Abstract
Background
Echocardiograms routinely sample pulmonary regurgitation signals from which it is possible to measure end diastolic gradients; these correlate with pulmonary artery diastolic pressures.
Methods
We performed echocardiograms in 741 ambulatory adults with coronary artery disease who were recruited for the Heart and Soul Study. We compared indicators of cardiac status among individuals with normal (0–5.0 mm Hg) and elevated (>5.0 mm Hg) end diastolic pulmonary regurgitation (EDPR) gradients.
Results
Of the 481 participants with measurable EDPR gradients, 21% had elevated EDPR gradients (>5.0 mm Hg). EDPR gradients >5.0 mm Hg were associated with higher New York Heart Association functional class (P = .002), higher brain natriuretic peptide (P = .002), fewer metabolic equivalents achieved on treadmill testing (P < 0.001), and higher left ventricular mass (P < 0.001). The EDPR gradient >5.0 mm Hg had a sensitivity of 25% (95% confidence interval 20–30%) and a specificity of 86% (80–91%) for detecting at least one of the following: systolic dysfunction, diastolic dysfunction, or abnormal wall motion score. The EDPR gradient >5.0 mm HG was statistically equivalent to the tricuspid regurgitation (TR) gradient >30 mm Hg in terms of diagnostic value (area under the receiver operating characteristic curve equaled 0.58 for each test). The EDPR gradient increased the yield of pulmonary artery pressures from 61% (TR gradient alone) to 84% (P < .0001).
Conclusion
The EDPR gradient provides valuable information independent of the TR gradient in evaluating pulmonary artery pressures and cardiac dysfunction.
Doppler echocardiographic evaluation of blood velocity gives reliable estimates of pressure gradients across heart valves. In particular, pulmonary regurgitation (PR) gradients provide accurate estimates of pulmonary artery diastolic pressures.1–4 We used prospectively collected data from the Heart and Soul Study to establish a normal range of PR gradients and to determine the degree to which the end-diastolic PR (EDPR) gradient is effective as a marker of clinical and echocardiographic indicators of cardiac disease. We hypothesized that the EDPR gradient is an informative general marker of cardiac impairment that can be useful independently or in supplement with the tricuspid regurgitation (TR) gradient.
METHODS
The Heart and Soul Study is a prospective cohort study investigating the influence of psychosocial factors on cardiovascular events. We used administrative databases to identify outpatients with documented coronary artery disease at two Department of Veterans Affairs (VA) medical center databases (San Francisco and Palo Alto, Calif), one University-based medical center (University of California Medical Center–San Francisco), and the Community Health Network of San Francisco, Calif. Criteria for enrollment were: (1) history of myocardial infarction; (2) angiographic evidence of at least 50% stenosis by area in at least one coronary vessel; (3) evidence of exercise-induced ischemia by treadmill electrocardiogram or stress nuclear perfusion imaging; (4) history of coronary revascularization; or (5) clinical diagnosis of coronary disease as documented by an internist or cardiologist. Individuals were excluded if they deemed themselves unable to walk one block or if they were planning to move out of the local area within 3 years. In the initial protocol design, the EDPR gradient was designated as a parameter of intent and was collected prospectively. After the study closed, we initiated a formal evaluation of the EDPR gradient in our analysis of the 741 participants who were enrolled between July 2001 and December 2002. The institutional review board at each of the sites approved our protocol, and all participants provided written informed consent.
Each participant completed a detailed interview or questionnaire that included age, sex, race, frequency of angina, medical history, level of physical activity, current smoking, and level of alcohol consumption. Study personnel recorded all current medications and measured height, weight, and blood pressure. Brain natriuretic peptide (BNP) levels were drawn for patients enrolled between July and December 2001. We performed a symptom-limited, graded exercise treadmill test according to a standard Bruce protocol. Peak exercise capacity was defined as total number of metabolic equivalents achieved. A single cardiologist (N. B. S.), blinded to clinical and laboratory information, evaluated each stress echocardiogram and each comprehensive resting echocardiogram. A single technician (L. W.) made all sonographic measurements.
