from the media of 293T cells transfected with the corresponding plasmids (Di - dimer, Mo - monomer). Mock represents the supernatant of 293T cells transfected with empty plasmid. E, SDS-PAGE of purified VEGF-Csf and its C137A mutant treated with varying concentrations of DTT. The proteins were stained with Coomassie Blue. Growth factor abbreviations are described in Figure 1 legend.
rAAV8-VEGF-C/D transgenes induce both angiogenesis and lymphangiogenesis
The plasmid vectors used in the in vitro studies were subsequently packaged into rAAV8 and injected into mouse t.a. muscle. Two or four weeks after the injection, the muscles were processed for immunohistochemical staining of PECAM-1 (endothelial cells), MECA32 (blood vascular endothelial cells only), LYVE-1 (lymphatic endothelial cells), SMA (smooth muscle cells and pericytes) and CD45 (leukocytes). Additional immunostaining was done for PROX-1 and VEGFR-3 to confirm the lymphatic vessel phenotype. Three different mouse strains (FvB/NJ, C57Bl/6J and ICR) were used in the experiments to validate the key findings as several studies have demonstrated significant interstrain differences in their responses to the angiogenic growth factors bFGF and VEGF.26,27
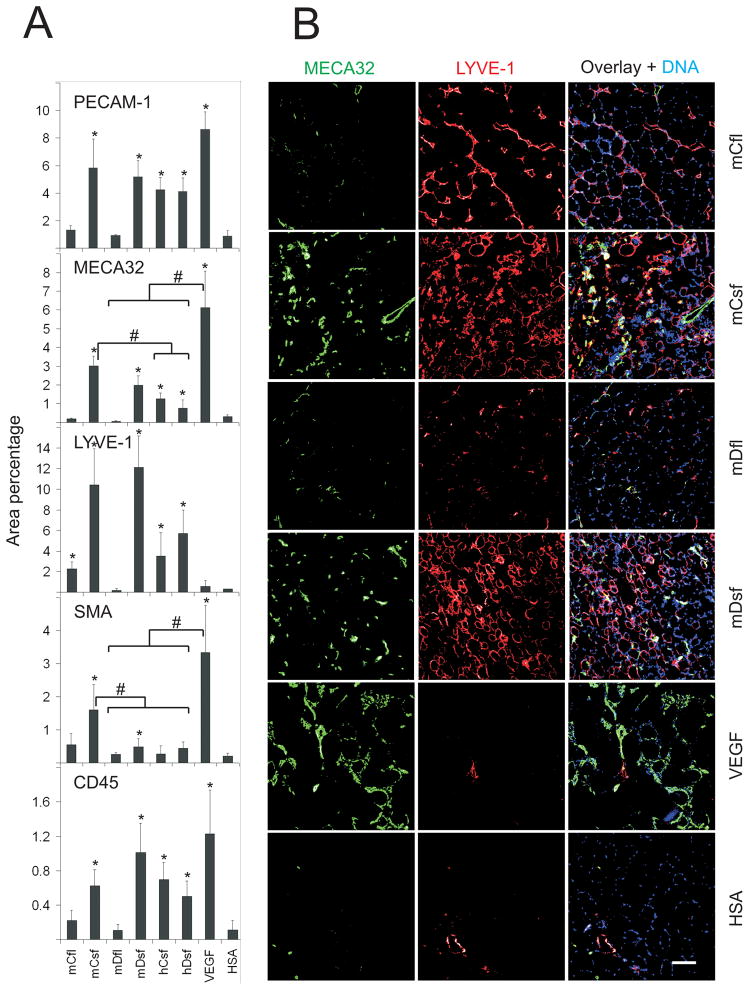
In muscles of the FvB/NJ and ICR mice, only small changes were observed in the blood or lymphatic vessels two weeks after injection of the vectors (data not shown). Analysis at four weeks, however, revealed both an angiogenic and a lymphangiogenic response to the short forms of the factors (Figure 3; Online Figure III, Online Figure IV). Double immunostaining of PECAM-1 and mVEGF-Dsf suggested a paracrine vascular effect around the growth factor producing myofibers (Online Figure V). Full-length mVEGF-C induced only some lymphangiogenesis, but no angiogenesis, while mVEGF-Dfl had no effect. The lymphangiogenic response was confirmed in parallel samples stained for PROX-1 (data not shown). Blood vessels (MECA32) with their associated perivascular smooth muscle (SMA) were notably increased in skeletal muscles injected with rAAV-mVEGF-Csf, which was also the most active among the wild-type factors in the VEGFR-2/BaF3 cellular assay (Figure 1 and Online Figure II). rAAV-VEGF165, used as a control, induced only angiogenesis (Figure 3).
Figure 3. Blood vascular and lymphatic endothelial staining of the target muscles four weeks after gene transduction.
