Summary
We have identified RORγt, a novel, thymus-specific isoform of the orphan nuclear receptor RORγ that is expressed predominantly in CD4+ CD8+ double-positive thymocytes. Ectopic expression of RORγt protects T cell hybridomas from activation-induced cell death by inhibiting the upregulation of Fas ligand. Following hybridoma stimulation, RORγt also inhibits IL-2 production but does not affect the induction of Nur-77 and Egr-3 nor the upregulation of CD69. Both the ligand-binding and DNA-binding domains of RORγt are required for this effect. We propose that the role of RORγt expression in immature thymocytes is to inhibit Fas ligand expression and cytokine secretion following engagement of their TCR during positive or negative selection.
Introduction
Only a small fraction of thymocytes meet certain selection criteria to mature and become peripheral CD4+ or CD8+ single-positive (SP) lymphocytes. Immature double-positive (DP) thymocytes expressing a TCR with intermediate affinity for self-MHC-peptide complexes are positively selected and complete maturation. Immature DP thymocytes that express TCR with too-low affinity undergo programmed cell death, referred to as “death by neglect,” while thymocytes expressing TCR with too-high affinity for self-MHC-peptide ligand are also deleted by programmed cell death, which is referred to as negative selection (Kisielow and von Boehmer, 1995; Jameson and Bevan, 1998). Although the mechanism by which signaling through a high-affinity TCR results in negative selection of thymocytes is unclear, certain pairs of cell surface molecules including CD40/gp39 and CD30/CD30 ligand have been implicated in thymocyte negative selection (Foy et al., 1995; Amakawa et al., 1996). With one exception (Castro et al., 1996), Fas/Fas ligand interaction has not been shown to be involved in immature DP negative selection in vivo. A recent study demonstrates that Fas-mediated clonal deletion of self-reactive T cells in the thymus occurs at a relatively late stage of thymocyte development at the “semimature” SP stage and is dependent on the dose of the antigen (Kishimoto et al., 1998). The interaction of Fas and Fas ligand also plays an important role in TCR-induced mature T cell apoptosis in the lymphoid periphery (Zheng et al., 1995; Sytwu et al., 1996). Defects in this pathway result in autoimmune diseases that are best exemplified by the lymphoproliferative and autoimmune phenotype in lpr and gld mice and autoimmune lymphoproliferative syndrome (ALPS) in humans (Nagata and Goldstein, 1995; Puck and Sneller, 1997).
The molecular mechanism of TCR-mediated cell death has been deduced mainly from studies on T cell hybridomas. TCR-mediated activation of T cell hybridomas leads to IL-2 production and upregulation of Fas and Fas ligand. The engagement of Fas by Fas ligand initiates the death program (Alderson et al., 1995; Brunner et al., 1995; Dhein et al., 1995; Ju et al., 1995). Several genes have been identified as regulators of Fas/Fas ligand death pathway such as c-myc (Hueber et al., 1997), TDAG51 (Park et al., 1996), ALG-2, ALG-3 (Vito et al., 1996), GILZ (D'Adamio et al., 1997), and Toso (Hitoshi et al., 1998).
The nuclear hormone receptor superfamily is composed of ligand-regulated transcription factors and orphan receptors, for which ligands are not identified or may not exist (Beato et al., 1995; Mangelsdorf and Evans, 1995; Laudet, 1997). These receptors perform extremely diverse functions in development, reproduction, and homeostasis by regulating cell growth, differentiation, and apoptosis (Kastner et al., 1995). Various hormones and their receptors have been shown to modulate a broad spectrum of immunological processes. Retinoids are important cofactors in T cell activation (Garbe et al., 1992). Vitamin D3 inhibits proliferation and immunoglobulin production in B cells (Provvedini et al., 1984). Both retinoic acid and glucocorticoids antagonize TCR-mediated cell death by inhibiting activation-induced Fas ligand upregulation (Yang et al., 1995). Nur-77, an orphan nuclear receptor, is required for TCR-induced apoptosis of T cell hybridomas (Liu et al., 1994; Woronicz et al., 1994). In transgenic thymocytes that overexpress Nur-77, Fas ligand mRNA expression was strongly upregulated (Weih et al., 1996), whereas a dominant-negative form of Nur-77 expressed in the thymus interferes with thymocyte negative selection, presumably by inhibiting the action of Nur-77 and the related orphan receptor Nor-1 (Calnan et al., 1995; Zhou et al., 1996). These studies suggest that a complex network involving multiple regulators controls the Fas/Fas ligand death program.
To further explore the molecular mechanism that underlies TCR-mediated cell death, we have employed an expression cloning strategy to identify genes that regulate this pathway. Here, we report the isolation of RORγt, a novel isoform of the orphan nuclear receptor RORγ (retinoic acid receptor-related orphan receptor) (Hirose et al., 1994; Ortiz et al., 1995), that is expressed primarily in immature DP thymocytes. Expression of RORγt protects T cell hybridomas from activation-induced cell death by inhibiting the upregulation of Fas ligand. RORγt also inhibits IL-2 production but does not affect the induction of Nur-77 and Egr-3 nor the upregulation of CD69 upon T cell activation. We propose that RORγt expression in DP immature thymocytes inhibits Fas ligand expression and cytokine secretion following engagement of their TCR during positive or negative selection.
Results
Cloning of RORγt
We used an expression cloning strategy to identify genes that are involved in TCR/CD3-mediated apoptosis. DO11.10 T cell hybridoma cells were infected with a thymocyte cDNA library cloned in a retroviral vector and selected for growth in the presence of phorbol myristate acetate (PMA) plus ionomycin, which induce the T cell hybridoma to undergo activation-induced apoptosis by direct activation of protein kinase C and elevation of Ca++. cDNA inserts from resistant clones were recloned and transduced into DO11.10 cells to confirm their anti-apoptotic effects. One 2.0 kb cDNA insert that conferred resistance to PMA plus ionomycin and to anti-CD3-induced cell death in DO11.10 cells was further investigated in this work.
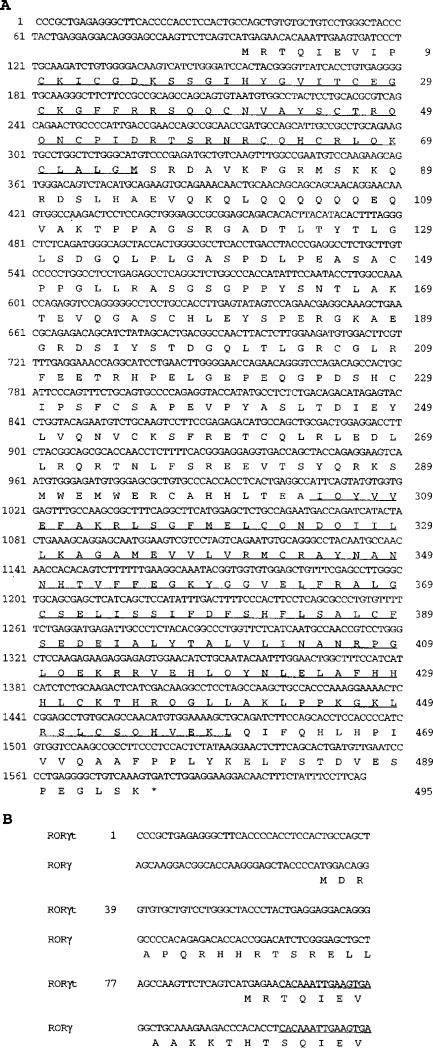
Sequence analysis of this cDNA revealed that it encodes an open reading frame of 495 amino acids (Figure 1A). The nucleotide sequence from nt 101 through the 3′ untranslated region is identical to RORγ/TOR, an orphan nuclear receptor with unknown function that was cloned independently from skeletal muscle and EL4 T cell libraries (Hirose et al., 1994; Ortiz et al., 1995; Medvedev et al., 1996). The matched sequence corresponds to exon 3 to exon 11 of RORγ (Medvedev et al., 1997). It has the same DNA-binding domain (DBD) and ligand-binding domain (LBD) as RORγ. The novel cDNA sequence differs from that of RORγ only in the first 100 nucleotides, which translate into distinct N-terminal amino acid sequences (Figure 1B). Therefore, this cDNA insert from the thymocyte library represents a new isoform of RORγ. The putative initiation codon begins at nt 93 and is surrounded by a standard Kozak consensus sequence. An in-frame termination codon lies 27 nt upstream of the putative initiation codon (Figure 1). Since it is primarily expressed in the thymus (see below), this novel isoform of RORγ was designated RORγt.