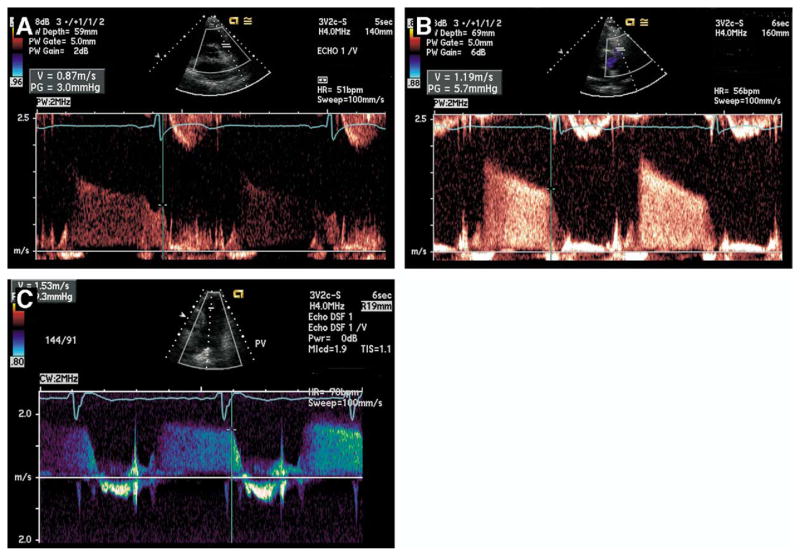
Echocardiographic studies were performed in the standard left lateral recumbent and supine positions with a commercially available ultrasound system with harmonic imaging (Acuson Sequoia, Siemens Corp, Mountain View, Calif). From the standard basal short-axis view, a spectral Doppler PR waveform was sought by color flow interrogation across the pulmonic valve. The velocity was measured at end diastole at termination of reverse flow after the “a” wave (Figure 1). The point of measurement corresponded with the first peak deflection of the QRS complex (usually at the R wave). In most cases, pulsed wave Doppler visualized an adequate waveform, but higher velocities (Figure 1, C) mandated occasional use of continuous wave Doppler.
Figure 1.
Measurement of end-diastolic pulmonary regurgitation (EDPR) velocity. On spectral Doppler signal from standard basal short-axis view, vertical line denotes termination of reverse flow across pulmonic valve at end diastole. Horizontal dash represents EDPR velocity. EDPR gradient is calculated by 4v2 where v equals EDPR velocity. A, EDPR is 3.0 mm Hg (pulse wave Doppler). Measurement is made at termination of “a” wave, corresponding with first peak deflection of QRS complex (in most cases R wave). EDPR gradient is 5.7 mm Hg (pulse wave Doppler) (B) and 9.3 mm Hg (continuous wave Doppler) (C).
Before digital data collection, the sonographer screened at least 10 diastolic cycles with the patient breathing quietly at rest. Although the value of the EDPR gradient changed with respiration, choosing the highest value out of several waveforms minimized the significance of beat-to-beat variability. The sonographer chose the highest EDPR velocity out of 10 cycles for measurement. We used the modified Bernoulli equation (ΔP = 4v2) to calculate EDPR pressure gradients from EDPR velocity. To determine interobserver variability of the EDPR gradient, we collected EDPR Doppler signals in 11 consecutive participants presenting to the VA hospital laboratory for echocardiography after completion of the study. Three observers independently measured EDPR signals, and the results were subjected to kappa analyses and calculation of correlation coefficients.
Patterns of diastolic dysfunction were based on mitral flow E/A ratios of peak velocities at early rapid filling (E) and late filling due to atrial contraction (A), mitral flow deceleration time (DT), and systolic or diastolic dominant pulmonary venous flow as modified from previous studies.5,6 A normal diastolic pattern was defined by E/A greater than 0.8, DT greater than 160 milliseconds, and systolic dominant pulmonary venous flow. An abnormal relaxation pattern was defined by E/A 0.8 or less, DT greater than 240 milliseconds, and systolic dominant flow. A pseudonormal pattern was defined by E/A greater than 1, DT greater than 160 milliseconds, and diastolic dominant flow. A restrictive pattern was defined by E/A greater than 1, DT less than 160 milliseconds, and diastolic dominant flow. We did not differentiate between reversible and irreversible restrictive patterns.
