Abstract
Background
Fetuin-A is a multifunctional hepatic secretory protein that inhibits the action of insulin in experimental animals. We evaluated the association between human serum fetuin-A and the metabolic syndrome (MetS) in a cohort of persons with coronary artery disease.
Methods and Results
We defined MetS by the National Cholesterol Education Program criteria among 711 nondiabetic outpatients with coronary artery disease. The mean age was 67 years, and 82% were male. We divided participants into quartiles by serum fetuin-A concentrations. A total of 45% of participants (80 of 177) in the highest quartile of fetuin-A had MetS compared with 24% of participants (42 of 177) in the lowest quartile (odds ratio, 2.7; 95% confidence interval, 1.7 to 4.2; P<0.001). This association persisted after adjustment for potential confounding variables, including hypertension, body mass index, and inflammatory biomarkers (adjusted odds ratio, 2.0; 95% confidence interval, 1.1 to 3.5; P=0.02). Higher fetuin-A quartiles were also strongly and independently associated with higher low-density lipoprotein, non–high-density lipoprotein (HDL), and triglyceride concentrations and lower HDL concentrations (all P<0.01).
Conclusions
Higher human fetuin-A concentrations are strongly associated with MetS and an atherogenic lipid profile. Future studies should evaluate whether fetuin-A predicts coronary artery disease risk.
Keywords: alpha2HS-glycoprotein, hypercholesterolemia, insulin, lipids, metabolism
The metabolic syndrome (MetS) is defined as a constellation of metabolic risk factors1 that are associated with cardiovascular events and all-cause mortality.2,3 MetS is estimated to affect 47 million Americans, including 40% of adults ≥60 years of age.4
Insulin resistance is thought to be the primary underlying abnormality leading to MetS.5,6 Animal studies have shown that human α2-HS-glycoprotein/fetuin-A, a protein secreted by the liver and found in high concentrations in serum, inhibits insulin receptor tyrosine kinase activity.7–13 Fetuin-A knockout mice have enhanced glucose clearance and insulin sensitivity, resistance to weight gain, and lower serum free fatty acid and triglyceride levels.10 The human fetuin-A gene resides on chromosome 3q27, which has been mapped as a type 2 diabetes susceptibility locus14 and is linked to a quantitative trait locus for MetS.15 Furthermore, the inhibitory function of fetuin-A on insulin receptor tyrosine kinase by bovine, mouse, sheep, and pig fetuin-A suggests a conserved function for fetuin-A homologs.13,16–18
We hypothesized that higher fetuin-A concentrations would be associated with MetS in humans. In a cross-sectional study of 711 nondiabetic outpatients with coronary artery disease (CAD), we evaluated the association of human fetuin-A with the presence of MetS and its individual components.
Methods
Participants
The Heart and Soul Study is a prospective cohort study designed to investigate the influence of psychosocial factors on CAD progression. Methods have been described previously.19–21 Participants were recruited from outpatient clinics in the San Francisco (Calif) Bay Area if they met one of the following inclusion criteria: history of myocardial infarction, angiographic evidence of >50% stenosis in ≥1 coronary vessels, evidence of exercise-induced ischemia by treadmill or nuclear testing, history of coronary revascularization, or documented diagnosis of CAD by an internist or cardiologist. Participants were excluded if they were not able to walk 1 block, had had a myocardial infarction in the preceding 6 months, or were likely to move out of the area within 3 years.
The study protocol was approved by the following institutional review boards: the Committee of Human Research at the University of California, San Francisco; the Research and Development Committee at the San Francisco VA Medical Center; the Medical Human Subjects Committee at Stanford University; the Human Subjects Committee at the VA Palo Alto Health Care System; and the Data Governance Board of the Community Health Network of San Francisco. All participants provided written informed consent.
Between September 2000 and December 2002, a total of 1024 participants enrolled and underwent a day-long baseline study appointment that included a medical history interview, a physical examination, an exercise treadmill test with stress echocardiogram, and a comprehensive health status questionnaire. Fasting (12-hour) serum samples were obtained and frozen at –70°C. Subjects with a history of diabetes mellitus (n=259) or for whom frozen serum was not available (n=54) were excluded, resulting in a final study sample of 711 subjects.
Measurements
Serum Fetuin-A
Serum fetuin-A was measured by means of a BNII nephelometric assay. Serum samples were centrifuged (60 minutes at 15 000g) and diluted 4-fold with PBS (N Diluent, Dade Behring Holdings, Liederbach, Germany). Serum was exposed to a polyclonal rabbit anti-human fetuin-A antibody identical to that used in the ELISA method that has been previously described.22 Particles agglutinated to increase the intensity of scattered light proportional to the amount of fetuin-A in the sample. A control solution of purified serum fetuin-A powder (Boehringer Mannheim GmbH, Mannheim, Dade Behring, Marburg, Germany) was used to prepare serial dilution curves, and serum concentrations of fetuin-A were calculated by regression analysis of standard curves. The assay was evaluated in a side-by-side comparison with immunoblot analyses to exclude cross-reactivity of the antibodies with other serum proteins and proteolytic fragments of fetuin-A. The assay does not cross-react with fetuin-B. The intra-assay coefficient of variation is 7.7%, and the interassay coefficient of variation is 8.1%. The assay range is from 0.05 to 3.5 g/L.
