Abstract
We examined the association between anemia (hemoglobin ≤12 g/dl) and 6 indexes of heart rate variability (HRV) as measured by 24-hour ambulatory electrocardiography in a cross-sectional study of 874 outpatients who had stable coronary heart disease. Of 90 participants who had anemia, 29% to 41% had low HRV, defined as the lowest quartile of each HRV index, compared with 23% to 25% of the 784 participants who did not have anemia (comparison p values <0.05 for all HRV indexes except high-frequency power). With the exception of high-frequency power, each 1 g/dl decrease in hemoglobin was associated with increased odds of having low HRV. This association remained strong after adjustment for potential confounding variables, including ischemia, left ventricular mass, left ventricular ejection fraction, and diastolic dysfunction. Thus, anemia is associated with low HRV in ambulatory patients who have stable coronary heart disease. Low HRV could potentially mediate the association of anemia with increased cardiac risk.
Several small studies have associated anemia due to vitamin B12 deficiency,1,2 thalassemia,3 and sickle cell anemia4 with low heart rate variability (HRV). However, no study has examined whether anemia is associated with HRV in patients who have heart disease. Several studies have shown that low HRV independently predicts sudden cardiac death and overall mortality in patients who have heart disease,5–10 suggesting that low HRV may contribute to the adverse cardiac outcomes associated with anemia. We hypothesized that anemia is associated with an imbalance of cardiac autonomic tone as measured by low HRV in patients who have coronary heart disease (CHD). To determine whether anemia is associated with HRV, we measured hemoglobin and HRV in a cross-sectional study of 874 ambulatory patients who had stable CHD.
The Heart and Soul Study is a prospective cohort study of psychosocial factors and health outcomes in patients who have CHD. Details regarding our methods have been previously published.11 Outpatients who had documented CHD were recruited from 2 veterans affairs medical centers (San Francisco VA Medical Center, San Francisco, California, and the VA Palo Alto Health Care System, Palo Alto, California), 1 university medical center (University of California, San Francisco, California), and 9 public health clinics in the Community Health Network of San Francisco. Patients were eligible to participate if they had ≥1 of the following: a history of myocardial infarction, angiographic evidence of ≥50% stenosis in ≥1 coronary vessel, previous evidence of exercise-induced ischemia by treadmill or nuclear testing, a history of coronary revascularization, or a diagnosis of CHD by an internist or cardiologist (based on a positive angiographic or exercise treadmill test result in >98% of cases). Patients were excluded if they were unable to walk 1 block or were planning to move from the local area within 3 years.
Between September 2000 and December 2002, 1,024 participants were enrolled and completed a day-long study appointment at the San Francisco VA Medical Center. A total of 150 participants was excluded from HRV analysis because they were not in sinus rhythm (n = 76) or had missing Holter data (n = 74), leaving 874 participants for this cross-sectional study. During their day-long appointment, all participants completed a comprehensive medical health interview and questionnaire and underwent exercise treadmill stress testing with echocardiographic imaging. Participants then underwent 24-hour ambulatory Holter electrocardiography for measurement of HRV. The protocol was approved by the appropriate institutional review boards, and all participants provided written informed consent.
After an overnight fast, venous blood samples were drawn into tubes that contained ethylenediaminetetraacetic acid. Hemoglobin values were obtained with the Beckman Coulter LH 750 (Fullerton, California); interassay coefficient of variation was 0.4%. The laboratory technicians who measured these values were blinded to the results of the stress echocardiogram. We defined anemia as a hemoglobin level ≤12 g/dl in accordance with previous studies.12–14 Hemoglobin was also examined as a continuous predictor variable.
We measured HRV indexes obtained by 3-channel, 24-hour, ambulatory Holter electrocardiographic recording as recommended by the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology.15 Holter recordings were scanned 500 times in real time, and electrocardiographic data were digitized at a frequency of 128 Hz. Software (GE Healthcare, Waukesha, Wisconsin) was used to detect and label each QRS complex. The software measures all cycles in which beats have normal morphologic characteristics and cycle lengths within 20% duration of the preceding cycle length. The processed electrocardiograms were carefully reviewed and modified as necessary by an editor who was blinded to hemoglobin levels.