To evaluate and quantify ventricular wall motion we used the 16-segment model recommended by the American Society of Echocardiography.7 Based on wall motion (normal to aneurysmal), each ventricular segment was assigned a number from 1 to 5; the mean of all the segments equaled the wall-motion score (normal wall motion = 1.0).
We selected a patient subgroup with normal clinical and echocardiographic characteristics to define the cutoff for normal and elevated EDPR gradients. We defined the upper limit of the normal EDPR gradient as the value at the mean plus 2SD. We selected the normal subgroup with the criteria listed in Table 1. We defined any EDPR gradient greater than the mean plus 2SD as elevated.
Table 1.
Inclusion criteria for establishing the numeric value of the normal end-diastolic pulmonary regurgitation gradient
| Measurable EDPR jet |
| Normotension (blood pressure < 140/90 mm Hg) after 10-minute rest |
| Normal in sinus rhythm |
| Left ventricular ejection fraction > 55% |
| Wall-motion index = 1.0 (both resting and postexercise) |
| Left ventricular mass index < 90 g/m2 |
| Normal Doppler parameters for diastolic function for age (systolic dominant pulmonary venous flow, mitral E/A ratio of 0.8–1.5, and E wave deceleration time of 170–250 ms) |
| Exercise time > 6 minutes on Bruce protocol |
EDPR, end-diastolic pulmonary regurgitation; mitral E/A, peak mitral flow velocity of early rapid filling wave (E)/peak velocity of late filling wave due to atrial contraction (A).
We determined the association of an elevated EDPR gradient with clinical and echocardiographic markers of cardiovascular disease. Differences in characteristics between participants with normal and abnormal EDPR gradients were determined using Student t test for continuous variables and χ2 tests for dichotomous variables. We used logistic regression to evaluate the association between our independent variable (the EDPR gradient) and dichotomous outcome variables. For these analyses, we report odds ratios with 95% confidence intervals. Analyses were performed using software (Statistical Analysis, Version 8, SAS Institute Inc, Cary, NC).
We calculated the sensitivity and specificity of the EDPR gradient greater than 5.0 mm Hg for detecting at least one of the following: systolic dysfunction, diastolic dysfunction, or abnormal wall-motion score. We categorized participants as having systolic dysfunction if the left ventricular (LV) ejection fraction (EF) was less than 55%, and we categorized participants as having diastolic dysfunction if pulmonary vein flow was diastolic dominant or if the mitral E/A ratio was less than 0.8 or greater than 1.5. We also determined the sensitivity and specificity of the TR gradient greater than 30 mm Hg and of left atrial (LA) end-systolic volume index greater than 32, and we used area under the receiver operating characteristic curves to compare EDPR, TR, and LA end-systolic volume index in terms of diagnostic value.
RESULTS
EDPR gradients were measurable in 481 of the 741 participants (65%). TR gradients were measurable in 454 participants (61%). The combination of EDPR and TR gradients increased the yield of estimates of pulmonary artery pressures to 619 participants (84%) (P < .0001). The complete categories of measurable gradients were 316 (43%) with EDPR and TR gradients, 165 (22%) with EDPR gradients only, and 138 (19%) with TR gradients only. Of the individuals without measurable TR gradients, EDPR measurements were made in 57% (165 of 287).
The physiologically healthy reference population that met the criteria in Table 1 consisted of 30 individuals, and the mean EDPR gradient in that group was 2.28 ± 1.27 mm Hg. The mean plus 2SD in the reference population was 4.82 mm Hg. We, therefore, chose 5.0 mm Hg as the upper limit of normal. Only 2 of 30 participants in the normal sample had an EDPR gradient greater than 5.0 mm Hg.
Elevated EDPR gradients (>5.0 mm Hg) occurred in 99 (21%) of the 481 participants with measurable EDPR gradients. The subset of 165 participants without measurable TR gradients included 29 participants (18%) with elevated EDPR gradients. Interobserver variability calculations based on 11 participants with 3 observers showed complete agreement with kappa equal to 1.0 in determining if the EDPR gradient was normal or elevated. The correlation coefficient for continuous measurements among 3 observers was 0.98, indicating that measurements of the EDPR gradient are reproducible.