Metabolic Syndrome
The primary outcome was the presence or absence of MetS as defined by the National Cholesterol Education Program.1 Subjects were considered to have MetS if they met ≥3 of the following criteria: abdominal obesity (waist circumference >40 in for men, >35 in for women), fasting serum triglycerides ≥150 mg/dL, low fasting serum high-density lipoprotein (HDL) cholesterol (<40 mg/dL in men, <50 mg/dL in women), blood pressure ≥130/85 mm Hg, and fasting serum glucose ≥110 mg/dL or use of diabetic medications. Because all subjects in our study sample had CAD and may have been taking antihypertensive medications for cardiac protective effects rather than for treatment of hypertension, we did not consider use of these medications as indicative of meeting the blood pressure cutoff for the MetS. Likewise, subjects who were on statins were not considered to meet the MetS criteria for elevated triglycerides or low HDL cholesterol.
Study participants underwent a complete physical examination that included blood pressure determination by trained study personnel using a calibrated sphygmomanometer. Pulse pressure was calculated (systolic blood pressure minus diastolic blood pressure). Waist circumference was measured on bare skin at the level of the umbilicus and repeated to ensure accuracy (within 0.2 cm between measurements). Height and weight were measured, and we calculated body mass index (kg/m2). Fasting serum samples were obtained the morning of the study appointment and measured for serum glucose, total and HDL cholesterol, and triglyceride concentrations. Low-density lipoprotein (LDL) concentrations were estimated from the Friedewald equation,23 and non-HDL cholesterol was calculated (total cholesterol minus HDL cholesterol).
Other Measurements
Self-reported age, medical history, and tobacco and alcohol use were determined by questionnaire. We performed exercise treadmill testing with stress echocardiography, and we defined inducible ischemia as the presence of ≥1 new wall motion abnormalities at peak exercise. Participants were instructed to bring their medication bottles to the study appointment, and study personnel recorded all current medications.
High-sensitivity C-reactive protein (CRP) was measured with the Roche Integra assay (Roche Diagnostics, Indianapolis, Ind) in 44 participants and, because of a change at the laboratory, with the Beckman Extended Range assay (Beckman Coulter, Inc, Fullerton, Calif) in the remaining 667 participants.19 This assay is highly correlated with the Roche Integra assay (Spearman rank correlation, 0.95; median for the Beckman and Roche assays were 2.1 [intraquartile range, 0.8 to 4.0] and 1.8 [intraquartile range, 0.9 to 3.7], respectively, in a sample of 185 Heart and Soul Study participants in whom we performed both assays). Results were corrected for systematic differences between assays. The interassay coefficient of variation was 6.7%; the intra-assay coefficient of variation was 6.2%.
Serum fibrinogen levels were determined by the Clauss assay.24 Dilutions of plasma standard (of known fibrinogen concentrations) are clotted with a high concentration of thrombin (–100 U/mL), with the resultant clotting time being proportional to the fibrinogen concentration. The clotting time of the participant's plasma was used to read the fibrinogen concentration from the standard curves. The standard assay range is from 60 to 1000 mg/dL, and the interassay and intra-assay coefficients of variation were 3%.
Serum creatinine was measured from serum taken at the study appointment, and creatinine clearance was estimated by means of the Cockroft-Gault equation.25 Urine microalbuminuria was defined as urine albumin-to-creatinine ratio ≥30 mg/g.
Statistical Analysis
We divided participants into quartiles based on serum fetuin-A concentrations, with quartile 1 representing the lowest. Differences in baseline characteristics were compared by use of ANOVA for continuous variables (or Kruskal-Wallis test for nonparametric variables) and χ2 tests (or Fisher exact equivalent) for categorical variables. We evaluated the unadjusted association of fetuin-A quartiles with the presence of the MetS using the χ2 test for trend.
To determine the adjusted association of fetuin-A and MetS, we used logistic regression analyses with quartiles of fetuin-A as the primary predictor variable and presence of MetS as the outcome variable. We selected covariates for multivariable adjustment on the basis of their reported associations with fetuin-A (creatinine clearance, albumin, fibrinogen, CRP) and MetS (hypertension, body mass index, LDL cholesterol, alcohol and tobacco use) in previously published research, in addition to demographic characteristics (age, sex, race). Finally, we evaluated for effect modification by certain key variables (age, sex, race, obesity, and statin use) on the association of fetuin-A with MetS.
We also evaluated the association of fetuin-A quartiles with the individual components of the MetS. We constructed logistic regression models in which fetuin-A was the predictor variable, and elevated fasting glucose (≥110 mg/dL), elevated blood pressure (systolic blood pressure ≥130 mm Hg or diastolic blood pressure ≥85 mm Hg), waist circumference (>40 in for men or >35 in for women), HDL (<40 mg/dL in men or <50 mg/dL in women), serum triglycerides (≥150 mg/dL), LDL (≥130 mg/dL), and non-HDL cholesterol levels (≥160 mg/dL)1,26 served as separate dichotomous outcome variables. These models were adjusted for the same covariates as in our primary multivariable models. Analyses were performed with Stata Statistical Software, version 9 (College Station, Tex).
The authors had full access to the data and take full responsibility for their integrity. All authors have read and agree to the manuscript as written.
