Bipolar kinesin-5 motors, essential in diverse organisms, can generate positive sliding forces between overlapped interpolar microtubules to push mitotic spindle poles apart. BMK-1, the sole Caenorhabditis elegans kinesin-5, is not essential. We have determined, by tracking pole movements in bmk-1 mutant C. elegans embryos, that BMK-1 actually resists pole separation during anaphase. This provides in vivo evidence that kinesin-5, when challenged by fast pole separation forces, can serve as a rate-limiting brake for interpolar microtubule sliding.
To organize and then accurately separate duplicated sets of chromosomes, eukaryotic cells construct a spindle. In many cell types, duplication of a centrosome creates two adjacent spindle poles that anchor microtubules by their minus ends to form aster-like radial arrays. Interpolar connections are formed between two asters when their microtubule plus ends associate laterally via cross-linking proteins to overlap in an anti-parallel fashion [1]. A classic model posits that plus-end-directed kinesin-5 type motors, which form bipolar heterotetramers, generate sliding forces between overlapped interpolar microtubules to push spindle poles apart [2,3]. Consistent with this, in vitro tests have shown that Xenopus kinesin-5 (Eg5) can crosslink antiparallel microtubules and forcefully slide minus-ends away from one another [4]. This agrees with in vivo kinesin-5 inhibition, which causes failure of pole separation, convergence of already separated poles, disruption of chromosome segregation and lethality in diverse organisms, from fungi to mammalian cells [5,6].
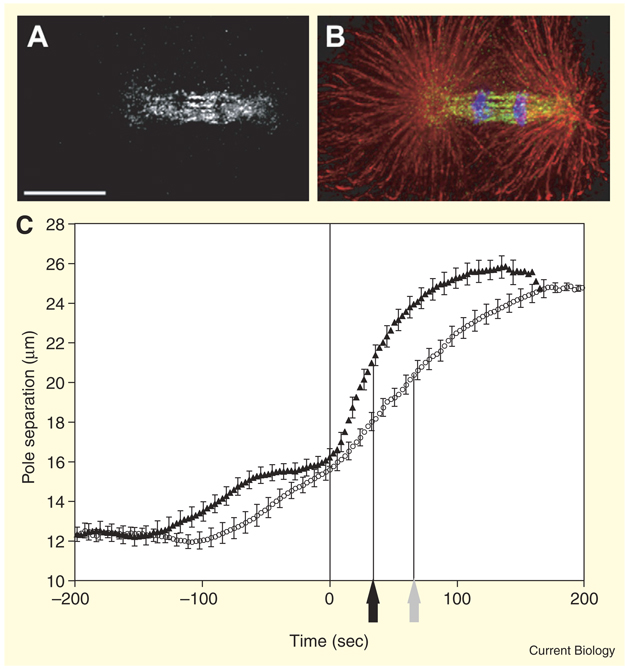
BMK-1, the sole C. elegans kinesin-5, provides a puzzling exception because inhibition does not block mitosis and is not lethal [7]. Such an exception could provide new insights, so we studied BMK-1 distribution and the consequences of disrupting its function on spindle pole behavior in early embryos (see Experimental procedures in Supplemental data published with this article online). BMK-1 distribution in mitosis parallels that of kinesin-5 in other organisms: at the poles and throughout the spindle before anaphase, then most concentrated during anaphase in the ‘interzone’ between separated chromosomes where interpolar microtubules overlap (Figure 1A,B) [7].
Figure 1.
Distribution of BMK-1 and its influence on spindle pole separation. (A) Anti-BMK-1 immunostaining of a 1-cell anaphase embryo (bar = 10 µm). (B) The same embryo with anti-BMK-1 in green, anti-α-tubulin in red, and DAPI staining of chromosomes in blue. (C) The distance between the centers of mitotic spindle poles as a function of time in living wild-type (circles) and bmk-1(ok391) mutant (triangles) embryos. Each data point represents the average pole-to-pole distance at a given time point before or after the last frame before start of anaphase chromosome separation (n = 20 embryos). Error bars indicate 95% confidence intervals (1.96 × σ/√n). Black and grey arrows indicate half-maximal anaphase pole separation for bmk-1 mutants and wild type, respectively.