Baseline characteristics of age, sex, current smoking, and alcohol consumption were similar between the groups with normal and elevated EDPR gradients (Table 2). Compared with those who had EDPR gradients 5.0 mm Hg or less, individuals with EDPR gradients greater than 5.0 mm Hg were more likely to be of black race and to have diabetes mellitus. Participants with elevated EDPR gradients were also more likely to be taking diuretics and angiotensin system inhibitors. Participants with EDPR gradients greater than 5.0 mm Hg were less physically active, had worse New York Heart Association functional status, and achieved fewer metabolic equivalents on treadmill testing. Creatinine clearance was lower in the group with abnormal EDPR gradients. Among the 153 participants with BNP measurements, mean BNP was markedly more elevated among those with abnormal EDPR gradients.
Table 2.
Comparison of clinical characteristics
| EDPR gradient ≤ 5.0 mm Hg n = 382 | EDPR gradient > 5.0 mm Hg n = 99 | P value | |
|---|---|---|---|
| Age, y | 64.8 ± 11.5 | 66.5 ± 11.3 | .18 |
| Male sex | 77% | 78% | .84 |
| Race: | |||
| White | 59% | 50% | .12 |
| Black | 16% | 25% | .03 |
| Asian | 15% | 14% | .75 |
| Other | 10% | 11% | .80 |
| History of: (%) | |||
| Hypertension | 66% | 75% | .09 |
| Myocardial infarction | 49% | 54% | .43 |
| Stroke | 13% | 17% | .39 |
| Chronic obstructive pulmonary disease | 16% | 17% | .65 |
| Diabetes | 19% | 34% | .001 |
| Revascularization | 58% | 54% | .42 |
| Angina ≥ once a week* | 21% | 20% | .82 |
| NYHA symptoms class II, III, or IV | 56% | 73% | .002 |
| Minimal or no physical activity† | 34% | 46% | .03 |
| Current smoking | 21% | 20% | .82 |
| Regular alcohol consumption | 29% | 26% | .55 |
| Measurements: | |||
| Body mass index | 27.6 ± 5.0 | 27.8 ± 5.0 | .78 |
| Body surface area | 1.93 ± 0.22 | 1.93 ± 0.20 | .99 |
| Metabolic equivalents on treadmill | 8.3 ± 3.7 | 6.1 ± 2.9 | <.001 |
| Serum brain natriuretic peptide | 53.7 ± 92.8 | 132.7 ± 162.1 | .002 |
EDPR, End-diastolic pulmonary regurgitation; NYHA, New York Heart Association.
Presence of angina is defined by individual self-assessment of angina occurring at least once a week.
Physical activity was determined using a multiple-choice question, “Which of the following statements best describes how physically active you have been during the last month, that is, done activities such as 15–20 minutes of brisk walking, swimming, general conditioning, or recreational sports?” Participants who answered “not at all” or “a little active” (vs “fairly,” “quite,” “very,” or “extremely”) were considered as performing minimal or no physical activity.
An elevated EDPR gradient was associated with an odds ratio of 3.7 (95% confidence interval 2.2–6.1) for having a LV EF less than 55% and an odds ratio of 2.7 (1.6–4.7) for having diastolic dominant pulmonary vein flow. An elevated EDPR gradient was associated with an odds ratio of 0.4 (0.3–0.6) for having normal resting wall motion.
Participants with elevated EDPR gradients had higher TR gradients, higher right atrial (RA) pressures, and larger RA end-systolic volumes (Table 3). Elevated EDPR gradients were associated with decreased LV EF, increased resting wall motion score, and increased LV mass index. Proportions of individuals with markers of diastolic dysfunction, including mitral DT less than 160 milliseconds and diastolic dominant pulmonary vein flow, were higher with elevated EDPR gradients. There were more individuals with a restrictive pattern of diastolic dysfunction in the group with elevated EDPR gradients. There were no variables that had trends toward better function among individuals with EDPR gradients greater than 5.0 mm Hg.