Immunostaining for PECAM-1 (endothelial cells), MECA32 (blood vessel endothelial cells), LYVE-1 (lymphatic endothelial cells), SMA (smooth muscle cells and pericytes), and CD45 (leukocytes) frozen sections of the t.a. muscles of FVB/NJ mice. Full-length forms of mouse VEGF-Cfl and VEGF-Dfl were tested along with mouse and human VEGF-Csf and VEGF-Dsf. A, Quantification of the immunostaining was perfomed as described in Materials and Methods. * marks statistical significance at P<0.05, when compared to the HSA control. Square brackets with # mark statistical significance at P<0.05 between the indicated experimental groups. B, Representative images of mouse factor immunostaining are shown. The corresponding PECAM-1/SMA immunostaining patterns are shown in Online Figure III. Note that only mVEGF-Csf and VEGF165 induced significant recruitment of smooth muscle cells. Scale bars here and in all other figures are 100 μm, unless otherwise indicated.
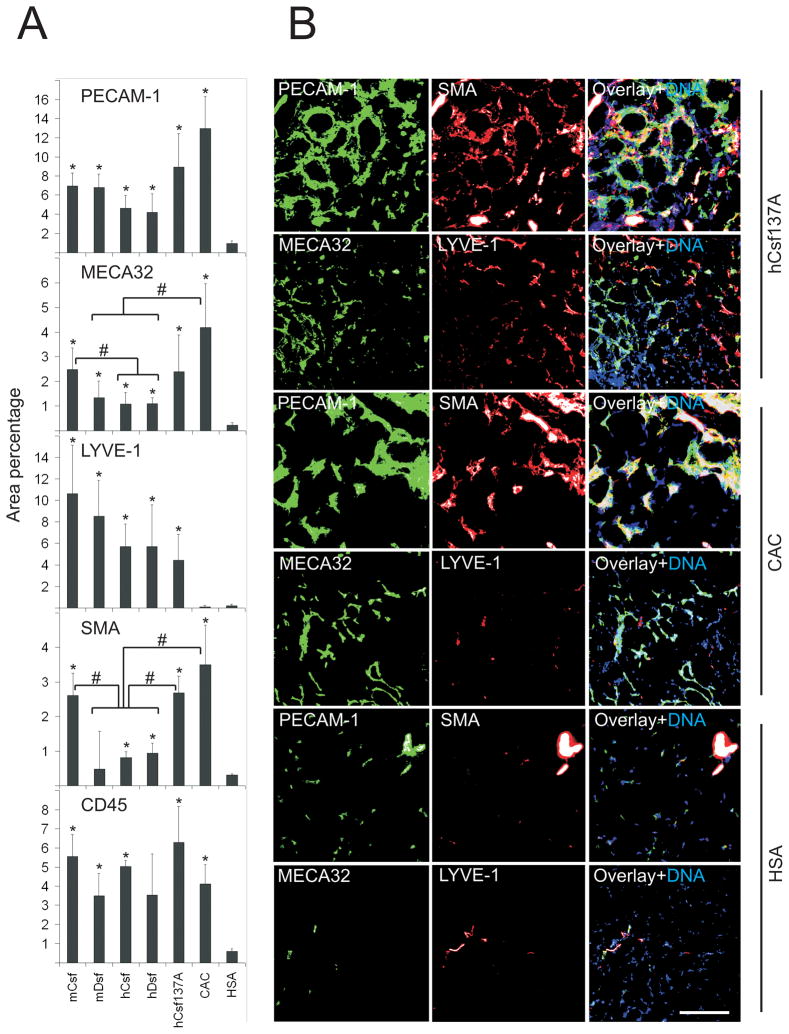
The vascular response in C57Bl/6J skeletal muscle was more rapid, being clear by two weeks after the injection of the vectors. As all mice of this strain injected with the corresponding concentrations of the control rAAV8-VEGF165 vector died or had to be euthanized (due to VEGF165-induced vascular leakage28), we instead used a chimeric VEGF-A called CAC that contains the VEGF165 residues 37 to 135 (VEGF homology domain) flanked with the propeptides of VEGF-C29. The mice tolerated treatment with this factor without overt symptoms despite the fact that rAAV-CAC induced significantly stronger angiogenic and arteriogenic responses than hVEGF-Csf, hVEGF-Dsf or mVEGF-Dsf in the skeletal muscles (MECA32 and SMA panels in Figure 4A). A vector encoding the hVEGF-Csf C137A mutant was also tested. Its arteriogenic effects were significantly stronger when compared to the parental factor hVEGF-Csf (Figure 4A), although its lymphangiogenic activity was not significantly different (see the LYVE-1 panel in Figure 4A: P>0.05, when hCsf137A is compared to any other experimental group). These effects were even more enhanced at week 11, the latest time point analyzed (data not shown). In all muscle samples analyzed, we observed a strong accumulation of CD45 positive cells with a tendency to concentrate in areas of neovesssel formation (Figure 4A and data not shown).
Figure 4. Blood vascular, lymphatic endothelial, and smooth muscle staining two weeks after gene transduction.
Double-immunostaining of the indicated antigens in frozen muscle sections from C57Bl/6J mice. A, Quantification of the immunostaining was performed as in Fig. 3. B, Representative images of hVEGF-Csf C137A-, CAC-, and HSA-treated muscle samples. Growth factor abbreviations are described in the Figure 1 legend. Note that while hVEGF-Csf C137A induces growth of both blood (MECA32-positive) and lymphatic (LYVE-1-positive) vessels, CAC induces only blood vessels. It should be noted that some of the inflammatory cells were also PECAM-1 positive.
