Abstract
As HIV-positive women live longer lives, and as testing for HIV becomes more routine, clinicians can expect to see more HIV-positive women in their practices. The need to be aware of management issues particular to this population becomes increasingly important. Metabolic dysregulation is a common, long-term complication associated with HIV and is one of the most difficult to manage. Hormonal contraception also is associated with metabolic dysregulation. As more HIV-positive women choose long-term, reversible contraception, the potential for concomitant and additive side effects, and the need for careful, proactive management strategies to avoid these complications, will become more important. This article reviews research detailing the metabolic dysfunction associated with hormonal contraception and with HIV-seropositivity. It highlights reasons for concern regarding the potential, although as yet theoretical, increased risk for metabolic dysfunction when hormonal contraception is used in the presence of HIV. Suggestions for management strategies for women living with HIV who choose to use hormonal contraception are presented. These strategies should be viewed as suggestions for management until substantitive research becomes available.
Keywords: glucose, HIV, hormonal contraception, insulin, lipids, metabolic dysregulation, osteoporosis
INTRODUCTION
Over the past 25 years, HIV disease has become a chronic health condition rather than a lethal diagnosis.1 Antiretroviral therapy has increased the lifespan of individuals infected with HIV in developed countries, and new treatment options promise further increases in the length and quality of life. In September 2006, the Centers for Disease Control and Prevention (CDC) recommended that screening for HIV be included in the general consent for medical care without the requirement of a separate, written consent for HIV screening.2 This change was made in recognition of the improved outcomes associated with antiretroviral therapy, and to better address the need to identify HIV-positive individuals who have not yet been diagnosed.2 For pregnant women, the routine panel of baseline prenatal screening should include HIV, with re-screening in the third trimester for those who are at high risk of acquisition, who present with symptoms of acute HIV infection, or who reside in a community with HIV incidence among women of childbearing age greater than or equal to 17 HIV cases per 100,000 person-years.2
Healthcare providers can anticipate seeing more HIV-positive individuals in their practices. While medical management of HIV should remain in the hands of specialists, primary care providers such as midwives may continue to manage the general and reproductive health concerns of HIV-positive women.
Most women living with HIV are of reproductive age.3 Because women constitute the majority of individuals younger than 25 years of age who are infected with HIV,4 control of fertility and the option of effective, reversible birth control will become a more significant issue as these women choose to have families and to space, rather than avoid, pregnancies. Midwives must be aware of the current clinical issues unique to HIV-positive populations, such as the long-term complications of HIV disease and its treatment. Metabolic dysregulation is a common and difficult-to-manage complication and may be of particular concern for HIV-positive women.
This article reviews the theoretical association between hormonal contraception, HIV and its treatment, and metabolic dysregulation, highlighting the potential for concomitant adverse effects. Suggestions for the management of hormonal contraception in HIV-positive women are presented. We review components of a baseline evaluation that may be particularly germane to evaluating metabolic risk. We then recommend additional laboratory and radiologic tests that providers may wish to consider, based on the theoretical concerns discussed. As concerns regarding increased risk of metabolic dysregulation in HIV-positive women using hormonal birth control are theoretical at present, there are no accepted standards for the management and follow-up of hormonal contraceptive use in this context. Definitive management strategies are expected to evolve as formative data become available.
BACKGROUND
Metabolic Dysregulation: Definition, Diagnosis, and Risk Factors
Metabolic dysregulation refers to a wide range of alterations in glucose, insulin, lipid, or bone metabolism, from precursor conditions such as impaired glucose tolerance, impaired fasting glucose, insulin resistance, dyslipidemia, and decreased bone mineral density (BMD), to clinically significant conditions such as type 2 diabetes, cardiovascular disease, or fractures. Glucose and lipid abnormalities are easily diagnosed via blood tests (Table 1). Decreased BMD may be diagnosed using dual energy x-ray absorptiometry.7 Currently, there is no practical way of identifying insulin resistance in the clinical setting.
Table 1.
Diagnosis of Glucose, Lipid, and Bone Mineral Density Abnormalities
| Biochemical Abnormalities | Diagnostic Criteria |
|---|---|
| Glucose abnormalitiesb | |
| Prediabetes | FPG 100–125 mg/dLa = impaired fasting glucose 2-hr post–50 g glucose load 140–199 mg/dLa = impaired glucose tolerance |
| Diabetes | Symptoms of diabetes plus random plasma glucose ≥200 mg/dLa or 2-hr post–75 g glucose load ≥200 mg/dLa |
| Fasting lipid abnormalitiesc | |
| Total cholesterol | 200–239 mg/dL = borderline high ≥240 mg/dL = high |
| HDL | <50 mg/dL |
| LDL | 100–129 mg/dL = near optimal/above optimal 130–159 mg/dL = borderline high 160–189 mg/dL = high ≥190 mg/dL = very high |
| Triglycerides | 150–199 mg/dL = borderline 200–499 mg/dL = high ≥500 mg/dL = very high |
| Bone mineral densityd | |
| Osteopenia | BMD between 1 and 2.5 SD below that of a “young normal” adult (T-score between −1 and −2.5) |
| Osteoporosis | BMD is 2.5 SD or more below that of a “young normal” adult (T-score ≤ −2.5). Women in this group who have already experienced one or more fractures are deemed to have severe or established osteoporosis. |
BMD = bone mineral density; FPG = fasting plasma glucose; HDL = high-density lipoprotein; LDL = low-density lipoprotein; SD = standard deviation; TG = triglycerides.
Note: Must be confirmed by repeat testing on a different day if abnormal.
Data taken from The American Diabetes Association.5
Data taken from The National Cholesterol Education Program.6
Data taken from the National Osteoporosis Foundation.61
Numerous clinical, behavioral, and sociodemographic factors are related to an increased risk of metabolic dysfunction. The key risk factors are summarized in Table 2. HIV infection and its treatment8,9 are also associated with an increased risk of metabolic dysfunction, as is the use of hormonal contraception.10
Table 2.