Results
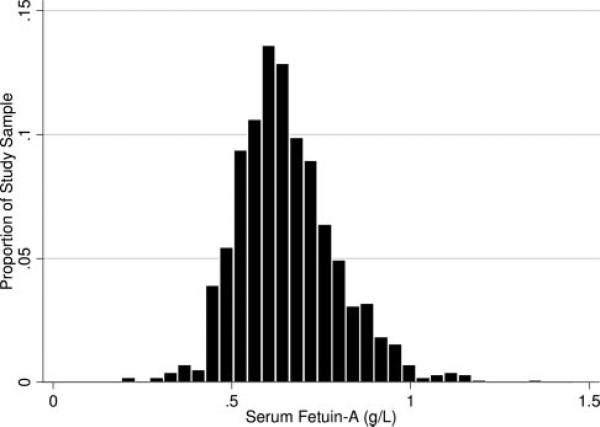
Fetuin-A concentrations were normally distributed among our study sample (mean, 0.64±0.13 g/L) (Figure 1). Compared with participants in the lowest quartile of fetuin-A, those in higher fetuin-A quartiles were younger, drank less alcohol, and had higher body mass index, hemoglobin, albumin, CRP, and fibrinogen levels (Table 1). In contrast, distributions of sex, ethnicity, tobacco use, and cardiac history were similar among fetuin-A quartiles. When we evaluated the association of these variables with fetuin-A as a linear outcome variable, age, sex, body mass index, hemoglobin, albumin, and fibrinogen were independently associated with fetuin-A (Table 2).
Figure 1.
Distribution of serum fetuin-A concentrations in 711 nondiabetic persons with CAD.
TABLE 1.
Characteristics of 711 Participants With Coronary Disease by Fetuin-A Quartiles
| Fetuin-A Quartile |
|||||
|---|---|---|---|---|---|
| 1 (≤0.55 g/L) | 2 (0.56–0.62 g/L) | 3 (0.63–0.70 g/L) | 4 (≥0.71 g/L) | P | |
| Demographics | |||||
| Age, y | 69±12 | 69±11 | 67±11 | 64±10 | <0.001 |
| Male sex, % | 151 (84) | 150 (85) | 143 (80) | 135 (77) | 0.14 |
| Ethnicity, n (%) | |||||
| White | 116 (65) | 118 (67) | 114 (64) | 112 (64) | 0.91 |
| Black | 37 (21) | 17 (10) | 24 (13) | 28 (16) | 0.03 |
| Other | 26 (15) | 41 (23) | 40 (22) | 36 (20) | 0.16 |
| Tobacco use, n (%) | 36 (20) | 39 (22) | 30 (17) | 40 (23) | 0.51 |
| Alcohol use, n (%) | 68 (38) | 65 (37) | 45 (25) | 53 (30) | 0.03 |
| Medical history, n (%) | |||||
| Hypertension | 128 (72) | 116 (66) | 112 (63) | 119 (68) | 0.28 |
| Myocardial infarction | 92 (52) | 93 (53) | 96 (54) | 92 (53) | 0.97 |
| Angioplasty | 77 (43) | 78 (44) | 64 (36) | 64 (36) | 0.23 |
| Coronary bypass | 66 (37) | 63 (36) | 60 (34) | 58 (33) | 0.83 |
| Measurements | |||||
| Creatinine clearance, mL/min | 78±31 | 81±29 | 82±28 | 84±24 | 0.25 |
| Microalbuminuria, n (%) | 20 (30) | 16 (24) | 19 (29) | 11 (17) | 0.32 |
| Body mass index, kg/m2 | 27±5 | 27±4 | 29±5 | 29±6 | <0.001 |
| Inducible ischemia, % | 39 (23) | 43 (25) | 43 (26) | 38 (24) | 0.24 |
| Hemoglobin, g/dL | 13.5±1.4 | 13.8±1.4 | 14.1±1.3 | 14.4±1.2 | <0.001 |
| Albumin, mg/dL | 3.8±0.4 | 3.9±0.3 | 3.9±0.3 | 4.0±0.3 | <0.001 |
| CRP, mg/dL* | 2.0 (0.6–4.5) | 1.8 (0.6–3.8) | 2.5 (0.9–5.3) | 2.8 (1.1–5.2) | 0.02 |
| Fibrinogen, mg/dL | 373±89 | 379±87 | 396±97 | 394±81 | 0.04 |
| Pulse pressure, mm Hg | 56±13 | 58±18 | 58±16 | 56±15 | 0.97 |
Values are mean±SD when appropriate.
Median (intraquartile range).
TABLE 2.
Unadjusted and Adjusted Correlates of Serum Fetuin-A Concentrations
| Unadjusted |
Adjusted* |
|||
|---|---|---|---|---|
| Variable | β | P | β | P |
| Demographics | ||||
| Age (per 10 y) | –0.022±0.004 | <0.001 | –0.021±0.008 | 0.006 |
| Male sex | –0.038±0.012 | 0.003 | –0.042±0.016 | 0.009 |
| White (vs nonwhite) | –0.019±0.010 | 0.30 | ||
| Tobacco use | 0.008±0.012 | 0.52 | ||
| Alcohol use | –0.022±0.011 | 0.04 | ||
| Medical history | ||||
| Hypertension | 0.004±0.011 | 0.65 | ||
| Myocardial infarction | –0.002±0.010 | 0.86 | ||
| Angioplasty | –0.013±0.010 | 0.20 | ||
| Coronary bypass | –0.010±0.010 | 0.36 | ||
| Measurements | ||||
| Creatinine clearance (per 30.7-mL/min increase)† | 0.024±0.005 | <0.001 | ||
| Microalbuminuria | –0.022±0.018 | 0.21 | ||
| Body mass index (per 5.0-kg/m2 increase)† | 0.025±0.005 | <0.0001 | 0.020±0.007 | 0.008 |
| Inducible ischemia | –0.019±0.012 | 0.12 | ||
| Hemoglobin (per 1.4-g/dL increase)† | 0.027±0.005 | <0.001 | 0.027±0.006 | <0.001 |
| Albumin (per 0.3-mg/dL increase)† | 0.027±0.005 | <0.001 | 0.022±0.006 | 0.001 |
| Log CRP (per 1.3-unit increase)† | 0.013±0.005 | 0.01 | ||
| Fibrinogen (per 89-mg/dL increase)† | 0.012±0.005 | 0.02 | 0.015±0.007 | 0.04 |
| Pulse pressure (per 15.8–mm Hg increase)† | 0.002±0.005 | 0.67 | ||
Coefficients are mean±SE and reflect change per 1 SD.