Increased blood vessel perfusion in the muscles expressing VEGF-C/D
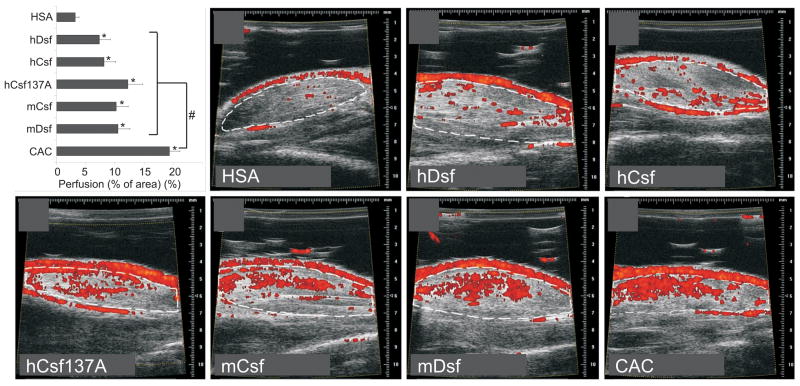
In order to observe any changes in blood perfusion in the treated muscles, we used Doppler ultrasound analysis to detect functional blood vessels with diameters of 30 μm or more. In C57Bl/6J skeletal muscles treated for two weeks with the various factors, significantly increased perfusion was recorded (Figure 5). The strongest effect was observed in muscles treated with rAAV-CAC (p<0.05). rAAV-hVEGF-C C137A also showed a strong angiogenic effect, although not statistically different from the effects of wild-type VEGF-C/Dsf proteins. The effect of mVEGF-Dsf seemed to be as strong as that of mVEGF-Csf, whereas the former was significantly weaker in inducing SMA-positive perivascular cells (Figure 4A).
Figure 5. Muscle perfusion two weeks after transduction of rAAV vectors.
C57Bl/6J mice were separated into 7 groups, and 4 animals per group were injected with vectors encoding the indicated growth factors. Blood flow in t.a. muscle was quantified by Doppler ultrasound. Significance values were determined between the test groups and the negative control group (HSA). The bars indicate ± s.e.m.; * marks statistically significant differences to HSA control. # marks statistical significance between CAC and any other experimental group. Representative 2-D images from the scanning of the muscles transduced with the various vectors are indicated.
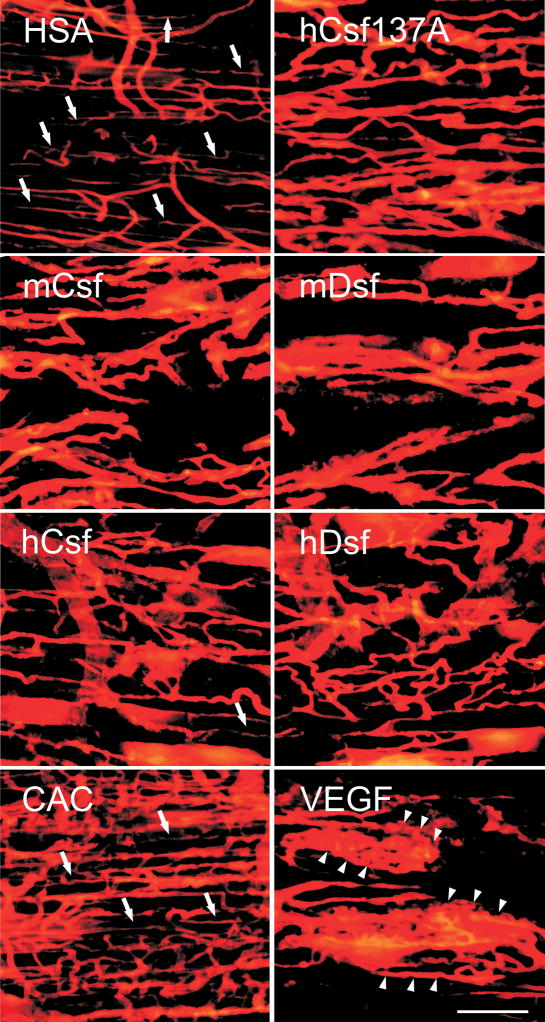
In order to further image the morphology and perfusion of vessels, the lipophilic dye DiI was injected to the left ventricle of the heart. This fluorescent dye is incorporated into blood vascular endothelial cell membranes, while unbound dye in the vessel lumen is washed away by subsequent perfusion-fixation.20 Confocal microscopy of thick muscle sections demonstrated that the AAV mediated VEGF-C/D expression leads to widening of preexisting vessels (Figure 6). Increased neovessel formation by sprouting was also observed in the hVEGF-Csf 137A treated samples and in the positive control (CAC). The CAC induced vessels differed greatly from those induced by VEGF which resulted in local angioma-like vascular patterns in the treated muscles (compare CAC and VEGF in Figure 6).
Figure 6. Comparison of perfusion staining of vessels induced by VEGF-C/-D, CAC and VEGF.