Risk Factors for Metabolic Dysfunction Found in the General Population
| Type 2 Diabetes Mellitus |
| Increasing age |
| Overweight or obesity as defined by increased waist circumference >35 inches or BMI ≥25 kg/m2 |
| Non-white race/ethnicity |
| Family history of type 2 diabetes mellitus |
| History of gestational diabetes or neonate weighing >9 pounds |
| History of polycystic ovarian syndrome (PCOS) |
| Hypertension (BP >130/80) |
| Physical inactivity |
| Dyslipidemia |
| Cardiovascular Disease |
| Increasing age |
| Overweight or obesity as defined by increased waist circumference >35 inches or BMI ≥25 kg/m2 |
| Non-white race/ethnicity |
| Family history of cardiovascular disease in first-degree relative |
| Osteoporosis |
| Increasing age |
| Female gender |
| Family/personal history of fractures as an adult |
| Race (white and Asian) |
| Small-boned and thin women |
| Menopause or cessation of menses |
| Lifestyle: cigarette smoking, excessive alcohol consumption (>2 drinks/day), inadequate calcium consumption, little or no weight- bearing exercise |
| Medications: glucocorticoids, excessive thyroid hormones, anticonvulsants, antacids containing aluminum, gonadotropin releasing hormones, methotrexate, heparin, or cholestyramine |
BMI = body mass index; BP = blood pressure.
Adapted from the National Osteoporosis Foundation.61
HIV
In 1985, 7% of AIDS cases in the United States were diagnosed in women; by 2003, the incidence of AIDS in women had increased to 26% of cases.11 Currently, more than 300,000 women in the United States are living with HIV/AIDS, and most are of reproductive age.3,12,13 Hispanic and African American women constitute 82% of HIV/AIDS cases among women in the United States.14 This issue is of particular importance to midwives, given their involvement in the care of under-served, vulnerable populations.
Contraception
Contraceptive use in the United States is very common, with 98% of all women who have ever had intercourse reporting having used at least one contraceptive method.15 Of the various methods available, hormonal contraception is used most frequently. In the 2002 National Survey of Family Growth, a survey of contraceptive use and method choice among men and women in the United States between the ages of 15 and 44 years, 82% of women reported ever having used oral contraceptive pills (OCPs).15 A growing number of women are using other forms of hormonal contraception, including depot medroxyprogesterone acetate (DMPA), progestin-only implants, contraceptive patches, vaginal rings, and progestin-containing intrauterine devices.
Few studies have investigated contraceptive use in women living with HIV. Contraceptive choice may differ between women who do and those who do not have HIV,16 but this conclusion may be confounded by the woman’s age, which impacts contraceptive choice.15,17 In a nationwide longitudinal cohort study of HIV-negative and -positive women, Massad et al.17 reported that among 20-year-old women, almost 40% use hormonal contraception, while 40% used barrier methods. The proportion using hormonal contraception decreases with increasing age. Sterilization and choosing not to contracept are more common among older members of the cohort.
Extensive literature supports an increased risk of dysregulated glucose, insulin, lipid, and bone metabolism associated with the use of hormonal contraception in HIV-negative populations.10,18–20 This risk may be more pronounced in women who are already at increased risk for metabolic dysregulation, including Hispanic and African American women,21 women with a history of gestational diabetes,22 and, theoretically, women living with HIV.
Metabolic Dysregulation in Women Who Are HIV-Positive and Who Use Hormonal Contraception
HIV-positive women find themselves at the intersection of multiple epidemics. Most US women who are HIV-positive are Hispanic or African American.14,23 In the general population, both Hispanics and African Americans are at increased risk for type 2 diabetes,24 cardiovascular disease,25 and metabolic syndrome, compared to whites.26 Therefore, these women may be at increased risk for metabolic dysfunction for a variety of reasons in addition to their HIV status.27
Hormonal contraception may represent an additional risk for metabolic dysfunction. The pattern of metabolic dysregulation associated with hormonal contraceptive use is very similar to the metabolic changes associated with HIV disease10,28 (Table 3). Both include glucose intolerance and insulin resistance. Changes in triglycerides, high density lipoprotein (HDL), and BMD demonstrate a more complex relationship to hormonal contraception: the alterations seen likely reflect the type and dose of the hormones used.
Table 3.
Metabolic Changes Associated with Contraceptive Hormones, HIV Disease, and Antiretroviral Medications
| Contraceptive Method or HIV-Related Disorder | Glucose Metabolism | Insulin | HDL | LDL | Triglycerides | BMD |
|---|---|---|---|---|---|---|
| Estrogen use | Intolerancea | Resistancea | Increased | Decreased | Elevated | Increased |
| Progestin use | Unclearb | |||||
| Gonane | Intolerancec | Resistancec | Decreasedd | Increased (first-generation) | Increasede Decreasedf | |
| Estrane | Minimal influenceg | Minimal influenceg | Increased—minimal influence | Minimal influence | May decrease | — |
| Drospirenone | Minimal influence | Minimal influence | Decreased—minimal influence | Minimal influence | Minimal influence | — |
| DMPA use | Intolerance | Resistance | Decreased | Increased | Increased | Decreased |
| HIV disease | Intolerance | Resistanceh | Decreased (host response) | Decreasedi | Increasedj | Decreasedk |
| HIV-related lipodystrophy | Intolerance | Resistance | — | N/A | Increased | — |
| Use of antiviral medications | N/A | Unclear | ||||
| Nucleoside reverse transcriptase inhibitors | Intolerance | Resistance | Minimal influence | — | May increase | — |
| Non-nucleoside reverse transcriptase inhibitors | Minimal influence | Minimal influence | Increased | — | Increasedl | — |
| Protease inhibitors | Intolerancel | Resistancel | Minimal influencem | — | Increasedl | — |
BMD = bone mineral density; DMPA = depot medroxyprogesterone acetate; HDL = high-density lipoprotein; LDL = low-density lipoprotein.
With supplemental estrogen, as in oral contraceptive preparations.
In combined formulations, the impact is unclear. Progestin-only formulations appear to have negative impact with prolonged use.
The third-generation gonane progestins, gestodene and desogestrel, have a less negative impact.
May be increased with third-generation gonane progestins.
Desogestrel and gestodene.
Levonorgestrel.
May contribute to glucose intolerance and/or insulin resistance in women with multiple risk factors for metabolic dysregulation.
Untreated HIV disease causes increased insulin sensitivity.
A decrease is seen in untreated HIV. The increase in LDL that may be seen in individuals on antiretroviral therapy is thought to reflect a return to health rather than a side effect of treatment.
With onset of AIDS.