Table 3.
Comparison of echocardiographic characteristics
| EDPR gradient ≤ 5.0 mm Hg n = 382 | EDPR gradient > 5.0 mm Hg n = 99 | P value | |
|---|---|---|---|
| EDPR gradient, mm Hg | 2.31 ± 1.35 | 7.42 ± 2.73 | <.001 |
| Tricuspid regurgitation gradient, mm Hg | 24.6 ± 7.5 | 30.5 ± 11.6 | <.001 |
| Mitral regurgitation, mild or worse | 18% | 28% | .03 |
| Right atrial pressure, mm Hg | 5.3 ± 1.4 | 6.1 ± 2.8 | <.001 |
| Left atrial end-systolic volume index | 31.0 ± 9.9 | 36.2 ± 11.4 | <.001 |
| Right atrial end-systolic volume index | 23.4 ± 8.8 | 27.6 ± 10.6 | <.001 |
| LV mass index | 91.1 ± 23.1 | 109.8 ± 30.4 | <.001 |
| LV end-diastolic volume index | 50.7 ± 18.0 | 56.4 ± 21.7 | .007 |
| LV end-systolic volume index | 19.9 ± 13.6 | 26.7 ± 17.2 | <.001 |
| LV outflow tract volume time integral | 0.22 ± 0.05 | 0.21 ± 0.06 | .05 |
| Resting LV ejection fraction | 0.63 ± 0.09 | 0.57 ± 0.11 | <.001 |
| Resting LV ejection fraction < 55% | 13% | 35% | <.001 |
| Postexercise wall-motion score = 1.0 | 69% | 49% | <.001 |
| Postexercise LV ejection fraction | 0.66 ± 0.11 | 0.59 ± 0.14 | <.001 |
| Diastolic dominant pulmonary vein flow | 11% | 25% | <.001 |
| Mitral E/A > 1.5 | 12% | 16% | .32 |
| DT < 160 ms | 5% | 12% | .007 |
| DT > 240 ms | 45% | 30% | .009 |
| Pulmonary vein systolic volume time integral | 0.17 ± 0.05 | 0.15 ± 0.06 | <.001 |
| Pulmonary vein diastolic volume time integral | 0.11 ± 0.04 | 0.11 ± 0.04 | .95 |
| Classification of diastolic function: | |||
| Normal | 224 (69%) | 43 (55%) | .02 |
| Impaired relaxation | 69 (21%) | 16 (20%) | .86 |
| Pseudonormal | 31 (9%) | 12 (15%) | .14 |
| Restrictive | 2 (1%) | 8 (10%) | <.001 |
DT, Deceleration time; EDPR, end-diastolic pulmonary regurgitation; LV, left ventricular; Mitral E/A, peak mitral flow velocity of early rapid filling wave (E)/peak velocity of late filling wave due to atrial contraction (A).
The EDPR gradient greater than 5.0 mm Hg had 25% sensitivity and 86% specificity for detecting at least one of the following: systolic dysfunction, diastolic dysfunction, or abnormal wall-motion score. By comparison, the TR gradient greater than 30 mm Hg had 20% sensitivity and 80% specificity, and LA end-systolic volume index greater than 32 had 48% sensitivity and 63% specificity. The positive predictive value of the EDPR gradient greater than 5.0 mm Hg for detecting abnormal cardiac function was 76%. The area under the receiver operating characteristic curve was the same for the EDPR gradient (0.58), TR gradient (0.58), and LA end-systolic volume index (0.59), indicating that all 3 tests were equivalent in detecting systolic dysfunction, diastolic dysfunction, or abnormal wall motion.
DISCUSSION
In a cohort of stable ambulatory individuals from the Heart and Soul Study, we found that an EDPR gradient greater than 5.0 mm Hg is a stand-alone Doppler parameter that strongly suggests cardiac dysfunction. In particular, we found that an elevated EDPR gradient correlates with decreased functional status, elevated serum BNP, elevated LV mass index, systolic dysfunction, and diastolic dysfunction.