Figure 1. RORγt Is a Novel Isoform of RORγ.
(A) cDNA sequence of RORγt and its predicted amino acid sequence. The cDNA sequence of RORγt was derived from sequencing the rescued cDNA insert from DO11.10 clones that were resistant to PMA plus ionomycin-induced cell death. Several independent RT-PCR from thymocyte RNA yielded the same sequence. Sequences for the DBD (aa 10–75) and LBD (aa 305–460) are underlined. The 3′ untranslated region is identical to that of RORγ (Medvedev et al., 1997) and is not included.
(B) Sequence comparison of RORγt and RORγ. Aligned are the first 114 nt and the predicted amino acid sequence for RORγt and RORγ. They share the same sequence beginning with the underlined nucleotides through the 3′ end of each cDNA.
Expression of RORγt
Previous studies demonstrated that RORγ is expressed in a variety of tissues, including thymus and skeletal muscle (Ortiz et al., 1995; Medvedev et al., 1996). However, these studies did not discriminate the expression of RORγt and RORγ. To investigate the tissue distribution of the two isoforms, we designed primers that are common to both or specific for one of the isoforms and examined their mRNA expression by reverse transcriptase polymerase chain reaction (RT-PCR) (Figure 2A). The RT-PCR products were then blotted and probed with a full-length RORγt cDNA.
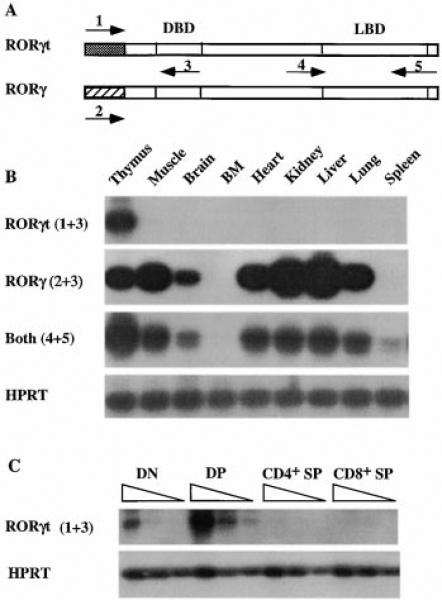
Figure 2. Differential Expression of RORγt and RORγ.
(A) Schematic representation of RORγt and RORγ cDNAs and the locations of the primers used in (B) and (C). The shaded region represents the distinct nucleotide sequences. Specific primers are 1 and 2. Common primers are 3, 4, and 5. Diagram is not to scale.
(B) Expression of RORγt and RORγ. RT-PCR products of different tissues were blotted and probed with a full-length RORγt cDNA. HPRT serves as an internal control. Numbers in parentheses indicate the primers shown in (A) used in the PCR.
(C) Expression of RORγt in thymocyte subpopulations. RT reactions of different thymocyte subpopulations were serially diluted at 1:3 and subjected to PCR with primers shown in (A). PCR products were blotted and probed as in (B). HPRT RT-PCR was performed from the same RT samples for cDNA template quantity control.
Among all the tissues examined, the RORγt isoform was detected solely in the thymus (Figure 2B). In contrast, RORγ was detected in the thymus, muscle, brain, heart, kidney, liver, and lung with strong signals in muscle, kidney, and liver. Interestingly, neither isoform was found in the spleen or bone marrow, indicating that mature T cells, as well as B cells at different stages of their development, do not express this nuclear receptor. Consistent with the expression pattern of RORγt and RORγ, a primer set common for both isoforms amplified signals from the above tissues shown to be positive for either isoform (Figure 2B). A weak signal was also detected in the spleen by the common primer set. This signal may represent a third isoform of RORγ (Figure 2B). The expression of RORγt in thymocyte subpopulations was further examined in fluorescence-activated cell sorting (FACS)-sorted immature DN and DP and mature CD4+ and CD8+ SP thymocytes. RORγt is predominantly expressed in immature DP thymocytes, with a low expression level in DN thymocytes and no detectable expression in either CD4+ or CD8+ SP thymocytes under these conditions (Figure 2C). In addition, RORγt is not expressed by the T cell hybridomas DO11.10 and KMIs-8.3.5 and is not induced upon activation of these cells either by anti-CD3 MAb or PMA plus ionomycin treatment (data not shown). These data indicate that the expression of RORγt is tightly regulated in the thymus and suggest a role for RORγt in thymocyte development.
RORγt Protects Hybridomas from TCR/CD3-Mediated Cell Death
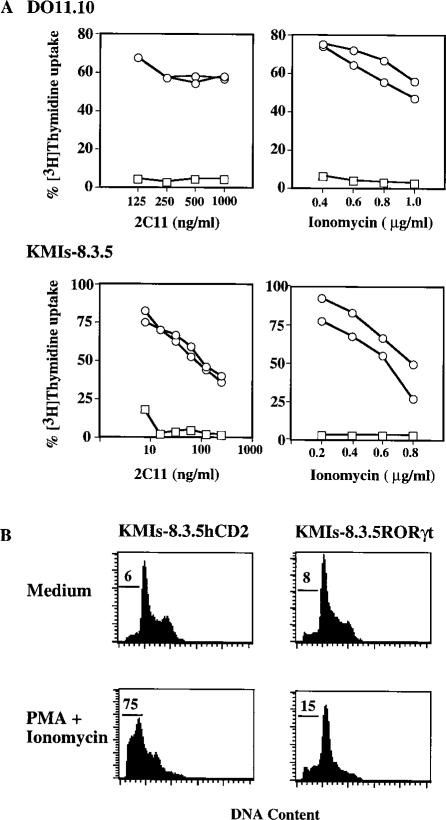
To study the function of RORγt, full-length RORγt cDNA was transduced into hybridoma cells using a pMI retrovirus vector. The pMI vector contains an IRES-hCD2 reporter construct downstream of the cDNA insert. cDNA cloned into this vector is transcribed into bicistronic mRNA that concomitantly directs translation of the insert and hCD2. Retrovirus-infected, transgene-positive polyclonal or monoclonal cell lines were established by either FACS or multiple rounds of panning for hCD2 positive cells. A pMX-hCD2 construct was used as a control vector and hCD2 positive hybridoma cells were isolated as control cells. Apoptosis induced by anti-CD3 MAb or PMA plus ionomycin was measured by comparing [3H]Thymidine uptake of these cells in the absence or presence of these stimuli (Figure 3A). The [3H]Thymidine incorporation by these cells closely correlated with their cellular viability measured by either propidium iodide uptake (Figure 3B) or trypan blue exclusion, which revealed that the viability for DO11.10hCD2 and DO11.10 RORγt are 1%–2% and 85%–95%, respectively, after 16–24 hr treatment with anti-CD3 MAb.
Figure 3. Expression of RORγt Protects T Cell Hybridomas from Activation-Induced Cell Death.