Coefficients for variables that remained independently associated with fetuin-A after adjustment for all other variables in the table.
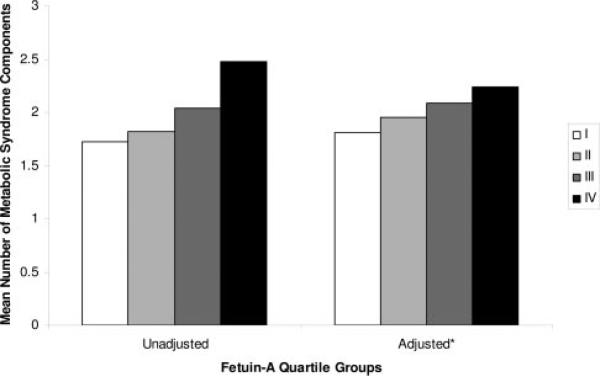
The highest fetuin-A quartile was associated with MetS in unadjusted and adjusted analyses (Table 3). However, the prevalence of MetS was not statistically different among persons in the second and third fetuin-A quartiles compared with those in the lowest quartile. When fetuin-A was entered as a continuous predictor variable, each 1-SD increase in fetuin-A (0.13 g/L) was associated with a 40% increased odds of MetS after adjustment for potential confounding variables (adjusted odds ratio [OR], 1.4; 95% confidence interval [CI], 1.1 to 1.7; P=0.002). Similarly, the number of MetS components increased linearly across increasing fetuin-A quartiles (Figure 2). We observed no effect modification with age, sex, race, obesity, or statin use for the association of fetuin-A and MetS (all P>0.35 for interaction).
TABLE 3.
Association of Fetuin-A With the Metabolic Syndrome in Persons with Coronary Disease
| Fetuin Quartiles | With Metabolic Syndrome, % | Unadjusted OR (95% CI) | P | Adjusted OR* (95% CI) | P |
|---|---|---|---|---|---|
| 1 | 24 (42/177) | 1.0 | Ref | 1.0 | Ref |
| 2 | 27 (49/179) | 1.2 (0.8–2.0) | 0.43 | 1.3 (0.8–2.3) | 0.3 |
| 3 | 29 (51/178) | 1.3 (0.8–2.1) | 0.32 | 1.0 (0.6–1.7) | 0.95 |
| 4 | 45 (80/177) | 2.7 (1.7–4.2) | <0.001 | 2.0 (1.1–3.5) | 0.02 |
| P for trend | <0.001 | 0.06 |
Ref indicates reference.
Adjusted for age, sex, race, tobacco use, alcohol use, creatinine clearance, body mass index, hypertension, albumin, LDL, fibrinogen, and CRP.
Figure 2.
Unadjusted and adjusted mean number of components of the metabolic syndrome among fetuin-A quartile groups. P for trend <0.001 for both comparisons. *Adjusted for age, sex, race, alcohol use, tobacco use, creatinine clearance, body mass index, hypertension, LDL cholesterol, fibrinogen, and CRP.
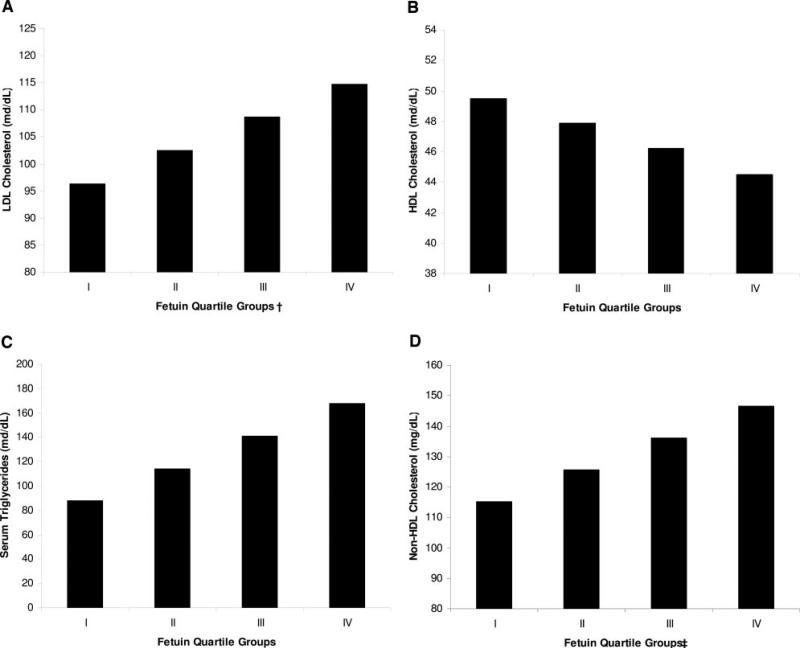
In univariate analyses, the highest quartile of fetuin-A was associated with each component of the MetS, except for elevated glucose and blood pressure, compared with the lowest quartile. However, after multivariable adjustment, fetuin-A remained associated only with the individual lipid parameters (Table 4). Increasing quartiles of fetuin-A were linearly associated with each lipid measure (Figure 3). The use of statins did not alter the association of high fetuin-A concentrations with any lipid parameter (all P>0.56 for interaction). Among statin users, the adjusted ORs of high fetuin-A were 2.6 (95% CI, 1.3 to 5.2; P=0.007) for low HDL, 5.5 (95% CI, 2.4 to 12.5; P<0.001) for high triglycerides, 3.9 (95% CI, 1.3 to 11.3; P=0.01) for high LDL, and 6.6 (95% CI, 0.47 to 8.3; P=0.35) for high non-HDL cholesterol. Among statin nonusers, the adjusted ORs were 1.7 (95% CI, 0.73 to 4.0; P=0.22) for low HDL, 8.5 (95% CI, 2.5 to 29; P=0.001) for high triglycerides, 6.9 (95% CI, 2.7 to 18; P<0.001) for high LDL, and 10.1 (95% CI, 3.3 to 30.8; P<0.001) for high non-HDL cholesterol.