Echocardiographic variables such as LV EF, right ventricular EF,8,9 TR,10 and LA volume11 have been established as markers of cardiovascular disease. To our knowledge, the significance of the EDPR gradient has not been established in a similar manner. The current study indicates that the EDPR gradient has statistically equivalent diagnostic value as the TR gradient and as LA end-systolic volume index in the detection of systolic dysfunction, diastolic dysfunction, or abnormal wall motion. We believe that the EDPR gradient should be useful in clinical practice as a general marker of cardiac status.
In addition to evaluating overall cardiac function, the EDPR gradient may be useful in evaluating pulmonary artery pressures. In most echocardiography laboratories, the TR velocity routinely estimates pulmonary artery systolic pressure.12,13 However, TR jets are not always present, and several studies suggest that measurable PR may be at least as common.14–19 In the current study, the EDPR gradient was able to identify normal and elevated pulmonary artery pressures in individuals without a measurable TR gradient.
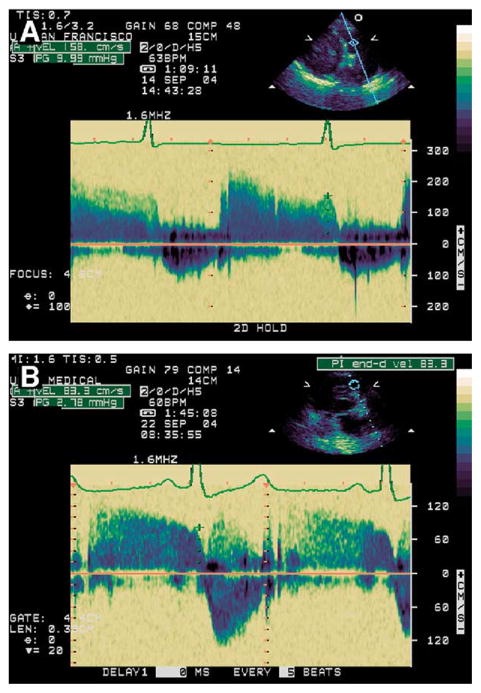
In our clinical practice, we routinely look at the EDPR gradient early in the transthoracic echocardiogram. An EDPR gradient greater than 5.0 mm Hg specifically guides our attention to a patient likely to have cardiac dysfunction. In Figure 2, we provide a case illustration of the use of the EDPR gradient. A 76-year-old woman presented with dizziness and hypoxia. The echocardiogram showed normal systolic function, LV hypertrophy, and the diastolic filling pattern of impaired relaxation. The mitral inflow E/A ratio was 0.8, the TR gradient plus RA pressure was 30 mm Hg, and the pulmonary venous signal was poor. The EDPR gradient was elevated at 10 mm Hg, providing evidence of elevated pulmonary artery diastolic pressure.
Figure 2.
Case illustrates elevated end-diastolic pulmonary regurgitation (EDPR) gradient of 10 mm Hg before treatment for heart failure (A) and improvement 2 weeks later to 2.8 mm Hg (B). Left ventricular systolic function was normal in both studies, and parameters of diastolic function were consistent with impaired relaxation. Peak mitral flow velocity of early rapid filling wave (E)/peak velocity of late filling wave due to atrial contraction (A) (mitral E/A ratio) was 0.8 before treatment and 0.3 after treatment, and pulmonary venous signal was poor in both studies. EDPR gradient provided only definitive echocardiographic evidence of hemodynamic improvement.
The patient received medical treatment for heart failure, and 2 weeks later her symptoms improved. Interpretation of the follow-up echocardiogram showed continued impaired relaxation, and the E/A ratio was 0.3. The pulmonary venous signal and the TR velocity could not be visualized. However, a measurable EDPR gradient was obtained and had decreased to a normal value (2.8 mm Hg), providing the only definitive echocardiographic evidence of hemodynamic improvement.