Annotated QRS data were processed by other software (GE Healthcare) to compute time-domain variables, including SD of NN intervals in milliseconds and SD of 5-minute mean NN intervals in milliseconds. The software also computed frequency-domain variables using a fast-Fourier transformation over the 24-hour period, including very-low-frequency power (0.0033 to 0.04 Hz), low-frequency power (0.04 to 0.15 Hz), high-frequency power (0.15 to 0.4 Hz), and wideband frequency power (0.0033 to 0.4 Hz) in square milliseconds.12,16,17 Very-low-frequency power and wideband frequency power were available for only 478 participants because the software was upgraded during the study. In a quality control check, we performed blinded repeat measurements of 20 tapes and found >99% concordance in readings between the 2 software programs.
Age, gender, ethnicity, marital status, smoking status, alcohol use, and medical history were determined by questionnaire. Participants were instructed to bring their medication bottles to the study appointment, and study personnel recorded all current medications. Participants were considered to be physically active if they answered fairly, quite, very, or extremely active (vs not at all or a little active) to the following multiple-choice question: “Which of the following statements best describes how physically active you have been during the last month, that is, done activities such as 15 to 20 minutes of brisk walking, swimming, general conditioning, or recreational sports?” We measured weight and height and calculated body mass index (kilograms per square meters).
Systolic and diastolic blood pressures were measured with a standard sphygmomanometer. We assessed left ventricular (LV) ejection fraction (systolic function) and diastolic pulmonary vein flow (diastolic function) on echocardiograms obtained at rest. Presence of ischemia was assessed by a symptom-limited, graded exercise treadmill test according to a standard Bruce's protocol, and wall motion score index at peak exercise using stress echocardiography was calculated.18 We also assessed the presence of inducible ischemia, defined as the presence of ≥1 new wall motion abnormality, at peak exercise. LV mass was determined by echocardiography, and LV mass index was calculated by dividing LV mass by body surface area. Creatinine clearance was assessed by 24-hour urine collection.
Differences in baseline characteristics between participants who had anemia and those who did not were compared with 2-tailed Student's t tests for continuous variables and chi-square tests for dichotomous variables. The frequency-domain HRV measurements were log-transformed to produce normal distributions. We used analysis of covariance to compare mean HRV values in participants who had anemia and those who did not after adjusting for potential confounding variables using a backward elimination procedure (p <0.05 for retention). We used logistic regression to determine the association of anemia with low HRV (defined as lowest quartile of each HRV index). All analyses were performed with SAS version 8 (SAS Institute, Cary, North Carolina).
Ninety of the 874 participants (10.3%) had anemia (hemoglobin ≤12 g/dl). Compared with participants who did not have anemia, those who did were less likely to be men, to be white, to drink alcohol, and to be physically active (Table 1). Participants who had anemia were more likely to have diabetes mellitus and congestive heart failure and to take diuretics. Compared with those who did not have anemia, participants who had anemia had higher LV mass index values, greater likelihood of diastolic pulmonary vein flow, lower diastolic blood pressure, and lower creatinine clearance.
Table 1.