TABLE 4.
Adjusted* Association of Fetuin-A Quartiles With Individual Components of the Metabolic Syndrome
| Fetuin-A Quartile Groups |
||||||||
|---|---|---|---|---|---|---|---|---|
| 1 |
2 |
3 |
4 |
|||||
| Reference | OR | 95% CI | OR | 95% CI | OR | 95% CI | P | |
| Fasting glucose ≥110 mg/dL or taking diabetes medication | 1 | 1.0 | 0.6–1.7 | 1.0 | 0.6–1.6 | 1.2 | 0.7–2.0 | 0.55 |
| SBP ≥130 or DBP ≥85 mm Hg | 1 | 1.0 | 0.6–1.5 | 0.9 | 0.6–1.5 | 1.0 | 0.6–1.7 | 0.99 |
| Waist circumference >40 in for men or >35 in for women | 1 | 0.4 | 0.2–0.9 | 0.5 | 0.3–1.2 | 0.6 | 0.3–1.3 | 0.35 |
| HDL <40 mg/dL in men or <50 mg/dL in women | 1 | 1.3 | 0.8–2.1 | 1.3 | 0.8–2.1 | 2.0 | 1.2–3.4 | 0.01 |
| Triglycerides ≥150 mg/dL | 1 | 3.1 | 1.6–6.0 | 3.2 | 1.7–6.1 | 5.6 | 2.9–10.8 | <0.001 |
| LDL ≥130 mg/dL† | 1 | 2.3 | 1.2–4.5 | 2.6 | 1.3–5.2 | 4.6 | 2.4–8.8 | <0.001 |
| Non-HDL cholesterol ≥160 mg/dL‡ | 1 | 3.6 | 1.5–8.4 | 3.5 | 1.5–8.2 | 7.8 | 3.4–17.5 | <0.001 |
SBP indicates systolic blood pressure; DBP, diastolic blood pressure.
Adjusted for age, sex, race, tobacco use, alcohol use, creatinine clearance, body mass index, hypertension, albumin, LDL, fibrinogen, and CRP.
LDL was not adjusted in this model because LDL ≥130 mg/dL served as the dependent variable.
LDL was not adjusted in this model because non-HDL is collinear with LDL and non-HDL cholesterol served as the dependent variable.
Figure 3.
Association of serum fetuin-A quartiles with adjusted mean lipid concentrations. *Adjusted for age, sex, race, tobacco use, alcohol use, creatinine clearance, body mass index, hypertension, albumin, LDL, fibrinogen, and CRP. †Not adjusted for LDL because LDL was the outcome variable. ‡Not adjusted for LDL because LDL and non-HDL cholesterol are collinear. P for trend <0.001 for all figures.
Discussion
Our study demonstrates a novel association between human fetuin-A and the MetS among 711 nondiabetic, predominantly male outpatients with coronary artery disease. Higher concentrations of human fetuin-A were associated with substantially increased odds of the MetS phenotype and an atherogenic lipid profile; these associations were independent of hypertension, body mass index, and inflammation. If confirmed in future studies, these observations may offer insights into potential mechanisms and targets for the prevention and treatment of MetS.
Prior research relating fetuin-A to insulin resistance has been limited to animal studies.7–13 These data suggest that fetuin-A interferes with insulin action at peripheral tissues through its interaction with the insulin receptor. In humans, the fetuin-A gene localizes to a site previously linked to the MetS quantitative trait locus.15 Because insulin resistance is thought to underlie the mechanisms leading to the MetS phenotype, our study would support the hypothesis that fetuin-A may directly promote the MetS phenotype in humans.
Among the components of MetS, we found a particularly strong association of human fetuin-A with an atherogenic lipid profile. These associations persisted after extensive statistical adjustment for potential confounding variables and in analyses stratified by statin use. The cross-sectional design of our study limits our ability to address direction of association, and we cannot exclude the possibility that hypercholesterolemia, hypertriglyceridemia, or hyperinsulinemia may trigger hepatic synthesis of fetuin-A. The mechanisms that explain the disproportionate association of fetuin-A with lipids compared with hyperglycemia remain unclear. Studies to evaluate whether this effect is mediated through tissue-specific actions of the inhibitory effect of fetuin-A on insulin receptor tyrosine kinase or through alternative pathways could yield novel insights into the regulatory mechanisms of hypercholesterolemia and hypertriglyceridemia. Our leading hypothesis is that fetuin-A directly or indirectly leads to these changes through its inhibitory effects on the insulin receptor tyrosine kinase. Among the most sensitive effects of insulin is its inhibition of lipolysis in adipose tissue.27 Inhibition of the insulin receptor by fetuin-A may lead to increased lipolysis and efflux of free fatty acids from adipose tissue. This may, in turn, lead to increased production of apolipoprotein B–containing very-low-density lipoprotein (VLDL).28 Furthermore, hypertriglyceridemia may lead to a decrease in the cholesterol content of HDL, which may enhance HDL clearance from the circulation,29 thereby potentially leading to the atherogenic lipid profile observed in this study. Future studies are needed to confirm our findings and to elucidate the underlying mechanisms.