Several limitations of our study must be considered. First, the study population was predominantly elderly men, and all participants had known coronary artery disease. Thus, the implications of high EDPR gradients in women, young individuals, or in individuals free of coronary disease were not addressed. Second, for simplification, we did not add RA pressure to our measurements of EDPR gradients. As is typical of an ambulatory population, most (92%) of the individuals in the current study had a normal RA pressure of 5 mm Hg or less. Third, our yield of a measurable EDPR gradient (65%) was less than the 67% to 93% reported in other studies to have detectable PR.14,16–19 Contrast enhancement Doppler echocardiography could presumably be expected to increase the yield of measurable EDPR gradients.20
To promote ease of clinical use, we did not adjust EDPR gradients for body mass index, which is known to have an influence on other echocardiographic variables.21 The current investigation is limited to cardiac testing at one point in time. Extended observation of this population is planned to determine the prognostic value of an elevated EDPR gradient on patient outcome and survival.
Conclusion
The EDPR gradient, found routinely on the Doppler echocardiographic examination, has high specificity and statistical equivalence with the TR gradient in evaluating the presence of at least one of the following: systolic dysfunction, diastolic dysfunction, or abnormal wall-motion score. An EDPR gradient greater than 5.0 mm Hg is associated with decreased functional capacity, increased serum BNP, increased LV mass index, and echocardiographic features of both systolic and diastolic dysfunction. The EDPR gradient, especially useful when the TR gradient is not available, provides a simple means to evaluate pulmonary artery pressures and cardiac dysfunction.
Acknowledgments
Supported by the Department of Veterans Affairs (VA) (Epidemiology Merit Review Program), the Robert Wood Johnson Foundation (Generalist Physician Faculty Scholars Program), the American Federation for Aging Research (Paul Beeson Faculty Scholars in Aging Research Program), the Ischemia Research and Education Foundation, and an equipment loan from Siemens Corp (Mountain View, Calif). Dr Whooley is supported by an Advanced Research Career Development Award from the VA Health Services Research and Development Service.
References
- 1.Ge Z, Zhang Y, Ji X, Fan D, Duran CM. Pulmonary artery diastolic pressure: a simultaneous Doppler echocardiography and catheterization study. Clin Cardiol. 1992;15:818–24. doi: 10.1002/clc.4960151106. [DOI] [PubMed] [Google Scholar]
- 2.Lee RT, Lord CP, Plappert T, Sutton MS. Prospective Doppler echocardiographic evaluation of pulmonary artery diastolic pressure in the medical intensive care unit. Am J Cardiol. 1989;64:1366–70. doi: 10.1016/0002-9149(89)90583-3. [DOI] [PubMed] [Google Scholar]
- 3.Masuyama T, Kodama K, Kitabatake A, Sato H, Nanto S, Inoue M. Continuous-wave Doppler echocardiographic detection of pulmonary regurgitation and its application to noninvasive estimation of pulmonary artery pressure. Circulation. 1986;74:484–92. doi: 10.1161/01.cir.74.3.484. [DOI] [PubMed] [Google Scholar]
- 4.Jiang L, Shen XD, He J. Quantitative assessment of pulmonary arterial diastolic and mean pressure using continuous wave Doppler [in Chinese] Zhonghua Xin Xue Guan Bing Za Zhi. 1989;17:3–6. 61. [PubMed] [Google Scholar]
- 5.Appleton CP, Jensen JL, Hatle LK, Oh JK. Doppler evaluation of left and right ventricular diastolic function: a technical guide for obtaining optimal flow velocity recordings. J Am Soc Echocardiogr. 1997;10:271–92. doi: 10.1016/s0894-7317(97)70063-4. [DOI] [PubMed] [Google Scholar]
- 6.Yamada H, Goh PP, Sun JP, et al. Prevalence of left ventricular diastolic dysfunction by Doppler echocardiography: clinical application of the Canadian consensus guidelines. J Am Soc Echocardiogr. 2002;15:1238–44. doi: 10.1067/mje.2002.124877. [DOI] [PubMed] [Google Scholar]