(A) Vector control cells and DO11.10 or KMIs-8.3.5 clones expressing RORγt were cultured in either 2C11-coated plates or in PMA (10 ng/ml) plus various amounts of ionomycin and measured for their [3H]Thymidine incorporation. The [3H]Thymidine uptake of individual cell lines cultured in medium alone is calculated as 100%. Shown are the results from two individual hybridoma clones expressing RORγt (circles) or control cells (squares).
(B) KMIs-8.3.5 vector control cells and a clone expressing RORγt were cultured in PMA (10 ng/ml) plus ionomycin (0.2 μg/ml) for 16 hr. Apoptotic cells with subdiploid DNA content were determined by propidium iodide uptake, and the percentage is indicated.
As shown in Figure 3A, DO11.10 cells expressing RORγt were protected from anti-CD3-induced cell death. In contrast, control cells were readily induced to undergo apoptosis. This anti-apoptotic effect by RORγt is not due to impaired TCR-CD3-mediated stimulation, since these DO11.10RORγt clones express comparable levels of CD3 on their surface relative to control cells (data not shown). Furthermore, DO11.10RORγt clones were also refractory to apoptosis induced by PMA plus ionomycin, which bypass the TCR (Figure 3A). The anti-apoptotic effect of RORγt was further tested on another T cell hybridoma, KMIs-8.3.5 (Park et al., 1996). Similarly to DO11.10RORγt, KMIs-8.3.5RORγt cells were protected from treatment by either anti-CD3 MAb or PMA plus ionomycin, whereas control KMIs-8.3.5hCD2 cells succumbed to cell death with almost no [3H]Thymidine uptake (Figure 3A). These data indicate that RORγt protected T cell hybridomas from activation-induced apoptosis.
Both the DBD and LBD Are Required for RORγt to Protect Hybridomas from Activation-Induced Cell Death
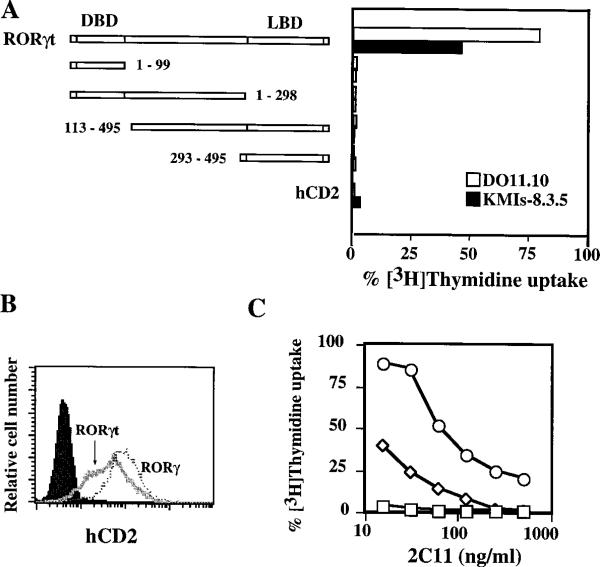
To map the regions of RORγt that are required for the anti-apoptotic effect, we made four constructs that consist of either the DBD or the LBD of RORγt and transduced them into both DO11.10 and KMIs-8.3.5 cells using the pMI vector (Figure 4A). None of these four deletion mutants of RORγt displayed an anti-apoptotic effect against anti-CD3 stimulation in either cell line (Figure 4A). These results indicate that both DBD and LBD are required for RORγt to protect cells from activation-induced cell death. One caveat to the interpretation of these data is that the protein expression levels of these constructs have not been confirmed due to the lack of an antibody to RORγt.
Figure 4. Protection of T Hybridomas from Activation-Induced Apopotosis by RORγt Deletion Mutants or RORγ.
(A) Both the DBD and the LBD of RORγt are required to protect T cell hybridomas from activation-induced cell death. DO11.10 or KMIs-8.3.5 cells expressing full-length or truncated RORγt or hCD2 were cultured in 2C11-coated plates (125 ng/ml) and tested for their [3H]Thymidine incorporation. Incorporation of cells cultured in medium alone is calculated as 100%. Numbers indicate the amino acids contained in each RORγt deletion mutant. An initiation methionine was added to RORγt deletion mutant 113–495.
(B) FACS analysis of hCD2 expression on KMIs-8.3.5 cells expressing RORγ or RORγt. Polyclonal KMIs-8.3.5 cell lines expressing RORγ or RORγt were generated by panning on anti-hCD2 MAb-coated plates, and adherent cells were stained with a FITC-anti-hCD2 as indicated. The profile at the far left represents parental KMIs-8.3.5 cells stained with anti-hCD2 MAb.
(C) Comparison of the anti-apopototic effects by RORγ and RORγt. KMIs-8.3.5 cells expressing RORγ (diamonds), RORγt (circles), or control cells (squares) shown in (B) were cultured in 2C11-coated plates and tested as described in (A).
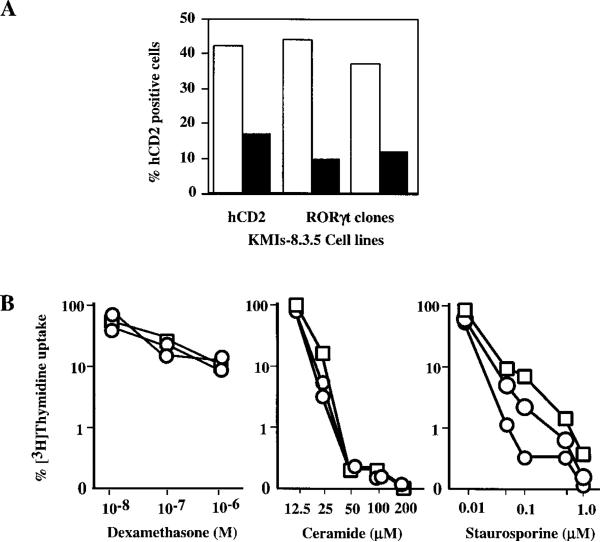
We further examined whether RORγ also protects T cell hybridoma from activation-induced apoptosis. KMIs-8.3.5 polyclonal cell lines expressing either RORγt or RORγ were stimulated with anti-CD3 MAb or PMA plus ionomycin. Even though RORγ was expressed at a higher level in the hybridoma cells than RORγt as assessed by the cell surface expression of the reporter hCD2 (Figure 4B), the protection against apoptosis mediated by RORγ was much less effective than that by RORγt when these cells were stimulated with either anti-CD3 (Figure 4C) or PMA plus ionomycin (data not shown). These results, together with their differential expression pattern, suggest that RORγ and RORγt perform distinct functions.
RORγt Negatively Regulates Fas Ligand Expression and IL-2 Production
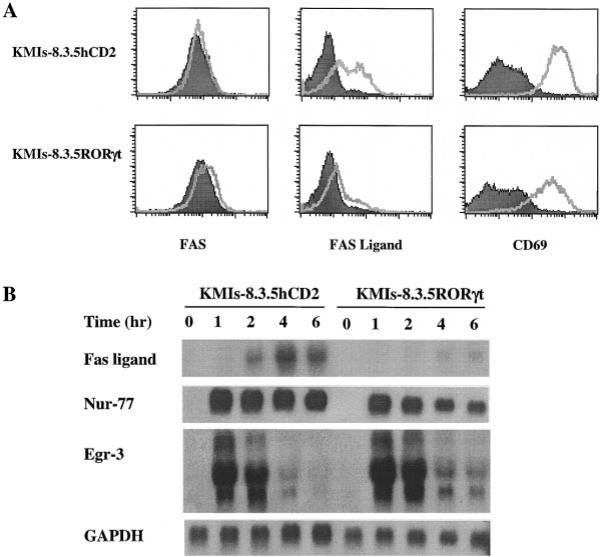
To explore the mechanism by which RORγt protects T cell hybridomas from activation-induced apoptosis, we first examined Fas and Fas ligand expression following activation. KMIs-8.3.5 cells were stimulated with anti-CD3 MAb for 4–5 hr and the surface expression of Fas and Fas ligand were determined by FACS analysis. Fas ligand was strongly induced and Fas was slightly upregulated in control KMIs-8.3.5hCD2 cells. In contrast, Fas ligand induction was dramatically inhibited in cells expressing RORγt (Figure 5A). The lack of Fas ligand induction was not due to insufficient stimulation and/or activation since the upregulation of the T cell activation marker CD69 was not affected by RORγt (Figure 5A). Furthermore, the upregulation of other surface molecules such as CD44 and the downregulation of CD62L upon TCR stimulation were similar in both KMIs-8.3.5hCD2 and KMIs-8.3.5RORγt cells (data not shown). To determine at which level RORγt negatively regulates Fas ligand expression, Northern blot analyses were performed. Following TCR stimulation, the expression of Fas ligand mRNA was induced as early as 2 hr and peaked at 4 hr in control KMIs-8.3.5hCD2 cells. In contrast, the induction of Fas ligand mRNA was strongly inhibited in KMIs-8.3.5RORγt cells, indicating that RORγt regulates Fas ligand expression at the transcriptional level (Figure 5B).