Intramuscular blood vessels were stained by perfusion with the lipophilic dye DiI two weeks after rAAV transduction. Note that both VEGF-C and VEGF-D increase vessel density and size. In CAC treated muscles, vessel density is markedly increased and vessel morphology most similar to that of muscle injected with the control vector (HSA), whereas VEGF induced the formation of angioma-like structures (arrowheads). Capillary-sized vessels are indicated by arrows.
VEGF-C and VEGF-D induced intramuscular lymphatic vessels are functional
Comparison of MECA32, LYVE-1 and PROX-1 immunostaining after four weeks of transgene expression in FvB/NJ mice demonstrated that the majority of the induced endothelium within the muscles was of lymphatic origin (Figure 3 and data not shown). In comparison, neither VEGF165 nor CAC induced lymphatic vessel growth (Figures 3 and 4).
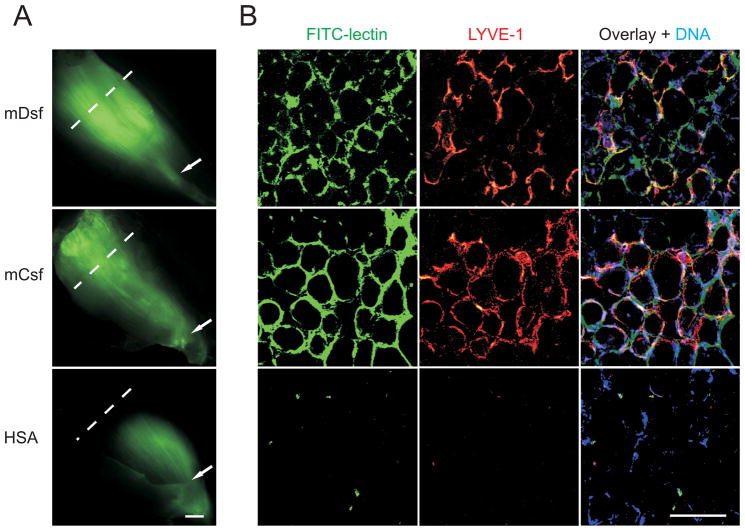
We used FITC-conjugated Lycopersicon esculentum lectin 30,31 to determine if the newly formed lymphatic vessels were functional, i.e. capable of absorbing macromolecules (such as FITC-lectin) and conducting fluid flow. Indeed, FITC-lectin injected to the distal end of the t.a. muscle was uniformly distributed within the newly formed lymphatic vessels 45 min after injection, although some was also present in between the myofibers (Figure 7), demonstrating that the vessels were functional.
Figure 7. Mouse VEGF-Csf and VEGF-Dsf induce formation of functional lymphatic vessels.
A, Lymphatic dye uptake and transfer were imaged 45 minutes after FITC-lectin injection into the distal (lower) part of t.a. muscles four weeks post-transduction with rAAVs encoding the indicated proteins. The stained sections were prepared from the regions indicated by the punctuated lines. Arrows indicate sites of FITC-lectin injection. The scale bar indicates 1 mm. B, FITC-lectin, nuclear (Hoechst 33258), and anti-LYVE-1 immunostaining were performed using muscle sections obtained from the muscle areas indicated in A.
Discussion
We show here that rAAV-delivered activated forms of VEGF-C and VEGF-D induce both angiogenesis and lymphangiogenesis in skeletal muscle and that the latter response tends to predominate at the four-week time-point used in our experiments.
Previously, adenovirally delivered human VEGF-DΔNΔC or VEGF165 were reported to induce transient angiogenic effects (increased vessel density and perfusion) in mouse skeletal muscle.9 These angiogenic effects peaked at four to seven days after injection, and then sharply decreased, reaching negligible levels at two weeks. The effects of VEGF165 were slightly more prolonged. Lymphatic vessels were only very weakly affected by hVEGF-D in this system. For the current studies we used rAAV-delivered transgene expression, as it was previously reported to provide more prolonged transgene expression combined with lower immunogenicity.17–19,32 Using immunohistochemical analysis we could observe a significant increase in lymphatic as well as blood vessels at two- and four-week time points, although the overall magnitude of the response depended on the mouse strain and in particular on the vascular growth factor used. Both effects were more profound when the activated, short forms were used, as compared with the full-length forms. Full-length mVEGF-D was not angiogenic or lymphangiogenic in our assays and full-length mVEGF-C was only able to induce formation of lymphatic vessels, apparently originating from the preexisting lymphatics found in between the myofibers.33
hVEGF-Dsf is significantly less active than hVEGF-Csf at inducing proliferation of VEGFR-2/BaF3 and VEGFR-3/BaF3 cells. Furthermore, neither mVEGF-Dfl nor mVEGF-Dsf was active in the VEGFR-2/BaF3 assay, consistent with the previously reported lack of mVEGF-D/mVEGFR-2 interaction.21 However, our in vivo data (immunohistochemistry for PECAM-1, MECA32 and LYVE-1, ultrasound analysis of muscle perfusion, visualization of blood vessels with DiI and studies of the functionality of newly formed lymphatic vessels by FITC-lectin uptake and transport) conclusively demonstrate that the angiogenic activity of rAAV-delivered VEGF-Dsf is comparable to that of VEGF-Csf, although mVEGF-Dsf shows significantly weaker smooth muscle cell recruiting activity than mVEGF-Csf, at least in the FvB/NJ and C57Bl/6J mice.