- 7.Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography: American Society of Echocardiography committee on standards, subcommittee on quantitation of two-dimensional echocardiograms. J Am Soc Echocardiogr. 1989;2:358–67. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 8.Di Salvo TG, Mathier M, Semigran MJ, Dec GW. Preserved right ventricular ejection fraction predicts exercise capacity and survival in advanced heart failure. J Am Coll Cardiol. 1995;25:1143–53. doi: 10.1016/0735-1097(94)00511-n. [DOI] [PubMed] [Google Scholar]
- 9.Polak JF, Holman BL, Wynne J, Colucci WS. Right ventricular ejection fraction: an indicator of increased mortality in patients with congestive heart failure associated with coronary artery disease. J Am Coll Cardiol. 1983;2:217–24. doi: 10.1016/s0735-1097(83)80156-9. [DOI] [PubMed] [Google Scholar]
- 10.Hung J, Koelling T, Semigran MJ, Dec GW, Levine RA, Di Salvo TG. Usefulness of echocardiographic determined tricuspid regurgitation in predicting event-free survival in severe heart failure secondary to idiopathic-dilated cardiomyopathy or to ischemic cardiomyopathy. Am J Cardiol. 1998;82:1301–3. A10. doi: 10.1016/s0002-9149(98)00624-9. [DOI] [PubMed] [Google Scholar]
- 11.Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol. 2002;90:1284–9. doi: 10.1016/s0002-9149(02)02864-3. [DOI] [PubMed] [Google Scholar]
- 12.Yock PG, Popp RL. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation. 1984;70:657–62. doi: 10.1161/01.cir.70.4.657. [DOI] [PubMed] [Google Scholar]
- 13.Currie PJ, Seward JB, Chan KL, et al. Continuous wave Doppler determination of right ventricular pressure: a simultaneous Doppler-catheterization study in 127 patients. J Am Coll Cardiol. 1985;6:750–6. doi: 10.1016/s0735-1097(85)80477-0. [DOI] [PubMed] [Google Scholar]
- 14.Borgeson DD, Seward JB, Miller FA, Jr, Oh JK, Tajik AJ. Frequency of Doppler measurable pulmonary artery pressures. J Am Soc Echocardiogr. 1996;9:832–7. doi: 10.1016/s0894-7317(96)90475-7. [DOI] [PubMed] [Google Scholar]
- 15.Macchi C, Orlandini SZ, Orlandini GE. An anatomical study of the healthy human heart by echocardiography with special reference to physiological valvular regurgitation. Ann Anat. 1994;176:81–6. doi: 10.1016/s0940-9602(11)80421-8. [DOI] [PubMed] [Google Scholar]
- 16.Maciel BC, Simpson IA, Valdes-Cruz LM, et al. Color flow Doppler mapping studies of “physiologic” pulmonary and tricuspid regurgitation: evidence for true regurgitation as opposed to a valve closing volume. J Am Soc Echocardiogr. 1991;4:589–97. doi: 10.1016/s0894-7317(14)80218-6. [DOI] [PubMed] [Google Scholar]
- 17.Kral J, Hradec J, Petrasek J. Valvular regurgitations in healthy young people. Cor Vasa. 1989;31:485–94. [PubMed] [Google Scholar]
- 18.Yoshida K, Yoshikawa J, Shakudo M, et al. Color Doppler evaluation of valvular regurgitation in normal subjects. Circulation. 1988;78:840–7. doi: 10.1161/01.cir.78.4.840. [DOI] [PubMed] [Google Scholar]
- 19.Takao S, Miyatake K, Izumi S, et al. Clinical implications of pulmonary regurgitation in healthy individuals: detection by cross sectional pulsed Doppler echocardiography. Br Heart J. 1988;59:542–50. doi: 10.1136/hrt.59.5.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dini FL, Traversi E, Franchini M, Micheli G, Cobelli F, Pozzoli M. Contrast-enhanced Doppler hemodynamics for noninvasive assessment of patients with chronic heart failure and left ventricular systolic dysfunction. J Am Soc Echocardiogr. 2003;16:124–31. doi: 10.1067/mje.2003.8. [DOI] [PubMed] [Google Scholar]
- 21.McQuillan BM, Picard MH, Leavitt M, Weyman AE. Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects. Circulation. 2001;104:2797–802. doi: 10.1161/hc4801.100076. [DOI] [PubMed] [Google Scholar]