Figure 5. RORγt Inhibits Activation-Induced Fas Ligand Upregulation in KMIs-8.3.5 Cells.
(A) FACS analysis of cell surface expression of Fas, Fas ligand, and CD69 in KMIs-8.3.5hCD2 control or KMIs-8.3.5RORγt cells after TCR-mediated activation. KMI-8.3.5 cells expressing RORγt and control cells were activated on 2C11-coated plates for 4.5 hr and the cell surface phenotype was examined by FACS (filled, before stimulation; unfilled, after stimulation). A similar pattern was observed when PMA (10 ng/ml) and ionomycin (0.2 μg/ml) were used as stimuli (data not shown).
(B) Northern blot analysis of RNA expression of Fas ligand, Nur-77, and Egr-3 in KMIs-8.3.5hCD2 control, or KMIs-8.3.5RORγt cells after TCR-mediated activation. Cells were activated in 2C11-coated plates (125 ng/ml) for the indicated time, harvested, and extracted for total RNA. 15 μg total RNA was used for each sample. GAPDH serves as a loading control.
Previous experiments showed that the orphan nuclear receptor Nur-77 positively regulates Fas ligand expression (Weih et al., 1996). To test whether the inhibition of Fas ligand induction by RORγt is due to an effect on Nur-77 expression, the same set of RNAs from anti-CD3-stimulated KMIs-8.3.5hCD2 and KMIs-8.3.5RORγt were probed with a full-length Nur-77 cDNA. Nur-77 was induced rapidly and remained strongly positive for 6 hr in both types of cells (Figure 5B). This result indicates that RORγt does not negatively regulate Nur-77 transcription. A recent study demonstrated that Egr-3, an early response gene in cell activation, binds to the Fas ligand promoter and ectopic expression of Egr-3 activates Fas ligand expression (Mittelstadt and Ashwell, 1998). Thus, we further evaluated whether expression of RORγt affects Egr-3 transcription. As shown in Figure 5B, Egr-3 was induced to the same level in KMIs-8.3.5RORγt cells as in KMIs-8.3.5hCD2 control cells.
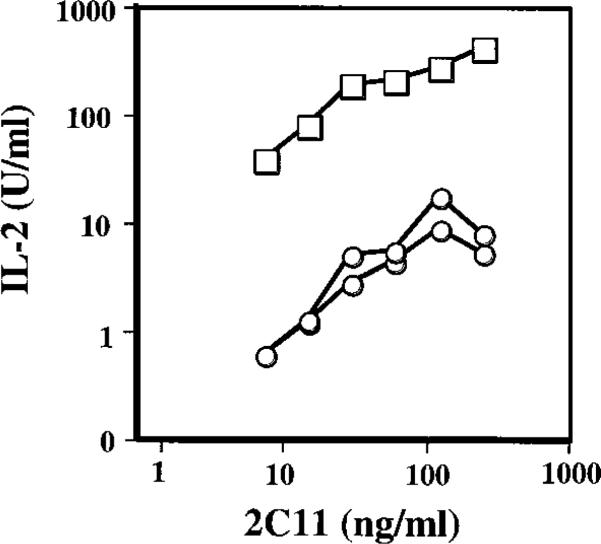
One hallmark of T cell hybridoma activation is IL-2 production. To examine the effect of RORγt on IL-2 production, cells were stimulated with various concentrations of anti-CD3 MAb for 20 hr and the culture supernatant assayed for IL-2. As expected, a significant amount of IL-2 was detected from KMIs-8.3.5hCD2 cells upon TCR stimulation, whereas only very low amounts of IL-2 were produced by KMIs-8.3.5RORγt cells (Figure 6). This inhibition of IL-2 production by RORγt could not be overcome by stimulation with PMA plus ionomycin (data not shown). Taken together, these data demonstrate that RORγt negatively regulates some, but not all, aspects of the activation program in T cell hybridomas.
Figure 6. Expression of RORγt Inhibits IL-2 Production by T Cell Hybridomas.
KMIs-8.3.5hCD2 control (squares) or KMIs-8.3.5RORγt cells (circles) were cultured for 20 hr in 96-well plates coated with the indicated amount of 2C11, and the supernatants were tested for IL-2 using a HT-2 bioassay. Shown are representative results from three similar experiments.
RORγt Does Not Inhibit Fas-Mediated or Other Forms of Apoptosis
To test whether RORγt inhibits T cell apoptosis induced by Fas signaling, KMIs-8.3.5hCD2 and KMIs-8.3.5RORγt cells were cocultured with L929 or L929FasL cells, and cell survival was quantitated by FACS analysis of hCD2 positive cells. Compared with cells cocultured with L929, both control and RORγt expressing KMIs-8.3.5 cells suffered significant loss when cultured with L929FasL (Figure 7A), indicating that expression of RORγt does not interfere with the Fas apoptotic pathway.
Figure 7. Expression of RORγt in T Cell Hybridoma Does Not Prevent Cell Death Induced by Fas Signaling or Other Stimuli.
(A) RORγt does not inhibit Fas-induced killing in KMIs-8.3.5 cells. KMIs-8.3.5hCD2 control or KMIs-8.3.5RORγt cells were cocultured with L929 or L929FasL cells at a ratio of 1:3. Two days later, the percentage of hCD2 positive cells from cells cocultured with L929 (empty column) or L929FasL (filled column) were determined by FACS.
(B) RORγt does not inhibit cell death induced by dexamethasone, ceramide, and staurosporine. KMIs-8.3.5hCD2 control or KMIs-8.3.5RORγt cells were cultured in the presence of indicated stimuli and [3H]Thymidine incorporation measured. [3H]Thymidine uptake of cells cultured in medium alone is calculated as 100%. Shown are results from two individual KMIs-8.3.5RORγt clones (circles) and KMIs-8.3.5hCD2 control cells (squares).
We also tested the effect of RORγt on apoptosis induced by other stimuli. Glucocorticoids induce immature thymocytes as well as T cell lines to undergo apoptosis (Zacharchuk et al., 1990; Cohen, 1992). Staurosporine is a broad-spectrum protein kinase inhibitor and induces apoptosis among various cell types (Raff et al., 1993). Ceramide is implicated in the signaling pathway that mediates apoptosis induced by Fas and TNFα (Cifone et al., 1994). As shown in Figure 7B, expression of RORγt had no effect on T cell apoptosis induced by any of these stimuli. These data demonstrate that RORγt specifically regulates genes that are related to T cell activation.