Characteristics of 874 Participants, Stratified by Presence of Anemia
| Hemoglobin (g/dL) | |||
|---|---|---|---|
| Variable | ≤12 (n = 90) |
>12 (n = 784) |
p Value |
| Age (yrs) | 68 ± 12 | 66 ±11 | 0.12 |
| Men | 59 (66%) | 657 (84%) | <0.0001 |
| White | 39 (43%) | 466 (60%) | 0.003 |
| Married | 30 (33%) | 335 (43%) | 0.08 |
| Smoking (current) | 14 (16%) | 156 (20%) | 0.32 |
| Alcohol (regular use) | 17 (19%) | 229 (29%) | 0.05 |
| Hypertension | 71 (79%) | 554 (71%) | 0.11 |
| Diabetes mellitus | 37 (41%) | 192 (25%) | 0.0007 |
| Congestive heart failure | 22 (25%) | 119 (15%) | 0.02 |
| Angina weekly or more | 18 (20%) | 145 (19%) | 0.74 |
| Previous myocardial infarction | 49 (55%) | 426 (55%) | 0.94 |
| Previous stroke | 15 (17%) | 112 (14%) | 0.55 |
| Previous coronary bypass | 27 (30%) | 290 (37%) | 0.20 |
| Previous angioplasty | 31 (35%) | 311 (40%) | 0.37 |
| Chronic lung disease | 13 (14%) | 125 (16%) | 0.70 |
| Physically active | 47 (52%) | 508 (65%) | 0.02 |
| β-Blocker therapy | 55 (61%) | 465 (59%) | 0.74 |
| Renin-angiotensin system inhibitor therapy | 51 (57%) | 377 (48%) | 0.12 |
| Diuretic (loop or thiazide) therapy | 41 (46%) | 204 (26%) | <0.0001 |
| Statin therapy | 56 (62%) | 513 (65%) | 0.54 |
| Aspirin therapy | 67 (74%) | 636 (81%) | 0.13 |
| Antidepressant therapy | 16 (18%) | 152 (19%) | 0.71 |
| LV mass index (g/m2) | 104 ± 26 | 97 ± 26 | 0.007 |
| LV ejection fraction (%) | 62 ± 10 | 62 ± 9 | 0.66 |
| Diastolic dominant pulmonary vein flow (%) | 20 (22%) | 79 (10%) | 0.0007 |
| Wall motion score index | 1.19 ± 0.37 | 1.16 ± 0.34 | 0.49 |
| Inducible ischemia | 21 (23%) | 164 (21%) | 0.60 |
| Heart rate at rest (beats/min) | 67 ± 13 | 67 ± 13 | 0.89 |
| Systolic blood pressure (mm Hg) | 137 ± 23 | 133 ±21 | 0.09 |
| Diastolic blood pressure (mm Hg) | 72 ± 12 | 75 ±11 | 0.01 |
| Body mass index (kg/m2) | 28 ± 5 | 28 ± 6 | 0.54 |
| Creatinine clearance (ml/min) | 61 ± 25 | 84 ± 28 | <0.0001 |
Values are mean ± SD or numbers of patients (percentages).
In age-adjusted analyses, time- and frequency-domain measurements of mean HRV were lower in participants who had anemia (Table 2). In multivariable analyses, anemia remained associated with lower mean HRV, but this association was statistically significant only for very-low-frequency and wideband frequency power measurements (Table 2).
TABLE 2.
Heart Rate Variability in 873 Participants, Stratified by the Presence of Anemia (hemoglobin ≤12 g/dl)
| Age-Adjusted Mean (95% CI) | Adjusted Mean (95% CI)† | |||||
|---|---|---|---|---|---|---|
| Variable* | Anemia (n = 90) |
No Anemia (n = 784) |
p Value | Anemia (n = 90) |
No Anemia (n = 784) |
p Value |
| SDNN (ms) | 112 (104–120) | 123 (120–125) | 0.02 | 105 (97–113) | 111 (107–115) | 0.10 |
| SDANN (ms) | 100 (92–107) | 110 (107–112) | 0.01 | 93 (86–101) | 100 (96–104) | 0.06 |
| LnVLF (ms2) | 6.0 (5.8–6.2) | 6.4 (6.3–6.4) | 0.002 | 5.7 (5.5–5.9) | 6.0 (5.9–6.1) | 0.009 |
| LnLF (ms2) | 5.0 (4.8–5.2) | 5.3 (5.2–5.4) | 0.004 | 5.1 (4.9–5.3) | 5.2 (5.1–5.3) | 0.22 |
| LnHF (ms2) | 4.4 (4.2–4.6) | 4.4 (4.3–4.4) | 0.73 | 4.4 (4.2–4.7) | 4.4 (4.3–4.6) | 0.82 |
| LnWBF (ms2) | 6.5 (6.3–6.7) | 6.9 (6.8–6.9) | 0.005 | 6.3 (6.1–6.6) | 6.6 (6.4–6.8) | 0.02 |
Values for VLF and WBF were available for only 478 participants.