HIV-disease overall is associated with decreased BMD, but whether it is the HIV-disease itself, antiretroviral therapy, or some other factor is unknown.
Individual drugs within the class may not have identical effects or may not have the same magnitude of effect.
Minimal influence directly, but may indirectly cause decrease, through the increase in triglyceride levels.
To date, there are no data regarding additive effects of hormonal contraception use in the presence of HIV on the degree or onset of metabolic dysfunction in HIV-positive individuals. Nonetheless, the potential for metabolic interactions theoretically exists. Therefore, the causes of metabolic dysregulation in HIV-positive women and in women who use hormonal contraception are reviewed here, with suggestions for clinical evaluation and management.
HIV AND METABOLIC DYSREGULATION
Insulin and Glucose Dysregulation
The cause of HIV-related insulin and glucose dysregulation is unclear but is likely multifactoral. The potential causes include body fat composition changes (lipodystrophy),29 factors related to the HIV disease process, including inflammation and direct viral effects,30 duration and severity of HIV disease,31 the choice of antiretroviral therapy (both class and individual drug choice) and duration of use,32 as well as risk factors found in the general population.30
Lipodystrophy and Insulin and Glucose Dysregulation
HIV-related lipodystrophy syndrome, which closely resembles the phenotype of the rare congenital and acquired lipodystrophy syndrome, was first described in 1997–199833,34 in patients using protease inhibitors. Until recently, research suggested two distinct forms of therapy-induced lipodystrophy. Lipoatrophy is the loss of subcutaneous fat throughout the body, especially in the face, limbs, and gluteal region.30 Total body fat may be normal or low, and patients may note a progressive weight loss.30 Lipohypertrophy, in contrast, consists of an increase in total weight and body fat, with an increase in waist size caused by intra-abdominal fat accumulation. Breast enlargement, as well as dorsocervical fat pads (buffalo hump or fat pad on the back of the neck) can also be seen.30 Lipoatrophy and lipodystrophy were reported to occur in isolation and in combination. However, recent reports comparing body fat changes over time in both HIV-positive and -negative women indicate that lipohypertrophy may not be directly associated with antiretroviral therapy, nor can it be routinely found in persons with peripheral lipoatrophy.35–38 Many authors now propose that HIV-related lipodystrophy involves peripheral as well as central lipoatrophy,39,40 while central fat accumulation may be more a product of non–HIV-related factors, such as aging or a restoration to health phenomenon after starting antiretroviral therapy.
In HIV-positive individuals who use highly active antiretroviral therapy (HAART), the prevalence of lipodystrophy has been reported to range from 2% to 84%.41 The large range may be a consequence of many factors, including inconsistencies in terminology and lack of a universally accepted case definition.36,42 Lipodystrophy typically appears after approximately 10 to 18 months of HAART treatment.43
HIV-related lipodystrophy has been reported to be associated with glucose and lipid dysregulation,44 although this is not always the case. Some have suggested that the increase in free fatty acid levels associated with lipodystrophy may be responsible for the increase in insulin resistance.45
HIV Disease-Related Factors and Insulin and Glucose Dysregulation
The role of HIV disease-related factors in metabolic dysregulation has also been considered. The literature supports a link between inflammation and insulin resistance as proinflammatory cytokines stimulate lipolysis and inhibit adipose tissue lipogenesis, resulting in lipids being deposited in other tissues, most notably in the liver or muscle, which can lead to insulin resistance in that tissue.46 In HIV-positive patients, increasing severity and longer duration of HIV disease, as well as a greater degree of immune system recovery, appear to be associated with metabolic dysregulation.47 Whether this dysregulation occurs because of stimulation of proinflammatory pathways, however, is unclear. What might stimulate these proinflammatory pathways in the HIV-positive individual is equally uncertain: lipodystrophy and metabolic dysregulation are seen most often in individuals who have complete viral suppression, making it difficult to link virologically induced inflammation with metabolic dysregulation. Furthermore, in studies done before the widespread use of antiretroviral therapy, HIV-positive men were found to be more insulin sensitive than their HIV-negative counterparts,48 thus calling into question the association between HIV-related inflammation and insulin resistance. While the precise mechanisms of HIV-related metabolic dysfunction are unclear, it has been well established to be associated with HIV disease.
Antiretroviral Therapy and Insulin and Glucose Dysregulation
The role of antiretroviral therapy in metabolic dysregulation has been the subject of intense scrutiny, and our understanding of the mechanisms involved has evolved over time.49–51 Many health practitioners initially believed that the new drug combinations, also referred to as “cocktails” or HAART, which included protease inhibitors, were particularly associated with metabolic dysfunction.52 Subsequently, the contribution of protease inhibitors to metabolic dysfunction vis-à-vis that of the other classes of antiretrovirals used in the cocktails, especially the nucleoside reverse transcriptase inhibitors, has been called into question.
Viewing glucose and insulin dysregulation as a class effect also has changed. Recent research suggests that the class effect, or the contribution of protease inhibitors versus nucleoside reverse transcriptase inhibitors, for example, may be less important than the individual contributions of each drug.8,9,50 For example, while indinavir (Crixivan; Merck & Co., Inc., Whitehouse Station, NJ), a protease inhibitor, has a significant impact on glucose metabolism, atazanavir (Reyataz; Bristol-Myers Squibb, New York, NY), another protease inhibitor, appears to be fairly benign. The one exception to this rule is non-nucleoside reverse transcriptase inhibitors; this class of drugs does not seem to contribute directly to glucose or insulin dysregulation.53
Research has elucidated multiple pathways, both direct and indirect, by which antiretroviral therapy alters glucose and insulin metabolism. Protease inhibitors directly interfere with glucose metabolism by blocking glucose transport into the cell, which is most likely a transient effect.32,50 The direct effects of nucleoside reverse transcriptase inhibitors on glucose metabolism have yet to be characterized. Direct effects of individual medications on metabolic dysregulation have not been established for all antiretroviral therapy agents, especially those belonging to the newly emerging drug classes.