One explanation for the apparent discrepancy between in vitro and in vivo data is that human or mouse VEGF-Dsf binds to VEGFR-3 in vitro with at least 20-fold lower affinity than VEGF-Csf.2,5,21 Thus, to obtain equal stimulatory activity of VEGF-C and VEGF-D on receptor-bearing BaF3 cells in vitro, the proteins have to be added into the cell cultures in significantly different quantities, as here demonstrated using the BaF3 cell-based assays. Yet, in vivo, rAAV-infected myofibers provide continuous expression and supply of the transgenic protein, which may accumulate in high enough local concentrations to stimulate endothelial cell growth.
In the current study, as well as in previous reports, VEGF-D was shown to induce an angiogenic response.9,13,14 Although VEGF-D does not activate mouse VEGFR-2,21,22 it is possible that VEGFR-3 is involved in the angiogenic response.10,34,35 On the other hand, we observed accumulation of CD45- and F4/80-positive cells, especially in the C57Bl/6J mice, that could produce angiogenic factors at sites of rAAV transgene expression. The accumulation of bone marrow-derived inflammatory cells capable of producing a range of biologically active molecules (including VEGF, TGFβ, PDGF-B, and others), can provide additional angiogenic and arteriogenic signals at sites of adult angiogenesis.36–38 Furthermore, inflammatory cells and especially macrophages can also promote lymphangiogenesis by synthesis and secretion of VEGF-C and VEGF-D.39–41
Our functional tests confirmed that the newly generated blood and lymphatic capillaries were perfused and thus functional. Transgene expression in muscle tissue at four weeks after vector injection was consistent with morphological and functional changes observed at that time. However, only some myofibers expressed the transgenes (unpublished data), similar to what has been reported for rAAV8-LacZ-injected canine muscle, where the initially high numbers of β-galactosidase positive myofibers were significantly reduced 4 to 8 weeks after injection.42 It has been reported, that the majority of stably transduced host cells apparently maintain recombinant AAV genome as extrachromosomal episomes.43
The strongest stimulator of BaF3 cell proliferation was the hVEGF-Csf C137A mutant, which showed improved dimer stability in multiple assays. Evidently, disulfide reducing agents that lead to full dissociation of the native hVEGF-Csf only partially affected hVEGF-Csf C137A homodimers. It should be noted that the cysteine residue corresponding to C137 in human VEGF-C is not conserved outside of the VEGF-C/D subfamily, and according to our structural model it is located next to the putative intermolecular disulfide bridge. We therefore suggest that this cysteine residue makes the intermolecular disulfide bond unstable, resulting in non-covalent homodimers of wild-type VEGF-Csf.6 Replacing cysteine-137 with alanine may lead to the stabilization of the intermolecular disulfide bond and ultimately to a more stable hVEGF-Csf C137A homodimer. As mentioned previously, this more stable dimeric form of VEGF-C can bind to and activate the VEGFR-3 and VEGFR-2 receptors more efficiently and the corresponding change in VEGF-D has a similar effect 44.
Our immunohistological data demonstrate that, compared to hVEGF-Csf, the hVEGF-CsfC137A mutant shows significantly increased arteriogenenic activity (SMA panel in Figure 4A), but not increased lymphangiogenic activity. In previous studies of adenovirally transfected mouse and rabbit skeletal muscles, human VEGF-D was also demonstrated to possess arteriogenic activity.9,13 The differences between previously published data and our own might be attributable to differences in target cells and to varying levels of transgene expression. – Unexpectedly, all C57Bl/6J mice transduced with rAAV8-VEGF165 died within 10 days, while mice transduced with corresponding doses of rAAV8-CAC survived and showed strong angiogenesis in the injected muscles. CAC was also a stronger angiogenic inducer than human VEGF-C or VEGF-D, but it did not promote lymphangiogenesis. Thus this chimeric factor has interesting properties that might be exploited for potential gene therapy.
In conclusion, we have found that rAAV-delivered activated VEGF-C and VEGF-D and an engineered VEGF-C variant can all induce blood and lymphatic vessels in mouse skeletal muscle. The high arteriogenic activity of the hVEGF-Csf C137A growth factor seems to be attributable to its enhanced dimer stability due to the formation of stable interchain disulfide bonds. The most active arteriogenic factors also demonstrated a trend towards higher angiogenic activity, and the newly generated blood and lymphatic vessels were determined to be functional. Future goals now will include determining the therapeutic benefit of these factors in conditions of ischemia or muscle edema.
Supplementary Material
Acknowledgments
We thank Professor Seppo Sarna and Drs. Paul Bromann, Caroline Heckman and Tuomas Tammela for critical comments on the manuscript; Tapio Tainola, for expert technical assistance; the Biomedicum Molecular Imaging Unit for microscope support and maintenance and the staff at the Biomedicum Helsinki and the Haartman Institute Animal Facilities for excellent animal husbandry.
Sources of Funding
This study was supported by NIH grant 5-R01-HL075183-02; the Finnish Technology Development Centre, the Academy of Finland Council of Health, the Sigrid Juselius Foundation, the Finnish and European Foundations for the Study of Diabetes and the Novo Nordisk Foundation.