Discussion
Nuclear receptors form a superfamily that consists of more than 60 members (Laudet, 1997). The majority of nuclear receptors share a common modular structure consisting of four principal domains. The amino-terminal domain encodes transactivating activity. The central DNA-binding domain is the most conserved among members of this superfamily and participates in either DNA–protein or protein–protein interactions. The carboxy-terminal ligand-binding domain, which is moderately conserved, serves for ligand binding, dimerization, and transcriptional activation or repression. The hinge domain located between the DBD and the LBD is poorly conserved and, in many cases, harbors nuclear localization signals (Beato et al., 1995; Mangelsdorf and Evans, 1995; Laudet, 1997).
RORγt is a novel isoform of RORγ. It shares with RORγ identical nucleotide sequence from exon 3 through the last exon, which contains the conserved DBD and putative LBD (Medvedev et al., 1997). RORγ belongs to the ROR/RZR orphan receptor subfamily of nuclear receptors. This subfamily consists of RORα (α1, α2, α3), RZRβ, and RORγ (Carlberg et al., 1994; Giguère et al., 1994; Hirose et al., 1994; Ortiz et al., 1995). RORγ shares 51% and 50% identity at the amino acid level with human RORα and rat RZRβ, respectively, with the highest identity in the DBD (89% and 91%, respectively). The distinct 5′ sequences of RORγt and RORγ are likely due to alternate RNA processing of a common transcript, rather than differential transcription of different genes, since Southern blot analysis revealed RORγ as a unique gene (Ortiz et al., 1995). Interestingly, the three isoforms of RORα are also generated by alternate RNA splicing (Giguère et al., 1994). Like RORγt and RORγ, these isoforms differ only in the region encoding the N terminus. In the case of RORγt and RORγ, this difference is functionally significant (see below).
The function of the widely expressed RORγ is not clear. DNA-binding studies demonstrated that RORγ binds specifically to a direct repeat of the half-site sequence PuGGTCA with a 4 or 5 nucleotide spacer (Ortiz et al., 1995; Medvedev et al., 1996). This core motif also serves as the binding site for other members of the ROR/RZR orphan receptor subfamily (Giguère et al., 1994), as well as thyroid hormone (TR) and retinoic acid receptors (RAR) (Laudet, 1997), because of the highly conserved DBD among these receptors. When cotransfected with TR and RAR, RORγ is able to repress the transcriptional activities of these receptors on their corresponding response elements (Ortiz et al., 1995). It is likely that RORγt is also able to bind to the same core sequence. However, the different efficiency of the repression of T cell hybridoma apoptosis by RORγt and RORγ suggests that these two isoforms may have different specificity for target genes, and the specificity is determined by the distinct N terminus in these two isoforms. In support of this, the three isoforms of RORα (α1, 2, and 3) showed distinct DNA-binding specificity (Giguère et al., 1994).
The mechanism by which RORγt negatively regulates Fas ligand expression and IL-2 production remains elusive. It may directly bind to the promoters for Fas ligand and IL-2 and repress their transcriptional activity. Alternatively, RORγt may indirectly regulate genes that are involved in the expression of these two genes by transcriptional control or protein interaction. Several genes including Nur-77 (Weih et al., 1996), Egr-3 (Mittelstadt and Ashwell, 1998), ALG-3 (Vito et al., 1996), GILZ (D'Adamio et al., 1997), NF-AT (Latinis et al., 1997), RAR, and GR (Yang et al., 1995) have been shown to regulate Fas ligand expression in vitro and in vivo. To date, the mechanisms by which these gene products control Fas ligand expression and how they interact with each other are not well understood. The rapid induction of Nur-77 mRNA was not changed in KMIs-8.3.5RORγt cells, and it remains to be determined whether RORγt affects Nur-77 function by inhibiting its DNA binding capacity to the same core motif PuGGTCA (Wilson et al., 1991) or by forming a heterodimer with Nur-77, since cross-talk among orphan nuclear receptors does occur (Forman et al., 1994). It has been shown that retinoic acid and glucocorticoids antagonize TCR-induced apoptosis of T cell hybridomas by inhibiting Fas ligand upregulation (Yang et al., 1995). However, this effect is not mediated through RORγt since neither retinoic acid nor glucocorticoids induced RORγt expression in DO11.10 or KMIs-8.3.5 hybridoma cells (He and Bevan, unpublished data).
One potential role of RORγt in thymocyte development is related to its ability to inhibit Fas ligand upregulation. Recent evidence suggests that Fas/Fas ligand signaling participates in thymocyte negative selection (Kishimoto et al., 1998). In both normal and transgenic mice, the deletion of a semimature thymocyte population, HSAhiCD4+8– cells, was shown to be dependent on Fas when high doses of antigen were applied. The deletion occurs in the thymic medulla, indicating Fas/Fas ligand-mediated thymocyte negative selection occurs at a relatively late stage of thymocyte differentiation (Kishimoto et al., 1998). This result is consistent with the expression pattern of both Fas and Fas ligand in the thymus. Although Fas is highly expressed in cortical immature DP thymocytes as well as CD4+ or CD8+ SP thymocytes (Drappa et al., 1993; Andjelic et al., 1994; Nishimura et al., 1995; Ogasawara et al., 1995), Fas ligand is primarily expressed in thymic epithelial and dendritic cells located within the medulla (French et al., 1997). Notably, among the three thymocyte subpopulations that highly express Fas, only immature DP are exquisitely sensitive to Fas-induced killing (Nishimura et al., 1995; Ogasawara et al., 1995). Accordingly, any expression of Fas ligand in this thymocyte subset would cause massive cell death before they are subjected to positive or negative selection. Given the primary expression of RORγt in immature DP thymocytes and its ability to inhibit Fas ligand upregulation in T cell hybridomas, we propose that one role of RORγt in the thymus is to repress Fas ligand expression at the DP stage. In support of this view, increased expression of Fas ligand in thymocytes of Nur-77 transgenic mice results in a dramatic reduction of DP as well as SP thymocytes due to the early onset of apoptosis. Furthermore, this massive cell death could be partially rescued in a gld mouse background that contains a mutation in Fas ligand (Weih et al., 1996).
The capacity of the different thymocyte subsets to produce IL-2 is developmentally regulated, and we suggest that it may be controlled by RORγt. DP thymocytes express a high level of RORγt and do not produce IL-2 upon stimulation (Fischer et al., 1991). When DP thymocytes mature into the SP stage, they gain the capacity to make IL-2 (Fischer et al., 1991). Correlating with this, SP thymocytes and splenic T cells do not express RORγt. DN thymocytes, which contain the precursor for DP thymocytes, are extremely heterogeneous in terms of their surface phenotype as defined by CD44 and CD25 (Godfrey et al., 1993) and in terms of their ability to produce IL-2 (Zlotnik et al., 1992). The most mature, CD44–CD25– subpopulation of DN thymocytes, which gives rise directly to DPs, are unable to produce IL-2, while the earlier subpopulations defined by these surface markers have the ability to make IL-2 (Zlotnik et al., 1992). Our RT-PCR data revealed a low level of RORγt expression in the DN subset, and it would be of interest in the future to determine whether this expression occurs solely in the CD44–CD25– subset. This tightly controlled ability by immature DP thymocytes to produce cytokine has been proposed to be a fail-safe mechanism that developing T cells go through before positive and negative selection (Fischer et al., 1991). Thus, any DP would not have effector function until the selection has been completed. The molecular mechanism for this phenomenon is not well understood. Several studies provide evidence suggesting that the inability to produce IL-2 by DP thymocytes is due to the lack of inducibility of two transcription factors, NF-AT and AP-1 (Chen and Rothenberg, 1993; Rincón and Flavell, 1996). The expression pattern of RORγt together with its inhibition on IL-2 production in T cell hybridomas strongly suggest that one role of RORγt in DP thymocytes is to prevent IL-2 production. The relations between RORγt and AP-1 or members of the NF-AT family remain to be determined.