Indirectly, medication-induced changes such as lipodystrophy likely contribute to glucose and insulin dysregulation. Both nucleoside reverse transcriptase inhibitors and protease inhibitors likely contribute to the development of HIV-related lipodystrophy. In addition, nucleoside reverse transcriptase inhibitors may exert an indirect influence on glucose homeostasis and insulin action via their toxic effect on mitochondria.54
It is well accepted that antiretroviral therapy is an important contributor to insulin and glucose dysregulation in HIV-positive patients, and our knowledge of how antiretroviral therapy contributes to this dysregulation has grown significantly. However, the multiplicity of mechanisms that link antiretroviral use with glucose and insulin dysregulation, and the fact that not all of the drugs within a class are identical in terms of the degree to which they contribute to the dysregulation, have made it difficult to determine the exact nature of the mechanisms involved.
HIV Disease-Related Factors and Lipid Dysregulation
Dyslipidemia, much like insulin resistance and glucose intolerance, likely results both from disease- and antiretroviral therapy-related processes. Untreated, HIV infection results in a substantial decrease in serum total cholesterol, HDL, and LDL levels, as is seen in other infectious diseases. Triglyceride levels, on the other hand, increase with the onset of AIDS.55 After the initiation of antiretroviral therapy, increases in LDL and total cholesterol are seen without a significant change in HDL. These increases in LDL and total cholesterol were originally associated with antiretroviral therapy, but are now understood to reflect a return to health and to preinfection lipid levels, in addition to specific age-related changes.55 The lack of response to antiretroviral therapy seen with HDL may indicate a host response to the virus that results in decreased HDL rather than a response to antiretroviral therapy.29
Antiretroviral Therapy and Lipid Dysregulation
Antiretroviral medications, especially protease inhibitors, are clearly associated with some of the lipid abnormalities seen in HIV-infected persons. The increase in triglycerides is attributable to specific therapies and to the development of lipodystrophy.44 Insulin resistance may play a role in the increase in triglycerides, but abnormal lipid pathways, exclusive of those related to insulin resistance, also have been proposed. Lipid abnormalities can also exist in the absence of measurable insulin resistance.44
Different protease inhibitors have dramatically different effects on hyperlipidemia, with ritonavir (Norvir; Abbott Laboratories, Abbott Park, IL) causing the most serious elevations.56 Among the non-nucleoside reverse transcriptase inhibitors, efavirenz (Sustiva; Bristol-Myers Squibb) may have a more negative effect than nevirapine (Viramune; Boehringer Ingelheim Pharmaceuticals, Ridgefield, CT) on triglyceride levels, although not all studies support this conclusion.50 Nevirapine has also been shown to boost HDL levels,57 and efavirenz may do the same.58
HIV and Dysregulation of Bone Metabolism
HIV-positive patients have lower BMD than their seronegative counterparts, although the severity of this decrease varies across studies. Prevalence rates of osteopenia, osteoporosis, or both in HIV-positive patients of all ages range from 16% to 66%.59,60 In the general population, 55% of individuals aged 50 years and older have either osteoporosis or osteopenia.61 Not only is decreased BMD more common among HIV-positive individuals than among persons who are not HIV-positive, but bone loss in seropositive populations also begins at a younger age than in seronegative populations.60 While decreased BMD appears to be common in HIV-positive populations, only a few reported cases of fractures exist.62
While the increased incidence of low BMD in HIV-positive populations is well documented, there is much less agreement regarding its etiology. Non-HIV-related risk factors, such as age, gender, family history, race, tobacco use, or inadequate calcium intake, play a role in these outcomes. However, controlling for these factors does not eliminate the association between HIV seropositivity and low BMD.59 The initial assumption was that HIV-related decreases in BMD were related to protease inhibitor use, but subsequent research questions this assumption.59,60 Some have suggested that increased levels of proinflammatory cytokines may play a role in osteoclast activation and bone resorption.63 Others have hypothesized a direct interaction between HIV and cells of the bone and bone marrow, chronic T-cell activation, HIV-related disturbances of calcium homeostasis, and altered vitamin D metabolism, as well as increased rates of opportunistic infections or neoplastic diseases.63 Hormonal deficiencies associated with HIV (hypoandrogenism), a history of wasting or poor nutrition, and past steroid use are also possible contributors,64 as is lactic acidosis related to nucleoside reverse transcriptase inhibitor-associated mitochondrial toxicity.65
Summary
HIV and its treatment can lead to increases in fasting insulin and insulin resistance, along with increases in blood glucose values. The etiology of this dysregulation is doubtless multifactoral. Antiretroviral therapy is a major contributor, but lipodystrophy and HIV disease-related factors likely contribute as well.
Different lipid subgroups are differently influenced by HIV disease and its treatment. Increased triglycerides are most closely associated with medication choice and lipodystrophy, while increases in LDL and total cholesterol more likely reflect a return to health and normal age-related alterations. The persistent decrease in HDL that begins with the onset of HIV is more likely related to a host response to the virus, although an increase in HDL may be associated with non-nucleoside reverse transcriptase inhibitor use. No definitive evidence links these lipid abnormalities to adverse clinical outcomes. However, there is evidence suggesting an association between LDL66 and HDL67 levels and abnormalities in intermediate endpoints, such as carotid intimal-medial thickness or endothelial dysfunction. Evidence linking HIV-related lipid abnormalities to myocardial infarctions or to other clinical manifestations of cardiovascular disease has not yet been established.
Finally, while a decrease in BMD is associated with HIV and its treatment, the etiology of this decrease remains unclear. Fortunately, while decreased BMD is relatively common in populations with HIV, fractures are not.
HORMONAL CONTRACEPTION AND METABOLIC DYSREGULATION
The literature investigating the impact of hormonal contraception on metabolic outcomes is vast, and much of it is contradictory or ambiguous. The mechanisms by which hormonal contraception influences metabolic processes are equally ambiguous.
Hormonal Contraception and Insulin and Glucose Dysregulation
Both estrogen and progesterone are implicated in the etiology of insulin resistance and the deterioration of glucose homeostasis during pregnancy; however, the specific contributions of each are unknown. The impact of synthetic contraceptive hormones, individually and synergistically, in nonpregnant women is even less clear.
Both estrogen deficiency and pharmacologic levels of estrogen are associated with insulin resistance. Estrogen deficiency is associated with deterioration in glucose homeostasis and with insulin resistance,68 while replacement to normal levels restores glucose homeostasis and insulin sensitivity.10 In pharmacologic doses, as can be found in contraceptive formulations, estrogen is often positively associated with insulin resistance68 in a dose-dependent fashion.