Whether or not fetuin-A independently predicts cardiovascular risk may be more complex than simply through its association with MetS and an atherogenic lipid profile. Previous data in select populations have suggested that low fetuin-A concentrations, rather than high concentrations, may predict increased cardiovascular risk.22 Fetuin-A has emerged as a potent inhibitor of vascular calcification in experimental animals.30–32 In this context, high fetuin-A levels may be protective for cardiovascular events by functioning to keep calcium and phosphorus solubilized in serum, thus preventing hydroxyapatite deposition in vessel walls. Indeed, in patients with end-stage renal disease, a population with altered calcium and phosphorus metabolism and exceedingly high cardiovascular risk, low fetuin-A concentrations were associated with higher rates of cardiovascular and all-cause mortality.22 If low concentrations of human fetuin-A also are associated with excess vascular calcification in nondialysis study populations, it is possible that the increased vascular calcification may offset the improvements in dyslipidemia and other features of the MetS with respect to risk of cardiovascular end points. However, in the absence of kidney disease, whether vascular calcification promotes atheromatous plaque stability33,34 or instability35 has been debated.
Inflammation, assessed by elevated CRP measurements, has been linked to excess cardiovascular risk36 and to MetS37 individually. Though not confirmed in our study, prior studies suggest that fetuin-A is a negative acute-phase reactant—levels fall during inflammation.38 Therefore, it remains plausible that there is a U-shaped relationship between fetuin-A and cardiovascular risk. Either a high fetuin-A level (associated with MetS and an atherogenic lipid profile) or low fetuin-A level (associated with vascular calcification and inflammation) may prove to predict incident cardiovascular events in the general population. Alternatively, given its multiple functions, fetuin-A may lack discriminatory ability for predicting cardiovascular risk. These novel questions await future studies with longitudinal cardiovascular outcomes.
Several limitations should be considered when our results are interpreted. First, the cross-sectional design does not allow causal inference or evaluation of direction of association. Although experimental animal data provide biological plausibility for the hypothesis that fetuin-A may interfere with insulin signaling in peripheral tissue that may have led to the observed associations, we cannot exclude the possibility that an unmeasured factor may lead to concomitant elevation in fetuin-A levels and the MetS phenotype or that MetS or hyperlipidemia led to elevated serum fetuin-A levels. However, to explain the strength of the observed association, any unmeasured confounder would need to be highly prevalent in the cohort and strongly associated with both fetuin-A and MetS and hyperlipidemia. We did not measure insulin levels, adiponectin, or lipoprotein subfractions in our study sample. Such measures may have extended our understanding of the mechanisms by which fetuin-A is associated to MetS. We performed multiple comparisons in evaluating the association of fetuin-A with each component of the MetS, LDL, and non-HDL cholesterol; this may have lead to spurious positive associations. Conversely, we potentially could have missed an underlying independent association of fetuin-A with glucose levels or body mass index. Inclusion criteria to our study required medical history of CAD, which may explain the high prevalence of MetS in our study sample, and the association of fetuin-A with MetS may differ in other clinical settings. Finally, our study participants were mostly elderly men; therefore, our results may not be generalizable to women or younger persons.
In summary, higher human fetuin-A concentrations have a strong and independent association with MetS in nondiabetic subjects with CAD. These findings, in conjunction with previous animal studies, raise the possibility that fetuin-A may directly promote the MetS phenotype in humans. Higher fetuin-A concentrations also were independently associated with atherogenic lipid profiles. Longitudinal studies should evaluate the direction of the observed associations, regulatory factors that alter serum fetuin-A concentrations, and the association of fetuin-A with subsequent cardiovascular events.
CLINICAL PERSPECTIVE.
In this cross-sectional study of 711 nondiabetic persons with coronary artery disease (CAD), we found a strong and independent association of human fetuin-A, an inhibitor of insulin receptor tyrosine kinase in animal models, and prevalent metabolic syndrome (MetS), as well as an atherogenic lipid profile. The associations were independent of hypertension, body mass index, inflammatory biomarkers, and statin use. In conjunction with existing animal data, these observations suggest that fetuin-A may promote the development of the MetS in humans. Future studies are required to evaluate whether fetuin-A independently predicts incident diabetes mellitus and cardiovascular disease events and to elucidate mechanisms for the observed associations of fetuin-A and an atherogenic lipid profile.
Acknowledgments
This work was supported by grants from the American Heart Association (Fellow-to-Faculty Transition Award) (J.H.I.), the Department of Veteran Affairs (M.W.), the American Federation for Aging Research (Paul Beeson Scholars Program) (M.W., M.G.S.), the Robert Wood Johnson Foundation (Faculty Scholars Program) (M.W., M.G.S.), the Ischemia Research Education Foundation (M.W.), and the IZKF BioMAT-TV B 67 (M.K.). We wish to thank Glenn M. Chertow, MD, MPH, for reviewing this manuscript.