Live imaging of spindles revealed abnormally fast pole separation at the start of anaphase in bmk-1 deletion mutants (Video S1 in Supplemental data). Anaphase poles in mutants reached half-maximal separation at an average of 34 sec as opposed to 66 sec in wild type (Figure 1C). Although there was a small decrease in GFP-tagged microtubule fluorescence in the anaphase interzones of bmk-1 mutants (24 ± 10%, n = 10), we observed no disconnection of half-spindles (‘pop-apart’) like that reported for inhibition of the microtubule depolymerase KLP-7 (MCAK) [8]. Similar but less severe phenotypes were seen after depletion of bmk-1 by RNAi. The implication of the fast initial pole separation in bmk-1 mutants is that BMK-1 normally resists anaphase pole separation forces generated by other motors.
Outward pulling by astral microtubules in C. elegans generates a prominent force in anaphase that can drive a peak pole separation velocity of 0.8–1.5 µm/sec when interzone connections are broken [8]. Kinesin-5 velocity along microtubules is only 0.02–0.10 µm/sec, even when pulled forward toward plus ends with an optical trap [4,9]. Our results suggest that, in the face of fast pole separation forces, BMK-1 regulates the interzone microtubule sliding rate. Although this might be indirect, e.g. via stabilization of interpolar microtubules, it could also reflect a direct effect of BMK-1 as a molecular brake [10], i.e. BMK-1 may govern sliding rates with its slow, but processive, ATP hydrolysis-driven step cycle [9]. Mixed-motor microtubule gliding assays suggest, however, that it also could generate drag via microtubule binding/release kinetics that are independent of ATP hydrolysis [11].
To test regulation of pole separation rate by BMK-1 in a different force-balance environment, we compared pole separation rates in bmk-1(+) and bmk-1 deletion mutant embryos after reducing outward pulling force on spindle poles by inhibition of GPR-1/2 [12,13]. In bmk-1(+) embryos, gpr-1/2 RNAi dramatically reduced the initial anaphase pole separation rate and the net separation that occurred during anaphase (Table 1). The bmk-1 mutation suppressed those gpr-1/2 RNAi phenotypes, allowing a near-normal initial rate and a partial restoration of net separation. This suggests that there is a gpr-1/2 RNAi-insensitive weak pole separation force that can be unmasked by eliminating the braking activity of BMK-1. It may be generated by a remnant GPR-1/2-dependent mechanism or by a novel GPR-1/2-independent mechanism. In either case, it is evident that pole separation rates are determined by a force-balance relationship [2] and that the C. elegans kinesin-5 homolog is a key factor for resisting fast outward pole separation forces.
Table 1.
Effects of gpr-1/2 RNAi on spindle pole separation in bmk-1(+) and bmk-1 deletion mutant embryos.
| Genotype and RNAi treatment |
1Initial pole separation rate (µm/s) 0-18s |
Net increase in separation during anaphase (µm) |
Maximum pole separation distance (µm) |
n |
|---|---|---|---|---|
| bmk-1(+) | 0.107 ± 0.008 | 8.33 ± 0.29 | 24.72 ± 0.21 | 10 |
| bmk-1(ok391) | 0.162 ± 0.012 | 8.98 ± 0.27 | 25.08 ± 0.41 | 10 |
| bmk-1(+) & gpr-1/2(RNAi) | 0.049 ± 0.007 | 4.46 ± 0.23 | 17.26 ± 0.29 | 9 |
| bmk-1(ok391) & gpr-1/2(RNAi) | 0.102 ± 0.010 | 7.00 ± 0.52 | 19.60 ± 0.61 | 8 |
All values represent mean ± SEM determined from pole-pole distance measurements starting in the last frame before detectable anaphase chromosome separation.
This role reversal for kinesin-5 in C. elegans, from a sliding motor to a sliding brake, supports the view that, despite conservation of basic structural and mechanistic principles in mitosis, the ways in which individual components of the mitotic machinery contribute can be shuffled by evolution. Such divergence may be allowed in mitosis because of the rich “layering” of partially redundant force generation and spindle assembly mechanisms. Where might the selective advantage lie in using BMK-1 as a brake? Perhaps it co-evolved with unusually strong outward astral pulling forces to create a balanced tension that helps ensure the precise control of metaphase-anaphase spindle position that is so critical for determining cell identities during C. elegans development.