The effects of progestins on glucose tolerance and insulin sensitivity differ according to progestin type, method of administration, and dose. Progestins fall into four different classes: gonane progestins, estrane progestins, a spironolactone-derived progestin, and pregnane progestins. First-generation gonane progestins, norgestrel and levonorgestrel, uniformly cause a progressive decrease in glucose tolerance as well as an increase in insulin resistance, both fasting and postglucose load, regardless of formulation, dose, or method of administration.20 The newer generation gonane progestins, gestodene and desogestrel, have demonstrated a significantly improved metabolic profile over that of their predecessors,69 although the tendency of desogestrel to increase the half-life of insulin may mask the presence of insulin resistance.10
Norethindrone, the most common of the estrane progestins used in contraceptive formulations, has minimal effects on glucose tolerance.70 Some authors, however, suggest that norethindrone use in women with metabolic risk factors may lead to clinically significant, detrimental outcomes.22 Kjos et al.22 found that in Hispanic mothers with a history of gestational diabetes mellitus, postpartum use of a norethindrone-only oral contraceptive, along with breastfeeding, increased the woman’s risk for type 2 diabetes as compared with nonusers and non-breastfeeding mothers using combined oral contraceptives. The negative impact of unopposed progestin could be aggravated by the hormone flux that occurs in the postpartum state and with breastfeeding, and thus one cannot extrapolate to women who are neither postpartum nor breastfeeding. However, the impact of hormonal contraception on metabolic outcomes may differ significantly between young, healthy women with few metabolic risk factors, and those with significant background risk for metabolic dysfunction.
Drospirenone, one of the newer progestins used in newer combined oral contraceptive formulations (e.g., Yasmin; Berlex Laboratories, Richmond, CA), has minimal effects on glucose and insulin levels in healthy women, even with prolonged use. It is more specific for the progesterone receptors than other progestins, which may account for its lower side-effect profile.69
In considering the impact of progestins, not only the type but the mode of administration may be important. The different modalities (pills, injection, and implants) often involve different progestins, so it is difficult to determine if it is the type of progestin, the mode of administration, or both that are responsible for the variations in outcomes. Oral, low-dose, progestin-only contraceptives are generally thought to have minimal metabolic effects, bearing in mind the qualification described above.71 However, intramuscular administration of DMPA, one of the pregnane progestins, and levonorgestrel implants are consistently associated with worsening glucose tolerance and insulin resistance.20 In clinical trials, duration of exposure to DMPA and levonorgestrel implants has contributed to the effect of these hormonal contraceptives on glucose metabolism and insulin action. Longer exposure leads to more severe dysregulation, especially in regard to insulin resistance.20 The risk of DMPA causing abnormal glucose tolerance seems to be particularly elevated in women with other risk factors for diabetes. In a cohort of Navajo women, a population at high risk for type 2 diabetes, users of DMPA were more likely to develop type 2 diabetes than nonusers, and longer use of DMPA was associated with greater risk.21
Hormonal Contraception and Lipid Dysregulation
Serum lipids and lipoproteins are also altered in women who use hormonal contraceptives, the nature of the change depending on the specific contraceptive used. Very low-density lipoproteins (VLDLs), HDL, and triglycerides are most significantly impacted. Estrogen increases serum levels of HDL and VLDL,72 while progestins appear to decrease HDL levels.72 The introduction of triphasic regimens provided formulations with reduced progestin doses and minimal impact on HDL.72
Elevations in triglycerides likely result from the estrogen-induced increase in hepatic VLDL synthesis.72 Estrogens may also alter lipoprotein lipase activity, shifting the flux of triglycerides from storage in adipose tissue to usage in skeletal muscle.73 Progestins also can contribute to this dysregulation: desogestrel and gestodene are associated with elevations in triglycerides, as is DMPA.74 Levonorgestrel implants, however, reduce triglyceride concentrations in a dose-dependent manner.72
LDL is also affected by exogenous hormones. Estrogen may increase LDL receptor activity, thus promoting LDL and remnant uptake by cells and decreasing plasma LDL levels.75 While first-generation progestins antagonize this effect, the newer progestins may not.72 There is some concern that OCPs, while not altering LDL levels, may actually increase the proportion of more atherogenic LDL subfractions.75 However, this concern may not be relevant: HDL and triglyceride levels, and not LDL, may be more predictive of cardiovascular disease risk in women than men.76
Hormonal Contraception and Dysregulation of Bone Metabolism
The relationship between OCP use and BMD is unclear. Clinical studies are inconsistent and have indicated a positive association between OCP use, decreased BMD, and the occurrence of fractures,77 no association between OCP use and any measure of bone metabolism,78 and in other studies, a positive association between OCP use and increased BMD.79 This inconsistency of outcomes may be indicative of a lack of association between OCP use and bone metabolism, or may be attributable to differences in study design or contraceptive formulation.
Progestin-only contraceptives have a more consistently negative impact on BMD than do OCPs, although levonorgestrel implants appear to have a neutral effect, possibly because of the androgenicity of levonorgestrel compared to other progestins. DMPA is of particular concern. The Food and Drug Administration (FDA) has published a black box warning highlighting the possibility that prolonged DMPA use results in a significant decrease in BMD. The degree of loss is proportional to the duration of DMPA use and may not be completely reversible, although this point remains controversial.80–82 DMPA may not necessarily be the culprit, because DMPA use is associated with other conditions that are confounding risk factors for low BMD, such as poor calcium intake, tobacco and alcohol use, and insufficient weight-bearing exercise.83
The impact of DMPA on BMD is likely the result of the consequent estrogen deficiency.84 In former DMPA users, BMD is identical to that of never users at menopause.85 In women using DMPA through the menopausal transition, DMPA-related bone loss does not seem to progress.82 However, there is little information regarding fracture risk in women who use DMPA through the menopausal transition as compared to nonusers. Thus the long-term impact of reduced BMD is uncertain. Of greater concern is the possibility that the use of DMPA during the early reproductive years may impair attainment of peak bone mass, which generally occurs around age 30, theoretically placing a woman at greater risk for postmenopausal osteopenia/osteoporosis and fractures.86
Summary
Despite the complex and sometimes contradictory nature of the data on hormonal contraception and metabolic dysregulation, some important conclusions emerge. Hormonal contraception may have an adverse impact on glucose metabolism and insulin resistance, regardless of the dose of estrogen or the type, dose, or mode of delivery of the progestin, although some of the low-dose oral formulations may actually improve insulin resistance. Insulin resistance may be present in women who use hormonal contraception, but in otherwise healthy, low-risk women, the short-term clinical significance appears to be negligible. In women already at risk for metabolic dysregulation, however, the additional metabolic stress caused by hormonal contraception may lead to adverse outcomes, including increased rates of type 2 diabetes.21,22 Progestin-only methods appear to be especially problematic.