All variables listed in Table 1 were entered into the multivariable models. Other variables associated with SDNN (at p <0.05) were smoking, diabetes, angina, CABG, asthma/COPD, β-blocker use, renin-angiotensin system inhibitor use, diuretic use, and heart rate at rest. Other variables associated with SDANN were smoking, diabetes, angina, CABG, asthma/COPD, β-blocker use, renin-angiotensin system inhibitor use, diuretic use, and heart rate at rest. Other variables associated with VLF were gender, current smoking, history of stroke, history of diabetes, heart rate at rest, and systolic blood pressure. Other variables associated with LF were current smoking, history of hypertension, history of diabetes, CABG, and heart rate at rest. Other variables associated with HF were age, history of diabetes, angina, previous CABG, heart rate at rest, and diastolic dominant pulmonary vein flow. Other variables associated with WBF were gender, current smoking, history of diabetes, history of stroke, renin-angiotensin system inhibitor use, previous CABG, heart rate at rest, systolic blood pressure, and diastolic dominant pulmonary vein flow.
CABG = coronary artery bypass grafting; CI = confidence interval; COPD = chronic obstructive pulmonary disease; LnHF = natural-log high frequency power (0.15 to 0.40 Hz); LnLF = natural-log low frequency power (0.04 to 0.15 Hz); LnVLF = natural-log very low frequency power (0.0033 to 0.04 Hz); LnWBF = natural-log wideband frequency power (0.0033 to 1.7070 Hz); SDANN = SD of 5-minute mean NN intervals; SDNN = SD of NN intervals.
When HRV was examined as a dichotomous outcome (defined as the lowest quartile of each HRV index), the presence of anemia remained associated with low HRV (Figure 1). Of the 90 participants who had anemia, 29% to 41% had low HRV compared with 23% to 25% of the 784 participants who did not have anemia (p values <0.05 for all HRV indexes except high-frequency power). With the exception of high-frequency power, each decrease in hemoglobin of 1 g/dl was associated with increased odds of being in the lowest quartile of HRV, and this association remained strong after adjusting for potential confounding variables (Table 3).
Figure 1.
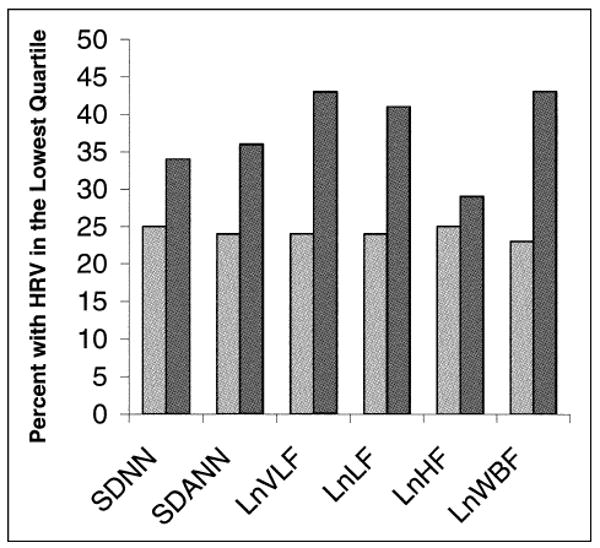
Proportion of participants who had HRV in the lowest quartile according to presence (hemoglobin ≤ 12 g/dl, n = 90) (dark gray bars) and absence (n = 784) (light gray bars) of anemia. p <0.05 for association of anemia with all HRV indexes except high frequency. LnHF = natural-log high-frequency power; LnLF = natural-log low-frequency power; LnVLF = natural-log very-low-frequency power; LnWBF = natural-log wideband frequency power; SDANN = SD of 5-minute mean NN intervals; SDNN = SD of NN intervals.
TABLE 3.
Association of Each Gram-per-Deciliter Decrease in Hemoglobin With Low Heart Rate Variability in Older Adults with Coronary Heart Disease*
| Unadjusted | Adjusted† | |||
|---|---|---|---|---|
| Variable | OR (95% CI) | p Value | OR (95% CI) | p Value |
| Lowest quartile SDNN (≤95 ms) | 1.1 (1.0–1.3) | 0.02 | 1.2 (1.0–1.3) | 0.008 |
| Lowest quartile SDANN (≤83 ms) | 1.2 (1.1–1.3) | 0.004 | 1.2 (1.1–1.3) | 0.002 |
| Lowest quartile LnVLF (≤5.85 ms2) | 1.3 (1.1–1.5) | 0.001 | 1.3 (1.1–1.5) | 0.001 |
| Lowest quartile LnLF (≤4.67 ms2) | 1.2 (1.1–1.4) | 0.0001 | 1.2 (1.1–1.4) | 0.0003 |
| Lowest quartile LnHF (≤3.66 ms2) | 1.0 (0.9–1.1) | 0.89 | 1.0 (0.9–1.2) | 0.51 |
| Lowest quartile LnWBF (≤6.29 ms2) | 1.2 (1.1–1.4) | 0.003 | 1.3 (1.1–1.4) | 0.002 |
This is the first reported association of anemia and low HRV in patients who have CHD. Several small studies have found decreased HRV in selected patients who have anemia.1–4 However, no study has examined the association between anemia and HRV in a broad spectrum of outpatients, nor has a study demonstrated an association between anemia and low HRV in patients who have CHD.