Footnotes
Disclosures
Dr Whooley has received significant research support from Roche Pharmaceuticals, Amgen, Inc, and Dade Behring Inc. Dr Shlipak has received a modest honorarium from Amgen, Inc.
References
- 1.Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 2.Klein BE, Klein R, Lee KE. Components of the metabolic syndrome and risk of cardiovascular disease and diabetes in beaver dam. Diabetes Care. 2002;25:1790–1794. doi: 10.2337/diacare.25.10.1790. [DOI] [PubMed] [Google Scholar]
- 3.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 4.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the Third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 5.Deedwania PC. Metabolic syndrome and vascular disease: is nature or nurture leading the new epidemic of cardiovascular disease? Circulation. 2004;109:2–4. doi: 10.1161/01.CIR.0000110642.73995.BF. [DOI] [PubMed] [Google Scholar]
- 6.Reaven GM. Banting Lecture 1988: role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 7.Auberger P, Falquerho L, Contreres JO, Pages G, Le Cam G, Rossi B, Le Cam A. Characterization of a natural inhibitor of the insulin receptor tyrosine kinase: cDNA cloning, purification, and anti-mitogenic activity. Cell. 1989;58:631–640. doi: 10.1016/0092-8674(89)90098-6. [DOI] [PubMed] [Google Scholar]
- 8.Haasemann M, Nawratil P, Muller-Esterl W. Rat tyrosine kinase inhibitor shows sequence similarity to human alpha 2-HS glycoprotein and bovine fetuin. Biochem J. 1991;274(pt 3):899–902. doi: 10.1042/bj2740899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalabay L, Chavin K, Lebreton JP, Robinson KA, Buse MG, Arnaud P. Human recombinant alpha 2-HS glycoprotein is produced in insect cells as a full length inhibitor of the insulin receptor tyrosine kinase. Horm Metab Res. 1998;30:1–6. doi: 10.1055/s-2007-978822. [DOI] [PubMed] [Google Scholar]
- 10.Mathews ST, Singh GP, Ranalletta M, Cintron VJ, Qiang X, Goustin AS, Jen KL, Charron MJ, Jahnen-Dechent W, Grunberger G. Improved insulin sensitivity and resistance to weight gain in mice null for the Ahsg gene. Diabetes. 2002;51:2450–2458. doi: 10.2337/diabetes.51.8.2450. [DOI] [PubMed] [Google Scholar]
- 11.Rauth G, Poschke O, Fink E, Eulitz M, Tippmer S, Kellerer M, Haring HU, Nawratil P, Haasemann M, Jahnen-Dechent W, Muller-Esterl W. The nucleotide and partial amino acid sequences of rat fetuin: identity with the natural tyrosine kinase inhibitor of the rat insulin receptor. Eur J Biochem. 1992;204:523–529. doi: 10.1111/j.1432-1033.1992.tb16663.x. [DOI] [PubMed] [Google Scholar]
- 12.Srinivas PR, Goustin AS, Grunberger G. Baculoviral expression of a natural inhibitor of the human insulin receptor tyrosine kinase. Biochem Biophys Res Commun. 1995;208:879–885. doi: 10.1006/bbrc.1995.1417. [DOI] [PubMed] [Google Scholar]
- 13.Srinivas PR, Wagner AS, Reddy LV, Deutsch DD, Leon MA, Goustin AS, Grunberger G. Serum alpha 2-HS-glycoprotein is an inhibitor of the human insulin receptor at the tyrosine kinase level. Mol Endocrinol. 1993;7:1445–1455. doi: 10.1210/mend.7.11.7906861. [DOI] [PubMed] [Google Scholar]
- 14.Vionnet N, Hani El H, Dupont S, Gallina S, Francke S, Dotte S, De Matos F, Durand E, Lepretre F, Lecoeur C, Gallina P, Zekiri L, Dina C, Froguel P. Genomewide search for type 2 diabetes-susceptibility genes in French whites: evidence for a novel susceptibility locus for early-onset diabetes on chromosome 3q27-qter and independent replication of a type 2-diabetes locus on chromosome 1q21-q24. Am J Hum Genet. 2000;67:1470–1480. doi: 10.1086/316887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kissebah AH, Sonnenberg GE, Myklebust J, Goldstein M, Broman K, James RG, Marks JA, Krakower GR, Jacob HJ, Weber J, Martin L, Blangero J, Comuzzie AG. Quantitative trait loci on chromosomes 3 and 17 influence phenotypes of the metabolic syndrome. Proc Natl Acad Sci USA. 2000;97:14478–14483. doi: 10.1073/pnas.97.26.14478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cintron VJ, Ko MS, Chi KD, Gross JP, Srinivas PR, Goustin AS, Grunberger G. Genetic mapping and functional studies of a natural inhibitor of the insulin receptor tyrosine kinase: the mouse ortholog of human alpha2-HS glycoprotein. Int J Exp Diabetes Res. 2001;1:249–263. doi: 10.1155/EDR.2000.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grunberger GMS, Deutsch DD. Insulin Signaling: From Cultured Cells to Animal Models: Frontiers in Animal Diabetes Research. Taylor and Francis; New York, NY: 2002. Tyrosine kinase inhibitors. [Google Scholar]
- 18.Mathews ST, Srinivas PR, Leon MA, Grunberger G. Bovine fetuin is an inhibitor of insulin receptor tyrosine kinase. Life Sci. 1997;61:1583–1592. doi: 10.1016/s0024-3205(97)00737-6. [DOI] [PubMed] [Google Scholar]