Footnotes
Disclosures
K.A. is the Chairman of VeGenics Scientific Advisory Board
References
- 1.Takahashi H, Shibuya M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin Sci (Lond) 2005;109:227–241. doi: 10.1042/CS20040370. [DOI] [PubMed] [Google Scholar]
- 2.Yamazaki Y, Morita T. Molecular and functional diversity of vascular endothelial growth factors. Mol Divers. 2006;10:515–527. doi: 10.1007/s11030-006-9027-3. [DOI] [PubMed] [Google Scholar]
- 3.Tirziu D, Simons M. Angiogenesis in the human heart: Gene and cell therapy. Angiogenesis. 2005;8:241–251. doi: 10.1007/s10456-005-9011-z. [DOI] [PubMed] [Google Scholar]
- 4.Karpanen T, Alitalo K. Molecular biology and pathology of lymphangiogenesis. Annu Rev Pathol. 2008;3:367–397. doi: 10.1146/annurev.pathmechdis.3.121806.151515. [DOI] [PubMed] [Google Scholar]
- 5.Makinen T, Veikkola T, Mustjoki S, Karpanen T, Catimel B, Nice EC, Wise L, Mercer A, Kowalski H, Kerjaschki D, Stacker SA, Achen MG, Alitalo K. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 2001;20:4762–4773. doi: 10.1093/emboj/20.17.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joukov V, Sorsa T, Kumar V, Jeltsch M, Claesson-Welsh L, Cao Y, Saksela O, Kalkkinen N, Alitalo K. Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J. 1997;16:3898–3911. doi: 10.1093/emboj/16.13.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stacker SA, Stenvers K, Caesar C, Vitali A, Domagala T, Nice E, Roufail S, Simpson RJ, Moritz R, Karpanen T, Alitalo K, Achen MG. Biosynthesis of vascular endothelial growth factor-D involves proteolytic processing which generates non-covalent homodimers. J Biol Chem. 1999;274:32127–32136. doi: 10.1074/jbc.274.45.32127. [DOI] [PubMed] [Google Scholar]
- 8.Benest AV, Harper SJ, Herttuala SY, Alitalo K, Bates DO. VEGF-C induced angiogenesis preferentially occurs at a distance from lymphangiogenesis. Cardiovasc Res. 2008;78:315–323. doi: 10.1093/cvr/cvm094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kholova I, Koota S, Kaskenpaa N, Leppanen P, Narvainen J, Kavec M, Rissanen TT, Hazes T, Korpisalo P, Grohn O, Yla-Herttuala S. Adenovirus-mediated gene transfer of human vascular endothelial growth factor-d induces transient angiogenic effects in mouse hind limb muscle. Hum Gene Ther. 2007;18:232–244. doi: 10.1089/hum.2006.100. [DOI] [PubMed] [Google Scholar]
- 10.Lohela M, Helotera H, Haiko P, Dumont DJ, Alitalo K. Transgenic induction of vascular endothelial growth factor-C is strongly angiogenic in mouse embryos but leads to persistent lymphatic hyperplasia in adult tissues. Am J Pathol. 2008;173:1891–1901. doi: 10.2353/ajpath.2008.080378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yla-Herttuala S, Alitalo K. Gene transfer as a tool to induce therapeutic vascular growth. Nat Med. 2003;9:694–701. doi: 10.1038/nm0603-694. [DOI] [PubMed] [Google Scholar]
- 12.Rutanen J, Rissanen TT, Markkanen JE, Gruchala M, Silvennoinen P, Kivela A, Hedman A, Hedman M, Heikura T, Orden MR, Stacker SA, Achen MG, Hartikainen J, Yla-Herttuala S. Adenoviral catheter-mediated intramyocardial gene transfer using the mature form of vascular endothelial growth factor-D induces transmural angiogenesis in porcine heart. Circulation. 2004;109:1029–1035. doi: 10.1161/01.CIR.0000115519.03688.A2. [DOI] [PubMed] [Google Scholar]
- 13.Rissanen TT, Markkanen JE, Gruchala M, Heikura T, Puranen A, Kettunen MI, Kholova I, Kauppinen RA, Achen MG, Stacker SA, Alitalo K, Yla-Herttuala S. VEGF-D is the strongest angiogenic and lymphangiogenic effector among VEGFs delivered into skeletal muscle via adenoviruses. Circ Res. 2003;92:1098–1106. doi: 10.1161/01.RES.0000073584.46059.E3. [DOI] [PubMed] [Google Scholar]
- 14.Bhardwaj S, Roy H, Gruchala M, Viita H, Kholova I, Kokina I, Achen MG, Stacker SA, Hedman M, Alitalo K, Yla-Herttuala S. Angiogenic responses of vascular endothelial growth factors in periadventitial tissue. Hum Gene Ther. 2003;14:1451–1462. doi: 10.1089/104303403769211664. [DOI] [PubMed] [Google Scholar]
- 15.Schultz BR, Chamberlain JS. Recombinant adeno-associated virus transduction and integration. Mol Ther. 2008;16:1189–1199. doi: 10.1038/mt.2008.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zentilin L, Giacca M. Adeno-associated virus vectors: Versatile tools for in vivo gene transfer. Contrib Nephrol. 2008;159:63–77. doi: 10.1159/000125584. [DOI] [PubMed] [Google Scholar]