In this study, anemia was associated with depressed very-low-frequency, low-frequency, and wideband frequency power but not with depressed high-frequency power. This finding is in accordance with previous studies that have shown depressed very-low frequency, low-frequency, and wideband frequency power but not high-frequency power to be predictive of ventricular tachycardia and cardiac events.8,9,19 Low-frequency power is believed to reflect the modulation of sympathetic and parasympathetic tones,16 whereas high-frequency power is believed to reflect pure parasympathetic tone.17 Although the precise physiologic meaning of very-low-frequency power is not completely understood, it has been suggested that very-low-frequency power is influenced by thermoregulation, fluctuation in the renin-angiotensin axis, function of peripheral chemoreceptors, and physical activity,9 all of which may be associated with adverse cardiovascular outcomes.
Acknowledgments
We are grateful to Gentiae Clinical Research, Inc., San Bruno, California, for analysis of the 24-hour ambulatory electrocardiographic recordings.
This work was supported by grants from the Department of Veterans Affairs (Epidemiology Merit Review Program), Washington, DC; the Robert Wood Johnson Foundation (Generalist Physician Faculty Scholars Program), Princeton, New Jersey; the American Federation for Aging Research (Paul Beeson Faculty Scholars in Aging Research Program), New York, New York; and the Ischemia Research and Education Foundation, South San Francisco, California. None of these funding sources had any role in the collection of data, interpretation of results, or preparation of this report.
References
- 1.Aytemir K, Aksoyek S, Buyukasik Y, Haznedaroglu I, Atalar E, Ozer N, Ovunc K, Ozmen F, Oto A. Assessment of autonomic nervous system functions in patients with vitamin B12 deficiency by power spectral analysis of heart rate variability. Pacing Clin Electrophysiol. 2000;23:975–978. doi: 10.1111/j.1540-8159.2000.tb00883.x. [DOI] [PubMed] [Google Scholar]
- 2.Sozen AB, Demirel S, Akkaya V, Kudat H, Tukek T, Yeneral M, Ozcan M, Guven O, Korkut F. Autonomic dysfunction in vitamin B12 deficiency: a heart rate variability study. J Auton Nerv Syst. 1998;71:25–27. doi: 10.1016/s0165-1838(98)00058-7. [DOI] [PubMed] [Google Scholar]
- 3.Franzoni F, Galetta F, Di Muro C, Buti G, Pentimone F, Santoro G. Heart rate variability and ventricular late potentials in beta-thalassemia major. Haematologica. 2004;89:233–234. [PubMed] [Google Scholar]
- 4.Romero Mestre JC, Hernandez A, Agramonte O, Hernandez P. Cardiovascular autonomic dysfunction in sickle cell anemia: a possible risk factor for sudden death? Clin Auton Res. 1997;7:121–125. doi: 10.1007/BF02308838. [DOI] [PubMed] [Google Scholar]
- 5.Aronson D, Mittleman MA, Burger AJ. Measures of heart period variability as predictors of mortality in hospitalized patients with decompensated congestive heart failure. Am J Cardiol. 2004;93:59–63. doi: 10.1016/j.amjcard.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Bilchick KC, Fetics B, Djoukeng R, Fisher SG, Fletcher RD, Singh SN, Nevo E, Berger RD. Prognostic value of heart rate variability in chronic congestive heart failure (Veterans Affairs' Survival Trial of Antiarrhythmic Therapy in Congestive Heart Failure) Am J Cardiol. 2002;90:24–28. doi: 10.1016/s0002-9149(02)02380-9. [DOI] [PubMed] [Google Scholar]