The impact of hormonal contraception on lipid metabolism is more straightforward, although any evidence of clinically important outcomes or disease is lacking. Estrogen increases serum levels of HDL and VLDL, the latter of which is at least partly responsible for the increase in triglycerides often seen in those using oral contraceptives. Estrogen also decreases plasma LDL. Progestins decrease HDL levels, but the lower doses of progestins found in triphasic regimens appear to have minimal impact on HDL. Some progestins also increase triglycerides, especially the newer generation gonane progestins and DMPA.
While there is much debate over the impact of combined hormonal contraception on BMD, DMPA has a clearly negative, albeit reversible, effect.
HIV, HORMONAL CONTRACEPTION, AND METABOLIC DYSREGULATION: THEORETICAL CONCERNS
While the actual combined risk for metabolic dysregulation in women who are both HIV seropositive and use hormonal contraception remains unknown, the potential for synergy exists. As the literature demonstrates, women who have risk factors for metabolic dysregulation are more likely to develop metabolic dysregulation while on hormonal contraception than those who do not. HIV-seropositivity certainly appears to be a risk factor for metabolic dysregulation. Of course, whether the relationship between hormonal contraception and HIV is similar to that between hormonal contraception and other risk factors for metabolic dysregulation has not been established.
Although there are no data-based recommendations regarding the use of hormonal contraception in women living with HIV infection, clinicians should consider the potential complications that may result from this combination of risks. Clinical management decisions in providing birth control for HIV-positive women should be made collaboratively by midwives, their consulting physicians, and an HIV specialist.
PROPOSED CLINICAL MANAGEMENT OF WOMEN LIVING WITH HIV WHO CHOOSE TO USE HORMONAL CONTRACEPTION
In the following section, we suggest information that may be assessed during a routine history and exam when an HIV-positive woman requests contraception, which particularly targets risk factors for metabolic dysregulation. In addition, we present laboratory and radiologic studies that providers may wish to consider. These tests can provide information on baseline metabolic abnormalities and provide a basis for later comparison, thus allowing prompt determination of adverse metabolic changes.
Baseline Evaluation
General History
Given the theoretical risk of increased metabolic dysregulation when hormonal contraception is used in the context of HIV, the authors suggest that HIV-positive women who present for birth control be evaluated at their baseline visit for all of the risk factors for metabolic dysregulation found in the general population (Table 2). In addition, information regarding complications with previous contraceptive use as well as complications during previous pregnancies (for example, a history of gestational diabetes) is important, as is a thorough behavioral history that would include past or present tobacco, alcohol and substance abuse, and any history of depression.
HIV-Specific History
A thorough HIV-related history should focus on those aspects of HIV and its treatment that have been found to be associated with metabolic dysregulation. The year of diagnosis of HIV may be important, because a longer duration may be associated with increased risk of metabolic dysfunction.9 A history of opportunistic infections suggests more severe HIV disease,11 and information about treatments, especially recent or ongoing steroid use, short-term for Pneumocystis jerovici pneumonia or longer-term for wasting, can help with making an individual risk assessment. Nadir CD4 count and peak viral load also will help determine the severity of HIV disease; knowing the current CD4 count and viral load will allow the provider to evaluate the degree of immune function and level of viral control. The larger the gap between nadir and current CD4 count, or between peak and current viral load, the greater the risk for metabolic dysfunction.9 A complete history of antiretroviral therapy use, including specific medications used, duration of use, and current regimens, can inform the provider about the nature and duration of antiretroviral therapy exposure, both by drug class and individual drug.
Information on current therapy is important in view of the fact that many antiretroviral medications, including but not limited to nevirapine (Viramune) and ritonavir (Norvir), can decrease ethinyl estradiol levels (reproductive hormones and antiretroviral therapy share a common metabolic pathway in the liver).87–89 This decrease in serum levels of ethinyl estradiol can potentially reduce the efficacy of OCPs. Unlike ethinyl estradiol, which is subject to first-pass metabolism, levonorgestrel implants, DMPA, and other non-oral formulations are not.90 However, all contraceptive steroid hormones are substrates of the CYP3A4 microsomal enzyme system, and if they are used in the presence of CYP3A4 enzyme inducers, their serum levels may be reduced.90–92 No studies have investigated serum levonorgestrel levels in the presence of antiretroviral therapy. DMPA levels have been studied in the presence of nelfinavir (Viracept; Pfizer US, New York, NY), efavirenz (Sustiva), and nevirapine (Viramune), and no significant changes in any of the hormone parameters were noted in comparison to control groups at 2, 4, 6, 8, 10, and 12 weeks after DMPA dosing.16 Studies of CYP450 interactions have not yet been conducted for the contraceptive patch or the ring.
Women should also be asked if they have noticed any changes in body fat distribution, especially an increase in bra size, the presence of a buffalo hump, or a loss of fat in the arms, legs, or face, because these changes may indicate the presence of lipodystrophy.
Physical Examination
A baseline physical examination is important, paying particular attention to the presence of hypertension (blood pressure >130/80), obesity (waist circumference >35 inches or BMI ≥25 kg/m2), and acanthosis nigricans, the presence of velvety, light brown-to-black markings, which are usually found on the neck, under the arms, or in the groin area, and that may indicate the presence of insulin resistance. The provider should also evaluate for the presence of lipodystrophy by looking for a buffalo hump and for loss of fat in the face, arms, and legs.
Education
Although the risks of hormonal contraception in the setting of HIV disease are only theoretical, education regarding prevention of metabolic complications, namely diet and exercise, is important for all women. The importance of follow-up must be emphasized so that problems can be identified and addressed in a timely manner. Adequate calcium and vitamin D intake, weight-bearing exercise, smoking cessation, and decreasing alcohol consumption are important for reducing the risk of osteoporosis.