Experimental Procedures
Cell Lines
DO11.10 (White et al., 1983) and KMIs-8.3.5 (Park et al., 1996) are T cell hybridomas. Subclones of DO11.10 cells were tested for their survival rate during culture in PMA plus ionomycin. A subclone of DO11.10 with a low survival rate under these conditions was selected for subsequent expression cloning. ΦNX-Ampho is a retrovirus packaging cell line (Hitoshi et al., 1998). L929FasL cells were derived by transduction of mFas ligand cDNA using the pMX vector, followed by FACS sorting for Fas ligand expression. Cells were cultured in DMEM containing 10% fetal calf serum, 2 mM glutamine, 25 mM HEPES, 50 μM β-mercaptoethanol, 100 U/ml penicillin, and 100 μg/ml streptomycin.
MAbs and Reagents
Anti-human CD2 MAb, 35.1 (American Type Culture Collection, Rockville, MD) was purified and labeled in our laboratory. The following MAbs were purchased from PharMingen (San Diego, CA): purified anti-CD3 (145–2C11), PE-anti-Fas (Jo2), PE-anti-CD69 (H1.2F3), and Biotin-anti-FasL (Kay-10). Dexamethasone was purchased from Sigma (St. Louis, MO) and ceramide and staurosporine from Calbiochem (La Jolla, CA).
Expression Cloning
A thymocyte cDNA library in the retrovirus vector pMX (Deftos et al., 1998 [this issue of Immunity]) was transfected into ΦNX-Ampho packaging cell line using CaPO4 precipitation, and retrovirus-containing supernatant was harvested 2 days after the transfection. DO11.10 cells were infected by centrifugation at 2500 rpm for 1.5 hr at 32°C with the retrovirus supernatant followed by incubation at 32°C overnight (Hitoshi et al., 1998). A total of 5 × 106 DO11.10 cells were infected as assessed using control pMX-hCD2 retrovirus infection. Two days after the infection, DO11.10 cells were placed into 96-well plates (5 × 104 cells/well) and selected for survival and growth in the presence of PMA (10 ng/ml) plus ionomycin (0.2 μg/ml). Resistant clones were identified after 2–4 weeks in culture. cDNA inserts were amplified by using RT-PCR with vector-specific primers that flank the polycloning site of pMX. PCR products were sequenced with nested vector-specific primers on an ABI automated sequencer.
Plasmid Construction and Generation of Transgene Positive Cell Lines
The pMI vector was constructed by cloning the internal ribosome entry site (IRES) from encephalomyocarditis virus and a cDNA encoding the extracellular and transmembrane domains of hCD2 into the pMX retrovirus vector (Onishi et al., 1996). The resulting vector contains a polycloning site upstream of an IRES-hCD2 cassette. cDNA cloned into this vector is transcribed into bicistronic mRNA that concomitantly directs translation of the cDNA and truncated hCD2. cDNAs for RORγt, RORγ, and the four deletion mutants of RORγt were made by RT-PCR using Pfu polymerase (Stratagene), sequenced, and directionally cloned into NotI/SalI sites of the pMI vector. DO11.10 and KMIs-8.3.5 cells were infected with retroviruses produced from these plasmids. Transgene positive cell lines were established by either multiple rounds of panning on tissue culture dishes coated with 50 μg/ml anti-hCD2 MAb or by FACS sorting for hCD2 positive cells.
RT-PCR and Northern Blot Analysis
Total RNA was extracted from different tissues of 6-week-old female C57BL/6 mice (The Jackson Lab, Bar Harbor, Maine) or cell lines using STAT-60 (Tel-Test, Friendswood, TX). cDNA was synthesized using Superscript II reverse transcriptase (GIBCO) with oligo dT primer. The sequences for primers used in Figure 2 are: primer 1, 5′-ACCTCCACTGCCAGCTGTGTGCTGTC-3′; primer 2, 5′-ATGGACAGGGCCCCACAGAGAC-3′; primer 3, 5′-TCATTTCTGCACTTCTGCATGTAGACTGTCCC-3′; primer 4, 5′-GGGAGATGTGGGAGCGCTGTGC-3′; and primer 5, 5′-TCCTTCCTCCAGATCACTTTGACAGCCC-3′. For the detection of RORγt and RORγ, PCR was performed with 30 cycles consisting of 1 min at 94°C, 1 min at 68°C, and 2 min at 72°C with a 10 min extension at 72°C for the last cycle. The RT-PCR products were then run on 1% agrose gel, blotted, and probed with a full-length RORγt cDNA. For rescuing cDNA from PMA and ionomycin-resistant DO11.10 clones, PCR was performed with the following conditions using the Advantage cDNA PCR Kit (Clontech, Palo Alto, CA): 30 sec at 94°C, 1 min at 58°C, and 3 min at 68°C for 30 cycles. 15 μg total RNA from unstimulated and stimulated cells was analyzed by Northern blot analysis using a standard protocol (Maniatis et al., 1989). cDNAs for Fas ligand, Nur-77, Egr-3, HPRT, and GAPDH derived from RT-PCR were used as probes.
Cell Separation and Flow Cytometric Analyses
Cell separation and flow cytometric analyses were performed essentially as previously described (He et al., 1997). In brief, thymocytes from 6-week-old C57BL/6 mice were subjected to complement killing to enrich for subpopulations followed by FACS sorting. The purity for sorted subpopulations of thymocytes was >98% in post-sort analyses. Cells were incubated with an excess of biotinylated MAb, PE-streptavidin, or PE-conjugated MAbs on ice for 30 min and washed with PBS containing 0.1% BSA. Data were collected on 1 × 104 cells on a FACScan flow cytometer (Becton Dickinson) using CellQuest software.
Induction of Apoptosis and Thymidine Incorparation Assay
Twenty-four or 96-well tissue culture plates were precoated with rabbit anti-hamster anti-serum (50 μg/ml) overnight at 37°C, washed with HBSS, and coated with anti-CD3 MAb (145–2C11) at the amount indicated in the text. Hybridoma cells were added to the plates for the indicated time, harvested, and analyzed. Alternatively, PMA plus ionomycin was added to the culture instead of anti-CD3 MAb. For the thymidine incorporation assay, 2 × 104 cells per well were added to 96-well plates, cultured for 20 hr, and labeled with [3H]Thymidine (1 μCi/well, 25 Ci/mmol) (NEN) for a further 4 hr. The cells were harvested on glass-fiber filters and counted in a β scintillation counter. Data were derived from the mean value of duplicate cultures.
IL-2 Bioassay
IL-2 was measured using HT-2 cells (Roehm et al., 1983). In brief, supernatants from stimulated T cell hybridomas were serially diluted, and HT-2 cells were added (4 × 103/well, 96-well plate). Cells were then cultured for 20 hr and [3H]Thymidine added for a further 4 hr. [3H]Thymidine incorporation was plotted against supernatant dilutions. One unit of IL-2 was defined as the dilution of supernatant that supports half-maximal proliferation of HT-2 cells, and the total units of IL-2 were extrapolated from the resulting curve.
Acknowledgments
We thank Yongwon Choi for the hybridoma KMIs-8.3.5, Ananda Goldrath for separation of thymocyte subpopulations, and Jacqueline Kirchner and Eugene Huang for reading this manuscript. These studies were supported by the National Institutes of Health and the Howard Hughes Medical Institute.