- 17.Nykanen AI, Pajusola K, Krebs R, Keranen MA, Raisky O, Koskinen PK, Alitalo K, Lemstrom KB. Common protective and diverse smooth muscle cell effects of AAV-mediated angiopoietin-1 and -2 expression in rat cardiac allograft vasculopathy. Circ Res. 2006;98:1373–1380. doi: 10.1161/01.RES.0000225987.52765.13. [DOI] [PubMed] [Google Scholar]
- 18.Rivera VM, Gao GP, Grant RL, Schnell MA, Zoltick PW, Rozamus LW, Clackson T, Wilson JM. Long-term pharmacologically regulated expression of erythropoietin in primates following AAV-mediated gene transfer. Blood. 2005;105:1424–1430. doi: 10.1182/blood-2004-06-2501. [DOI] [PubMed] [Google Scholar]
- 19.Rivera VM, Ye X, Courage NL, Sachar J, Cerasoli F, Jr, Wilson JM, Gilman M. Long-term regulated expression of growth hormone in mice after intramuscular gene transfer. Proc Natl Acad Sci U S A. 1999;96:8657–8662. doi: 10.1073/pnas.96.15.8657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Song Y, Zhao L, Gaidosh G, Laties AM, Wen R. Direct labeling and visualization of blood vessels with lipophilic carbocyanine dye DiI. Nat Protoc. 2008;3:1703–1708. doi: 10.1038/nprot.2008.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baldwin ME, Catimel B, Nice EC, Roufail S, Hall NE, Stenvers KL, Karkkainen MJ, Alitalo K, Stacker SA, Achen MG. The specificity of receptor binding by vascular endothelial growth factor-d is different in mouse and man. J Biol Chem. 2001;276:19166–19171. doi: 10.1074/jbc.M100097200. [DOI] [PubMed] [Google Scholar]
- 22.Alam A, Herault JP, Barron P, Favier B, Fons P, Delesque-Touchard N, Senegas I, Laboudie P, Bonnin J, Cassan C, Savi P, Ruggeri B, Carmeliet P, Bono F, Herbert JM. Heterodimerization with vascular endothelial growth factor receptor-2 (VEGFR-2) is necessary for VEGFR-3 activity. Biochem Biophys Res Commun. 2004;324:909–915. doi: 10.1016/j.bbrc.2004.08.237. [DOI] [PubMed] [Google Scholar]
- 23.Muller YA, Li B, Christinger HW, Wells JA, Cunningham BC, de Vos AM. Vascular endothelial growth factor: Crystal structure and functional mapping of the kinase domain receptor binding site. Proc Natl Acad Sci U S A. 1997;94:7192–7197. doi: 10.1073/pnas.94.14.7192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iyer S, Leonidas DD, Swaminathan GJ, Maglione D, Battisti M, Tucci M, Persico MG, Acharya KR. The crystal structure of human placenta growth factor-1 (PlGF-1), an angiogenic protein, at 2.0 A resolution. J Biol Chem. 2001;276:12153–12161. doi: 10.1074/jbc.M008055200. [DOI] [PubMed] [Google Scholar]
- 25.Iyer S, Scotney PD, Nash AD, Ravi Acharya K. Crystal structure of human vascular endothelial growth factor-B: Identification of amino acids important for receptor binding. J Mol Biol. 2006;359:76–85. doi: 10.1016/j.jmb.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Rohan RM, Fernandez A, Udagawa T, Yuan J, D’Amato RJ. Genetic heterogeneity of angiogenesis in mice. FASEB J. 2000;14:871–876. doi: 10.1096/fasebj.14.7.871. [DOI] [PubMed] [Google Scholar]
- 27.Shaked Y, Bertolini F, Man S, Rogers MS, Cervi D, Foutz T, Rawn K, Voskas D, Dumont DJ, Ben-David Y, Lawler J, Henkin J, Huber J, Hicklin DJ, D’Amato RJ, Kerbel RS. Genetic heterogeneity of the vasculogenic phenotype parallels angiogenesis; implications for cellular surrogate marker analysis of antiangiogenesis. Cancer Cell. 2005;7:101–111. doi: 10.1016/j.ccr.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 28.Nagy JA, Benjamin L, Zeng H, Dvorak AM, Dvorak HF. Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis. 2008;11:109–119. doi: 10.1007/s10456-008-9099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keskitalo S, Tammela T, Lyytikka J, Karpanen T, Jeltsch M, Markkanen J, Yla-Herttuala S, Alitalo K. Enhanced capillary formation stimulated by a chimeric vascular endothelial growth factor/vascular endothelial growth factor-C silk domain fusion protein. Circ Res. 2007;100:1460–1467. doi: 10.1161/01.RES.0000269042.58594.f6. [DOI] [PubMed] [Google Scholar]
- 30.Tammela T, Saaristo A, Holopainen T, Lyytikka J, Kotronen A, Pitkonen M, Abo-Ramadan U, Yla-Herttuala S, Petrova TV, Alitalo K. Therapeutic differentiation and maturation of lymphatic vessels after lymph node dissection and transplantation. Nat Med. 2007;13:1458–1466. doi: 10.1038/nm1689. [DOI] [PubMed] [Google Scholar]