Alternative forms of contraception should always be discussed. Metabolic dysregulation may only be a potential adverse effect associated with use of OCPs, the contraceptive patch, levonorgestrel implants, and DMPA. Whether or not the systemic levels of levonorgestrel associated with use of the levonorgestrel-releasing intrauterine system93 are sufficient to cause metabolic dysregulation has not been adequately studied.94
Emergency contraception in the context of HIV has received minimal research attention.95 Because anti-retrovirals, progestins, and ethinyl estradiol use a common liver metabolic pathway, concern regarding the adequacy of hormone levels and efficacy in the presence of the antiretroviral medication is legitimate. These concerns should not lead to the avoidance of emergency contraception, but should prompt, instead, more stringent follow-up for potential ongoing pregnancy. Consultation with a contraceptive specialist may also be indicated.
Women who choose oral contraceptives should be informed of the necessity of using condoms (male or female) in addition to OCPs, in view of potentially decreased plasma levels of ethinyl estradiol/progestins in the face of antiretroviral therapy. Of course, all HIV-positive patients should be encouraged to use condoms even in seroconcordant relationships to protect themselves from other HIV strains as well as other sexually transmitted infections.
Additional Recommendations
Laboratory and Radiologic Studies
Given the theoretical increased risk for metabolic dysfunction in HIV-positive women who use hormonal contraception, we propose the performance of specific laboratory and radiologic studies. Although they are not part of any standard recommendation, we feel that these laboratory and radiologic studies are reasonable given the biologic plausibility of the theoretical concern herein presented. Furthermore, these studies will provide a useful baseline for later comparison and will identify metabolic dysfunction before beginning hormonal contraception. Fasting plasma glucose or a 75-gram oral glucose tolerance test, as well as fasting total cholesterol, LDL, HDL and triglycerides, are recommended. Obtaining a dual energy x-ray absorptiometry scan to identify pre-existing decreased BMD is controversial because it is unclear what the results mean for young women regarding current as well as postmenopausal fracture risk. Some studies have indicated an increase in BMD in HIV seropositive men who use alendronate, but the incidence of fractures in HIV-positive patients with osteoporosis is still quite low. The long-term benefits of this therapy, especially for women, as well as the long-term risks, are unknown. However, for women who were diagnosed with HIV at a young age, or who were infected at birth, who have multiple risk factors for decreased BMD (Table 2), and who are considering using DMPA before the attainment of peak bone mass, a baseline measure of BMD may be of value. Performance of such tests should be done in consultation with the HIV specialist.
If laboratory and/or radiologic studies are ordered, the initiation of hormonal contraception would ideally be deferred until the results of the laboratory studies are available. However, if the midwife feels that the patient is at risk for becoming pregnant before follow-up, the initiation of hormonal contraception at the baseline visit may be reasonable, along with an appointment for a return visit in 3 months, and a limited number of refills if OCPs are the contraceptive of choice.
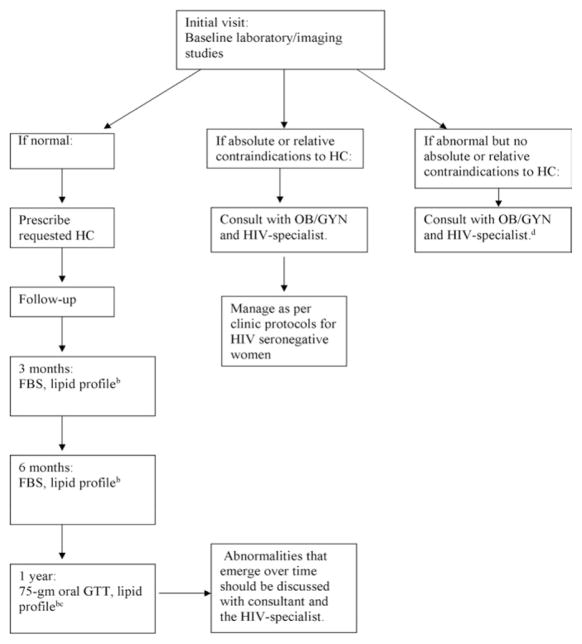
If the baseline physical exam and laboratory studies are normal, the woman can be started on the hormonal contraception of her choice and followed as indicated in Figure 1. Good communication with the HIV provider will be most helpful in providing adequate follow-up without redundant blood draws. Most HIV providers follow stable patients every 3 months, and laboratory studies could be coordinated allowing the midwife and the HIV provider to obtain the necessary tests without duplication of cost and effort.
Figure 1.
Proposed management plan if laboratory evaluation is included in management paradigm.a
aThese protocols are suggested for consideration only and should not be considered as definitive practice recommendations.
bLipid profile includes total cholesterol, high density lipoprotein, low density lipoprotein, and triglycerides.
cIf all results remain within normal limits, repeat every 6 months, alternating between FBS and GTT. Consider repeating dual energy x-ray absorptiometry in DMPA users every 3 years in patients with significant risk factors for osteoporosis, especially in those who started DMPA before 30 years of age.
dFollow-up as indicated for normal lab results may provide a useful baseline for the management of these women.
FBS = fasting blood sugar; HC = hormonal contraception; GTT = glucose tolerance test.
Severe hypercholesterolemia or hypertriglyceridemia are absolute contraindications to OCP use and relative contraindications to levonorgestrel implant use.96 Diabetes mellitus with evidence of end-organ damage is a relative contraindication to both OCP use and levonorgestrel implant use.96 Management of these relative or absolute contraindications should be the same in HIV-positive and HIV-negative women.
Laboratory abnormalities that do not constitute a relative or absolute contraindication to hormonal contraception use are more difficult to address. However, a discussion with both the consulting physician and the HIV specialist to determine the best approach for prescribing hormonal contraception and the appropriate follow-up is important. Equally central is a frank discussion with the patient to discuss the theoretical risks. Once the appropriateness of hormonal contraception and the patient’s preference have been determined and documented, the recommendations outlined in Figure 1 for the follow-up of women without lab abnormalities might be used as a starting point for the management discussion. Laboratory or radiographic abnormalities that emerge while a woman is using hormonal contraception should be managed on an individual basis and should be discussed both with the consultant and the HIV specialist.