References
- Alderson MR, Tough TW, Davis-Smith T, Braddy S, Falk B, Schooley KA, Goodwin RG, Smith CA, Ramsdell F, Lynch DH. Fas ligand mediates activation-induced cell death in human T lymphocytes. J. Exp. Med. 1995;181:71–77. doi: 10.1084/jem.181.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amakawa R, Hakem A, Kundig TM, Matsuyama R, Simard JJL, Timms E, Wakeham A, Mittruecker H, Griesser H, Takimoto HR, et al. Impaired negative selection of T cells in Hodgkin's disease antigen CD30-deficient mice. Cell. 1996;84:551–562. doi: 10.1016/s0092-8674(00)81031-4. [DOI] [PubMed] [Google Scholar]
- Andjelic S, Drappa J, Lacy E, Elkon KB, Nikolic-Zugic J. The onset of Fas expression parallels the acquisition of CD8 and CD4 in fetal and adult αβ thymocytes. Int. Immunol. 1994;6:73–79. doi: 10.1093/intimm/6.1.73. [DOI] [PubMed] [Google Scholar]
- Beato M, Herrlich P, Schütz G. Steroid hormone receptors: many actors in search of a plot. Cell. 1995;83:851–857. doi: 10.1016/0092-8674(95)90201-5. [DOI] [PubMed] [Google Scholar]
- Brunner T, Mogil RJ, LaFace D, Yoo NJ, Mahboubl A, Echeverri F, Martin SJ, Force WR, Lynch DH, Ware CF, et al. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature. 1995;373:441–443. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- Calnan BJ, Szychowski S, Chan FK-M, Cado D, Winoto A. A role for the orphan steroid receptor Nur77 in apoptosis accompanying antigen-induced negative selection. Immunity. 1995;3:273–282. doi: 10.1016/1074-7613(95)90113-2. [DOI] [PubMed] [Google Scholar]
- Carlberg C, Hooft van Huijsduijnen R, Staple JK, DeLarmarter JF, Becker-Andre M. RZRs, a new family of retinoid-related orphan receptors that function as both monomers and homo-dimers. Mol. Endocrinol. 1994;8:757–770. doi: 10.1210/mend.8.6.7935491. [DOI] [PubMed] [Google Scholar]
- Castro JE, Listman JA, Jacobson BA, Wang Y, Lopez PA, Ju S, Finn PW, Perkins DL. Fas modulation of apoptosis during negative selection of thymocytes. Immunity. 1996;5:617–627. doi: 10.1016/s1074-7613(00)80275-7. [DOI] [PubMed] [Google Scholar]
- Chen D, Rothenberg EV. Molecular basis for developmental changes in interleukin-2 gene inducibility. Mol. Cell. Biol. 1993;13:228–237. doi: 10.1128/mcb.13.1.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifone MG, De Maria R, Roncaioli P, Rippo MR, Azuma M, Lanier LL, Santoni A, Testi R. Apoptotic signaling through CD95 (Fas/Apo-1) activates an acidic sphingomyelinase. J. Exp. Med. 1994;180:1547–1552. doi: 10.1084/jem.180.4.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JJ. Glucocorticoid-induced apoptosis in the thymus. Semin. Immunol. 1992;4:363–369. [PubMed] [Google Scholar]
- D'Adamio F, Zollo O, Moraca R, Ayroldi E, Bruscoli S, Bartoli A, Cannarile L, Migliorati G, Riccardi C. A new dexamethasone-induced gene of the leucine zipper family protects T lymphocytes from TCR/CD3-activated cell death. Immunity. 1997;7:803–812. doi: 10.1016/s1074-7613(00)80398-2. [DOI] [PubMed] [Google Scholar]
- Deftos ML, He Y-W, Ojala EW, Bevan MJ. Correlating Notch signaling with thymocyte maturation. Immunity. 1998;9:777–786. doi: 10.1016/s1074-7613(00)80643-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhein J, Walczak H, Baumler C, Debatin K-M, Krammer PH. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95). Nature. 1995;73:438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- Drappa J, Brot N, Elkon KB. The Fas protein is expressed at high levels on CD4+CD8+ thymocytes and activated mature lymphocytes in normal mice but not in the lupus-prone strain. Proc. Natl. Acad. Sci. USA. 1993;90:10340–10344. doi: 10.1073/pnas.90.21.10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M, MacNeil I, Suda T, Cupp JE, Shortman K, Zlotnik A. Cytokine production by mature and immature thymocytes. J. Immunol. 1991;146:3452–3456. [PubMed] [Google Scholar]
- Forman BM, Chen J, Blumberg B, Kliewer SA, Henshaw R, Ong ES, Evans RM. Cross-talk among RORα1 and the Rev-erb family of orphan nuclear receptors. Mol. Endocrinol. 1994;94:1253–1261. doi: 10.1210/mend.8.9.7838158. [DOI] [PubMed] [Google Scholar]
- Foy TM, Page SM, Waldschmidt TJ, Schoneveld A, Laman JD, Masters SR, Tygrett L, Ledbetter JA, Aruffo A, Classen E, et al. An essential role for gp39, the ligand for CD40, in thymic selection. J. Exp. Med. 1995;182:1377–1388. doi: 10.1084/jem.182.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French LE, Wilson A, Hahne M, Viard I, Tschopp J, Mac-Donald HR. Fas ligand expression is restricted to nonlymphoid thymic components in situ. J. Immunol. 1997;159:2196–2202. [PubMed] [Google Scholar]
- Garbe A, Buck J, Hämmerling U. Retinoids are important cofactors in T cell activation. J. Exp. Med. 1992;176:109–117. doi: 10.1084/jem.176.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguère V, Tini M, Flock G, Ong E, Evans RM, Otulakowski G. Isoform-specific amino-terminal domains dictate DNA-binding properties of RORα, a novel family of orphan hormone nuclear receptors. Genes Dev. 1994;8:538–553. doi: 10.1101/gad.8.5.538. [DOI] [PubMed] [Google Scholar]
- Godfrey DI, Kennedy J, Suda T, Zlotnik A. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3-CD4-CD8- triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J. Immunol. 1993;150:4244–4252. [PubMed] [Google Scholar]
- He Y-W, Nakajima H, Leonard WJ, Adkins B, Malek TR. The common γ-chain of cytokine receptors regulates intrathymic T cell development at multiple stages. J. Immunol. 1997;158:2592–2599. [PubMed] [Google Scholar]
- Hirose T, Smith RJ, Jetten AM. RORγ: the third member of ROR/RZR orphan receptor subfamily that is highly expressed in skeletal muscle. Biochem. Biophys. Res. Commun. 1994;205:1976–1983. doi: 10.1006/bbrc.1994.2902. [DOI] [PubMed] [Google Scholar]
- Hitoshi Y, Lorens J, Kitada S-I, Fisher J, LaBarge M, Ring HZ, Francke U, Reed JC, Kinoshita S, Nolan GP. Toso, a cell surface, specific regulator of Fas-induced apoptosis in T cells. Immunity. 1998;8:461–471. doi: 10.1016/s1074-7613(00)80551-8. [DOI] [PubMed] [Google Scholar]
- Hueber AO, Zornig M, Lyon D, Suda T, Nagata S, Evan GI. Requirement for the CD95 receptor-ligand pathway in c-Myc-induced apoptosis. Science. 1997;278:1305–1309. doi: 10.1126/science.278.5341.1305. [DOI] [PubMed] [Google Scholar]
- Jameson SC, Bevan MJ. T-cell selection. Curr. Opin. Immunol. 1998;10:214–219. doi: 10.1016/s0952-7915(98)80251-3. [DOI] [PubMed] [Google Scholar]
- Ju S-T, Panka DJ, Cul H, Ettinger R, El-Khatib M, Sherr DH, Stanger BZ, Marshak-Rothstein A. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373:444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- Kastner P, Mark M, Chambon P. Nonsteroid nuclear receptors: what are genetic studies telling us about their role in real life? Cell. 1995;83:859–869. doi: 10.1016/0092-8674(95)90202-3. [DOI] [PubMed] [Google Scholar]