- 31.Thurston G, Baluk P, Hirata A, McDonald DM. Permeability-related changes revealed at endothelial cell borders in inflamed venules by lectin binding. Am J Physiol. 1996;271:H2547–H2562. doi: 10.1152/ajpheart.1996.271.6.H2547. [DOI] [PubMed] [Google Scholar]
- 32.Gruchala M, Bhardwaj S, Pajusola K, Roy H, Rissanen TT, Kokina I, Kholova I, Markkanen JE, Rutanen J, Heikura T, Alitalo K, Bueler H, Yla-Herttuala S. Gene transfer into rabbit arteries with adeno-associated virus and adenovirus vectors. J Gene Med. 2004;6:545–554. doi: 10.1002/jgm.535. [DOI] [PubMed] [Google Scholar]
- 33.Kivela R, Havas E, Vihko V. Localisation of lymphatic vessels and vascular endothelial growth factors-C and -D in human and mouse skeletal muscle with immunohistochemistry. Histochem Cell Biol. 2007;127:31–40. doi: 10.1007/s00418-006-0226-x. [DOI] [PubMed] [Google Scholar]
- 34.Laakkonen P, Waltari M, Holopainen T, Takahashi T, Pytowski B, Steiner P, Hicklin D, Persaud K, Tonra JR, Witte L, Alitalo K. Vascular endothelial growth factor receptor 3 is involved in tumor angiogenesis and growth. Cancer Res. 2007;67:593–599. doi: 10.1158/0008-5472.CAN-06-3567. [DOI] [PubMed] [Google Scholar]
- 35.Tammela T, Zarkada G, Wallgard E, Murtomaki A, Suchting S, Wirzenius M, Waltari M, Hellstrom M, Schomber T, Peltonen R, Freitas C, Duarte A, Isoniemi H, Laakkonen P, Christofori G, Yla-Herttuala S, Shibuya M, Pytowski B, Eichmann A, Betsholtz C, Alitalo K. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature. 2008;454:656–660. doi: 10.1038/nature07083. [DOI] [PubMed] [Google Scholar]
- 36.De Palma M, Murdoch C, Venneri MA, Naldini L, Lewis CE. Tie2-expressing monocytes: Regulation of tumor angiogenesis and therapeutic implications. Trends Immunol. 2007;28:519–524. doi: 10.1016/j.it.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Zacchigna S, Pattarini L, Zentilin L, Moimas S, Carrer A, Sinigaglia M, Arsic N, Tafuro S, Sinagra G, Giacca M. Bone marrow cells recruited through the neuropilin-1 receptor promote arterial formation at the sites of adult neoangiogenesis in mice. J Clin Invest. 2008;118:2062–2075. doi: 10.1172/JCI32832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 39.Murakami M, Zheng Y, Hirashima M, Suda T, Morita Y, Ooehara J, Ema H, Fong GH, Shibuya M. VEGFR1 tyrosine kinase signaling promotes lymphangiogenesis as well as angiogenesis indirectly via macrophage recruitment. Arterioscler Thromb Vasc Biol. 2008;28:658–664. doi: 10.1161/ATVBAHA.107.150433. [DOI] [PubMed] [Google Scholar]
- 40.Jeon BH, Jang C, Han J, Kataru RP, Piao L, Jung K, Cha HJ, Schwendener RA, Jang KY, Kim KS, Alitalo K, Koh GY. Profound but dysfunctional lymphangiogenesis via vascular endothelial growth factor ligands from CD11b+ macrophages in advanced ovarian cancer. Cancer Res. 2008;68:1100–1109. doi: 10.1158/0008-5472.CAN-07-2572. [DOI] [PubMed] [Google Scholar]
- 41.Schoppmann SF, Birner P, Stockl J, Kalt R, Ullrich R, Caucig C, Kriehuber E, Nagy K, Alitalo K, Kerjaschki D. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol. 2002;161:947–956. doi: 10.1016/S0002-9440(10)64255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohshima S, Shin JH, Yuasa K, Nishiyama A, Kira J, Okada T, Takeda S. Transduction efficiency and immune response associated with the administration of AAV8 vector into dog skeletal muscle. Mol Ther. 2008 doi: 10.1038/mt.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakai H, Yant SR, Storm TA, Fuess S, Meuse L, Kay MA. Extrachromosomal recombinant adeno-associated virus vector genomes are primarily responsible for stable liver transduction in vivo. J Virol. 2001;75:6969–6976. doi: 10.1128/JVI.75.15.6969-6976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Toivanen PI, Nieminen T, Viitanen L, Alitalo A, Roschier M, Jauhiainen S, Markkanen JE, Laitinen OH, Airenne TT, Salminen TA, Johnson MS, Airenne KJ, Ylä-Herttuala S. Novel vascular endothelial growth factor D (VEGF-D) variants with increased biological activity. J Biol Chem. 2009;284:16037–16048. doi: 10.1074/jbc.M109.001123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: A web-based environment for protein structure homology modelling. Bioinformatics. 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.