- 19.Beattie MS, Shlipak MG, Liu H, Browner WS, Schiller NB, Whooley MA. C-reactive protein and ischemia in users and nonusers of beta-blockers and statins: data from the Heart and Soul Study. Circulation. 2003;107:245–250. doi: 10.1161/01.cir.0000044387.23578.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ix JH, Shlipak MG, Liu HH, Schiller NB, Whooley MA. Association between renal insufficiency and inducible ischemia in patients with coronary artery disease: the Heart and Soul Study. J Am Soc Nephrol. 2003;14:3233–3238. doi: 10.1097/01.asn.0000095642.25603.7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health-related quality of life: the Heart and Soul Study. JAMA. 2003;290:215–221. doi: 10.1001/jama.290.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ketteler M, Bongartz P, Westenfeld R, Wildberger JE, Mahnken AH, Bohm R, Metzger T, Wanner C, Jahnen-Dechent W, Floege J. Association of low fetuin-A (AHSG) concentrations in serum with cardiovascular mortality in patients on dialysis: a cross-sectional study. Lancet. 2003;361:827–833. doi: 10.1016/S0140-6736(03)12710-9. [DOI] [PubMed] [Google Scholar]
- 23.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 24.Clauss A. Rapid physiological coagulation method in determination of fibrinogen [in German]. Acta Haematol. 1957;17:237–246. doi: 10.1159/000205234. [DOI] [PubMed] [Google Scholar]
- 25.Cockroft DL. Comparison of in vitro and in vivo development of rat foetuses. Dev Biol. 1976;48:163–172. doi: 10.1016/0012-1606(76)90054-3. [DOI] [PubMed] [Google Scholar]
- 26.Ridker PM, Rifai N, Cook NR, Bradwin G, Buring JE. Non-HDL cholesterol, apolipoproteins A-I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. JAMA. 2005;294:326–333. doi: 10.1001/jama.294.3.326. [DOI] [PubMed] [Google Scholar]
- 27.Jensen MD, Caruso M, Heiling V, Miles JM. Insulin regulation of lipolysis in nondiabetic and IDDM subjects. Diabetes. 1989;38:1595–1601. doi: 10.2337/diab.38.12.1595. [DOI] [PubMed] [Google Scholar]
- 28.Lewis GF, Uffelman KD, Szeto LW, Weller B, Steiner G. Interaction between free fatty acids and insulin in the acute control of very low density lipoprotein production in humans. J Clin Invest. 1995;95:158–166. doi: 10.1172/JCI117633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brinton EA, Eisenberg S, Breslow JL. Increased apo A-I and apo A-II fractional catabolic rate in patients with low high density lipoprotein-cholesterol levels with or without hypertriglyceridemia. J Clin Invest. 1991;87:536–544. doi: 10.1172/JCI115028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jahnen-Dechent W, Schinke T, Trindl A, Muller-Esterl W, Sablitzky F, Kaiser S, Blessing M. Cloning and targeted deletion of the mouse fetuin gene. J Biol Chem. 1997;272:31496–31503. doi: 10.1074/jbc.272.50.31496. [DOI] [PubMed] [Google Scholar]
- 31.Price PA, Thomas GR, Pardini AW, Figueira WF, Caputo JM, Williamson MK. Discovery of a high molecular weight complex of calcium, phosphate, fetuin, and matrix gamma-carboxyglutamic acid protein in the serum of etidronate-treated rats. J Biol Chem. 2002;277:3926–3934. doi: 10.1074/jbc.M106366200. [DOI] [PubMed] [Google Scholar]
- 32.Schinke T, Amendt C, Trindl A, Poschke O, Muller-Esterl W, Jahnen-Dechent W. The serum protein alpha2-HS glycoprotein/fetuin inhibits apatite formation in vitro and in mineralizing calvaria cells: a possible role in mineralization and calcium homeostasis. J Biol Chem. 1996;271:20789–20796. doi: 10.1074/jbc.271.34.20789. [DOI] [PubMed] [Google Scholar]
- 33.Beckman JA, Ganz J, Creager MA, Ganz P, Kinlay S. Relationship of clinical presentation and calcification of culprit coronary artery stenoses. Arterioscler Thromb Vasc Biol. 2001;21:1618–1622. doi: 10.1161/hq0901.095554. [DOI] [PubMed] [Google Scholar]
- 34.Huang H, Virmani R, Younis H, Burke AP, Kamm RD, Lee RT. The impact of calcification on the biomechanical stability of atherosclerotic plaques. Circulation. 2001;103:1051–1056. doi: 10.1161/01.cir.103.8.1051. [DOI] [PubMed] [Google Scholar]
- 35.Cheng GC, Loree HM, Kamm RD, Fishbein MC, Lee RT. Distribution of circumferential stress in ruptured and stable atherosclerotic lesions: a structural analysis with histopathological correlation. Circulation. 1993;87:1179–1187. doi: 10.1161/01.cir.87.4.1179. [DOI] [PubMed] [Google Scholar]
- 36.Ridker PM. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation. 2001;103:1813–1818. doi: 10.1161/01.cir.103.13.1813. [DOI] [PubMed] [Google Scholar]
- 37.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107:391–397. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 38.Lebreton JP, Joisel F, Raoult JP, Lannuzel B, Rogez JP, Humbert G. Serum concentration of human alpha 2 HS glycoprotein during the inflammatory process: evidence that alpha 2 HS glycoprotein is a negative acute-phase reactant. J Clin Invest. 1979;64:1118–1129. doi: 10.1172/JCI109551. [DOI] [PMC free article] [PubMed] [Google Scholar]