- 7.Bonaduce D, Petretta M, Marciano F, Vicario ML, Apicella C, Rao MA, Nicolai E, Volpe M. Independent and incremental prognostic value of heart rate variability in patients with chronic heart failure. Am Heart J. 1999;138:273–284. doi: 10.1016/s0002-8703(99)70112-2. [DOI] [PubMed] [Google Scholar]
- 8.Galinier M, Pathak A, Fourcade J, Androdias C, Curnier D, Varnous S, Boveda S, Massabuau P, Fauvel M, Senard JM, Bounhoure JP. Depressed low frequency power of heart rate variability as an independent predictor of sudden death in chronic heart failure. Eur Heart J. 2000;21:475–482. doi: 10.1053/euhj.1999.1875. [DOI] [PubMed] [Google Scholar]
- 9.Hadase M, Azuma A, Zen K, Asada S, Kawasaki T, Kamitani T, Kawasaki S, Sugihara H, Matsubara H. Very low frequency power of heart rate variability is a powerful predictor of clinical prognosis in patients with congestive heart failure. Circ J. 2004;68:343–347. doi: 10.1253/circj.68.343. [DOI] [PubMed] [Google Scholar]
- 10.Nolan J, Batin PD, Andrews R, Lindsay SJ, Brooksby P, Mullen M, Baig W, Flapan AD, Cowley A, Prescott RJ, et al. Prospective study of heart rate variability and mortality in chronic heart failure: results of the United Kingdom heart failure evaluation and assessment of risk trial (UK-heart) Circulation. 1998;98:1510–1516. doi: 10.1161/01.cir.98.15.1510. [DOI] [PubMed] [Google Scholar]
- 11.Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health-related quality of life: the Heart and Soul Study. JAMA. 2003;290:215–221. doi: 10.1001/jama.290.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silverberg DS, Wexler D, Blum M, Keren G, Sheps D, Leibovitch E, Brosh D, Laniado S, Schwartz D, Yachnin T, et al. The use of subcutaneous erythropoietin and intravenous iron for the treatment of the anemia of severe, resistant congestive heart failure improves cardiac and renal function and functional cardiac class, and markedly reduces hospitalizations. J Am Coll Cardiol. 2000;35:1737–1744. doi: 10.1016/s0735-1097(00)00613-6. [DOI] [PubMed] [Google Scholar]
- 13.Tanner H, Moschovitis G, Kuster GM, Hullin R, Pfiiffner D, Hess OM, Mohacsi P. The prevalence of anemia in chronic heart failure. Int J Cardiol. 2002;86:115–121. doi: 10.1016/s0167-5273(02)00273-5. [DOI] [PubMed] [Google Scholar]
- 14.Silverberg DS, Wexler D, Iaina A. The importance of anemia and its correction in the management of severe congestive heart failure. Eur J Heart Fail. 2002;4:681–686. doi: 10.1016/s1388-9842(02)00115-0. [DOI] [PubMed] [Google Scholar]
- 15.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 16.Koizumi K, Terui N, Kollai M. Effect of cardiac vagal and sympathetic nerve activity on heart rate in rhythmic fluctuations. J Auton Nerv Syst. 1985;12:251–259. doi: 10.1016/0165-1838(85)90065-7. [DOI] [PubMed] [Google Scholar]
- 17.Fouad FM, Tarazi RC, Ferrario CM, Fighaly S, Alicandri C. Assessment of parasympathetic control of heart rate by a noninvasive method. Am J Physiol. 1984;246:H838–H842. doi: 10.1152/ajpheart.1984.246.6.H838. [DOI] [PubMed] [Google Scholar]
- 18.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 19.Huikuri HV, Koistinen MJ, Yli-Mayry S, Airaksinen KE, Seppanen T, Ikaheimo MJ, Myerburg RJ. Impaired low-frequency oscillations of heart rate in patients with prior acute myocardial infarction and life-threatening arrhythmias. Am J Cardiol. 1995;76:56–60. doi: 10.1016/s0002-9149(99)80801-7. [DOI] [PubMed] [Google Scholar]