Supplementary Material
Acknowledgements
The bmk-1 deletion strain was a product of the C. elegans Gene Knockout Consortium. All other starting strains were obtained from the Caenorhabditis Genetics Center, except for WH204 for which we thank Chris Malone and John White. This work was supported by funds from MetaCYT of Indiana, NIH (GM58811 and GM46295) and by an HHMI Capstone undergraduate research award to A.M.S.
Footnotes
Supplemental data
Supplemental data, including experimental procedures and a movie, are available at http://www.current-biology.com/cgi/content/full/17/12/R453/DC1
References
- 1.Mastronarde DN, McDonald KL, Ding R, McIntosh JR. Interpolar spindle microtubules in PTK cells. J. Cell Biol. 1993;123:1475–1489. doi: 10.1083/jcb.123.6.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brust-Mascher I, Civelekoglu-Scholey G, Kwon M, Mogilner A, Scholey JM. Model for anaphase B: role of three mitotic motors in a switch from poleward flux to spindle elongation. Proc. Natl. Acad. Sci. USA. 2004;101:15938–15943. doi: 10.1073/pnas.0407044101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kashina AS, Baskin RJ, Cole DG, Wedaman KP, Saxton WM, Scholey JM. A bipolar kinesin. Nature. 1996;379:270–272. doi: 10.1038/379270a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kapitein LC, Peterman EJ, Kwok BH, Kim JH, Kapoor TM, Schmidt CF. The bipolar mitotic kinesin Eg5 moves on both microtubules that it crosslinks. Nature. 2005;435:114–118. doi: 10.1038/nature03503. [DOI] [PubMed] [Google Scholar]
- 5.Enos AP, Morris NR. Mutation of a gene that encodes a kinesin-like protein blocks nuclear division in A. nidulans. Cell. 1990;60:1019–1027. doi: 10.1016/0092-8674(90)90350-n. [DOI] [PubMed] [Google Scholar]
- 6.Mayer TU, Kapoor TM, Haggarty SJ, King RW, Schreiber SL, Mitchison TJ. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science. 1999;286:971–974. doi: 10.1126/science.286.5441.971. [DOI] [PubMed] [Google Scholar]
- 7.Bishop JD, Han Z, Schumacher JM. The Caenorhabditis elegans Aurora B kinase AIR-2 phosphorylates and is required for the localization of a BimC kinesin to meiotic and mitotic spindles. Mol. Biol. Cell. 2005;16:742–756. doi: 10.1091/mbc.E04-08-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grill SW, Gonczy P, Stelzer EH, Hyman AA. Polarity controls forces governing asymmetric spindle positioning in the Caenorhabditis elegans embryo. Nature. 2001;409:630–633. doi: 10.1038/35054572. [DOI] [PubMed] [Google Scholar]
- 9.Valentine MT, Fordyce PM, Krzysiak TC, Gilbert SP, Block SM. Individual dimers of the mitotic kinesin motor Eg5 step processively and support substantial loads in vitro. Nat. Cell Biol. 2006;8:470–476. doi: 10.1038/ncb1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crevel IM, Alonso MC, Cross RA. Monastrol stabilises an attached low-friction mode of Eg5. Curr. Biol. 2004;14:R411–R412. doi: 10.1016/j.cub.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 11.Tao L, Mogilner A, Civelekoglu-Scholey G, Wollman R, Evans J, Stahlberg H, Scholey JM. A homotetrameric kinesin-5, KLP61F, bundles microtubules and antagonizes Ncd in motility assays. Curr. Biol. 2006;16:2293–2302. doi: 10.1016/j.cub.2006.09.064. [DOI] [PubMed] [Google Scholar]
- 12.Colombo K, Grill SW, Kimple RJ, Willard FS, Siderovski DP, Gonczy P. Translation of polarity cues into asymmetric spindle positioning in Caenorhabditis elegans embryos. Science. 2003;300:1957–1961. doi: 10.1126/science.1084146. [DOI] [PubMed] [Google Scholar]
- 13.Gotta M, Dong Y, Peterson YK, Lanier SM, Ahringer J. Asymmetrically distributed C. elegans homologs of AGS3/PINS control spindle position in the early embryo. Curr. Biol. 2003;13:1029–1037. doi: 10.1016/s0960-9822(03)00371-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.