- Kishimoto H, Surh CD, Sprent J. A role for Fas in negative selection of thymocytes in vivo. J. Exp. Med. 1998;187:1427–1438. doi: 10.1084/jem.187.9.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisielow P, von Boehmer H. Development and selection of T cells: facts and puzzles. Adv. Immunol. 1995;58:87–209. doi: 10.1016/s0065-2776(08)60620-3. [DOI] [PubMed] [Google Scholar]
- Latinis KM, Norian LA, Eliason SL, Koretzky GA. Two NFAT transcription factor binding sites participate in the regulation of CD95 (Fas) ligand expression in activated human T cells. J. Biol. Chem. 1997;272:31427–31434. doi: 10.1074/jbc.272.50.31427. [DOI] [PubMed] [Google Scholar]
- Laudet V. Evolution of the nuclear receptor superfamily: early diversification from an ancestral orphan receptor. J. Mol. Endocrinol. 1997;19:207–226. doi: 10.1677/jme.0.0190207. [DOI] [PubMed] [Google Scholar]
- Liu Z-G, Smith SW, McLaughlin KA, Schwartz LM, Osborne BA. Apoptotic signals delivered through the T-cell receptor of a T-cell hybrid require the immediate-early gene nur77. Nature. 1994;367:281–284. doi: 10.1038/367281a0. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83:841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- Maniatis T, Fritsch EF, Sambrook J. Molecular Cloning: A Laboratory Manual. Second Edition Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1989. [Google Scholar]
- Medvedev A, Chistokhina A, Hirose T, Jetten AM. Genomic structure and chromosomal mapping of the nuclear orphan receptor RORγ (RORC) gene. Genomics. 1997;46:93–102. doi: 10.1006/geno.1997.4980. [DOI] [PubMed] [Google Scholar]
- Medvedev A, Yan Z-H, Hirose T, Giguère V, Jetten AM. Cloning of a cDNA encoding the murine orphan receptor RZR/RORγ and characterization of its response element. Gene. 1996;181:199–206. doi: 10.1016/s0378-1119(96)00504-5. [DOI] [PubMed] [Google Scholar]
- Mittelstadt PR, Ashwell JD. Cyclosporin A-sensitive transcription factor Egr-3 regulates Fas ligand expression. Mol. Cell. Biol. 1998;18:3744–3751. doi: 10.1128/mcb.18.7.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata S, Goldstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- Nishimura Y, Ishii A, Kobayashi Y, Yamasaki Y, Yonehara S. Expression and function of mouse Fas antigen on immature and mature T cells. J. Immunol. 1995;154:4395–4403. [PubMed] [Google Scholar]
- Ogasawara J, Suda T, Nagata S. Selective apoptosis of CD4+CD8+ thymocytes by the anti-Fas antibody. J. Exp. Med. 1995;181:485–491. doi: 10.1084/jem.181.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi M, Kinoshita S, Morikawa Y, Shibuya A, Phillips J, Lanier LL, Gorman DM, Nolan GP, Miyajima A, Kitamura T. Applications of retrovirus-mediated expression cloning. Exp. Hematol. 1996;24:324–329. [PubMed] [Google Scholar]
- Ortiz MA, Piedrafita FJ, Pfahl M, Maki R. TOR: a new orphan receptor expressed in the thymus that can modulate retinoid and thyroid hormone signals. Mol. Endocrinol. 1995;9:1679–1691. doi: 10.1210/mend.9.12.8614404. [DOI] [PubMed] [Google Scholar]
- Park CG, Lee SY, Kandala G, Lee SY, Choi Y. A novel gene product that couples TCR signaling to Fas(CD95) expression in activation-induced cell death. Immunity. 1996;4:583–591. doi: 10.1016/s1074-7613(00)80484-7. [DOI] [PubMed] [Google Scholar]
- Puck JM, Sneller MC. ALPS: an autoimmune human lymphoproliferative syndrome associated with abnormal lymphocyte apoptosis. Semin. Immunol. 1997;9:77–84. doi: 10.1006/smim.1996.0056. [DOI] [PubMed] [Google Scholar]
- Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1a, 25 dihydroxyvitamin D3-binding macromolecules in human B lymphocytes: effects on immunoglobulin production. Science. 1984;224:1438–1440. [PubMed] [Google Scholar]
- Raff MC, Barres BA, Burne JF, Coles HS, Ishizaki Y, Jacobson MD. Programmed cell death and the control of cell survival: lessons from the nervous system. Science. 1993;262:695–700. doi: 10.1126/science.8235590. [DOI] [PubMed] [Google Scholar]
- Rincón M, Flavell RA. Regulation of AP-1 and NFAT transcription factors during thymic selection of T cells. Mol. Cell. Biol. 1996;16:1074–1084. doi: 10.1128/mcb.16.3.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehm NW, Marrack P, Kappler JW. Helper signals in the plaque-forming cell response to protein-bound haptens. J. Exp. Med. 1983;158:317–333. doi: 10.1084/jem.158.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sytwu H-K, Liblau RS, McDevitt HO. The roles of Fas/APO-1 (CD95) and TNF in antigen-induced programmed cell death in T cell receptor transgenic mice. Immunity. 1996;5:17–30. doi: 10.1016/s1074-7613(00)80306-4. [DOI] [PubMed] [Google Scholar]
- Vito P, Lacaná E, D'Adamio L. Interfering with apoptosis: Ca2+-binding protein ALG-2 and Alzheimer's disease gene ALG-3. Science. 1996;271:521–525.. doi: 10.1126/science.271.5248.521. [DOI] [PubMed] [Google Scholar]
- Weih F, Ryseck R-P, Chen L, Bravo R. Apoptosis of nur77/N10-transgenic thymocytes involves the Fas/Fas ligand pathway. Proc. Natl. Acad. Sci. USA. 1996;93:5533–5538. doi: 10.1073/pnas.93.11.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J, Haskins KM, Marrack P, Kappler J. Use of I region-restricted, antigen-specific T cell hybridomas to produce idiotypically specific anti-receptor antibodies. J. Immunol. 1983;130:1033–1037. [PubMed] [Google Scholar]
- Wilson TE, Fahrner TJ, Johnston M, Milbrandt J. Identification of the DNA binding site for NGFI-B by genetic selection in yeast. Science. 1991;252:1296–1300. doi: 10.1126/science.1925541. [DOI] [PubMed] [Google Scholar]
- Woronicz JD, Calnan B, Ngo V, Winoto A. Requirement for the orphan steroid receptor Nur77 in apoptosis of T-cell hybridomas. Nature. 1994;367:277–280. doi: 10.1038/367277a0. [DOI] [PubMed] [Google Scholar]
- Yang Y, Mercep M, Ware CF, Ashwell JD. Fas and activation-induced Fas ligand mediate apoptosis of T cell hybridomas: inhibition of Fas ligand expression by retinoic acid and glucocorticoids. J. Exp. Med. 1995;181:1673–1682. doi: 10.1084/jem.181.5.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharchuk CM, Mercep M, Chakraborti PK, Simons SS, Jr., Ashwell JD. Programmed T lymphocyte death. Cell activation and steroid induced pathways are mutually antagonistic. J. Immunol. 1990;145:4037–4045. [PubMed] [Google Scholar]
- Zheng L, Fisher G, Miller RE, Peschon J, Lynch DH, Lenardo MJ. Induction of apoptosis in mature T cells by tumour necrosis factor. Nature. 1995;377:348–351. doi: 10.1038/377348a0. [DOI] [PubMed] [Google Scholar]
- Zhou T, Cheng J, Yang P, Wang Z, Liu C, Su X, Bluethmann H, Mountz JD. Inhibition of Nur77/Nurr1 leads to inefficient clonal deletion of self-reactive T cells. J. Exp. Med. 1996;183:1879–1892. doi: 10.1084/jem.183.4.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik A, Godfrey DI, Fischer M, Suda T. Cytokine production by mature and immature CD4-CD8- T cells αβ-T cell receptor+ CD4-CD8- T cells produce IL-4. J. Immunol. 1992;149:1211–1215. [PubMed] [Google Scholar]