CONCLUSIONS
Research suggests the potential for overlap of metabolic complications between HIV and its management, and the use of hormonal contraception. Both HIV and hormonal contraception use share a common risk profile for metabolic abnormalities, and while there is insufficient evidence that hormonal contraception aggravates the metabolic dysregulation associated with HIV, the literature suggests a need for caution and careful, conservative management. Denying a woman her choice of contraceptive because of an as-yet-hypothetical concern is inappropriate, but so is ignoring the potential for iatrogenic side effects. Research is needed to investigate these issues and to provide evidence-based practice guidelines for hormonal contraception management in HIV-positive women. Until then, conservative management that focuses on early identification of problems and close cooperation with and sharing of information among midwives, HIV providers, consulting physicians, and the women involved, seems appropriate.
Acknowledgments
Supported by the National Institute of Nursing Research (F31-NR-009886). We would like to thank Gina Novick, CNM, for her comments on an earlier draft of this article.
Contributor Information
Julie Womack, Doctoral candidate at the Yale School of Nursing, New Haven, CT.
Susan Richman, Assistant professor at the Yale School of Medicine, and section chief for the Yale Family Planning Program, New Haven, CT.
Phyllis C. Tien, Assistant professor with the Department of Medicine, University of California, San Francisco, and San Francisco Department of Veterans Affairs, San Francisco, CA.
Margaret Grey, Dean and Annie Goodrich Professor at the Yale School of Nursing, New Haven, CT.
Ann Williams, Professor of nursing and professor of medicine, Yale University, New Haven, CT.
References
- 1.Fang CT, Chang YY, Hsu HM, Twu SJ, Chen KT, Lin CC, et al. Life expectancy of patients with newly-diagnosed HIV infection in the era of highly active antiretroviral therapy. QJM. 2007;100:97–105. doi: 10.1093/qjmed/hcl141. [DOI] [PubMed] [Google Scholar]
- 2.Branson BM, Handsfield HH, Lampe MA, Janssen RS, Taylor AW, Lyss SB, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55:1–17. [PubMed] [Google Scholar]
- 3.Kaiser FF. [Accessed September 18, 2007];Fact sheet: Women and HIV/AIDS in the United States. Available from: www.kff.org/hivaids/upload/6092-04.pdf.
- 4.Centers for Disease Control and Prevention Web site. [Accessed February 7, 2008];HIV/AIDS surveillance in adolescents and young adults (through 2005) Available from: www.cdc.gov/hiv/topics/surveillance/resources/slides/adolescents/index.htm.
- 5.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2007;30(Suppl 1):S42–7. doi: 10.2337/dc07-S042. [DOI] [PubMed] [Google Scholar]
- 6.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 7.National Osteoporosis Foundation Web site. [Accessed May 31, 2007];Physician’s guide: Diagnosis. Available from: www.nof.org/physguide/diagnosis.htm.
- 8.Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352:48–62. doi: 10.1056/NEJMra041811. [DOI] [PubMed] [Google Scholar]
- 9.Nolan D. Metabolic complications associated with HIV protease inhibitor therapy. Drugs. 2003;63:2555–74. doi: 10.2165/00003495-200363230-00001. [DOI] [PubMed] [Google Scholar]
- 10.Godsland IF. The influence of female sex steroids on glucose metabolism and insulin action. J Intern Med Suppl. 1996;738:1–60. [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. HIV/AIDS Surveillance Report, 2003. Atlanta, GA: Centers for Disease Control and Prevention; 2004. [Google Scholar]
- 12.Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, et al. The Women’s Interagency HIV Study. WIHS Collaborative Study Group. Epidemiol. 1998;9:117–25. [PubMed] [Google Scholar]
- 13.Chiao EY, Ries KM, Sande MA. AIDS and the elderly. Clin Infect Dis. 1999;28:740–5. doi: 10.1086/515219. [DOI] [PubMed] [Google Scholar]
- 14.United Nations AIDS Programme Web site. UNAIDS/WHO. [Accessed January 2, 2007];AIDS epidemic update. Available from: http://data.unaids.org/pub/EpiReport/2006/2006_EpiUpdate_en.pdf.
- 15.Mosher WD, Martinez GM, Chandra A, Abma JC, Willson SJ. Use of contraception and use of family planning services in the United States: 1982–2002. Adv Data. 2004;10:1–36. [PubMed] [Google Scholar]
- 16.Stanwood NL, Cohn SE, Heiser JR, Pugliese M. Contraception and fertility plans in a cohort of HIV-positive women in care. Contraception. 2007;75:294–8. doi: 10.1016/j.contraception.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Massad LS, Evans CT, Wilson TE, Golub ET, Sanchez-Keeland L, Minkoff H, et al. Contraceptive use among U.S. women with HIV. J Womens Health (Larchmt) 2007;16:657–66. doi: 10.1089/jwh.2006.0204. [DOI] [PubMed] [Google Scholar]
- 18.Crook D, Godsland IF, Worthington M, Felton CV, Proudler AJ, Stevenson JC. A comparative metabolic study of two low-estrogen-dose oral contraceptives containing desogestrel or gestodene progestins. Am J Obstet Gynecol. 1993;169:1183–9. doi: 10.1016/0002-9378(93)90279-r. [DOI] [PubMed] [Google Scholar]
- 19.Wynn V, Godsland I. Effects of oral contraceptives on carbohydrate metabolism. J Reprod Med. 1986;31(9 Suppl):892–7. [PubMed] [Google Scholar]
- 20.Kahn HS, Curtis KM, Marchbanks PA. Effects of injectable or implantable progestin-only contraceptives on insulin-glucose metabolism and diabetes risk. Diabetes Care. 2003;26:216–25. doi: 10.2337/diacare.26.1.216. [DOI] [PubMed] [Google Scholar]
- 21.Kim C, Seidel KW, Begier EA, Kwok YS. Diabetes and depot medroxyprogesterone contraception in Navajo women. Arch Intern Med. 2001;161:1766–71. doi: 10.1001/archinte.161.14.1766. [DOI] [PubMed] [Google Scholar]
- 22.Kjos SL, Peters RK, Xiang A, Thomas D, Schaefer U, Buchanan TA. Contraception and the risk of type 2 diabetes mellitus in Latina women with prior gestational diabetes mellitus. JAMA. 1998;280:533–8. doi: 10.1001/jama.280.6.533. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention Web site. [Accessed February 7, 2008];HIV/AIDS surveillance report 2002. Available from: www.cdc.gov/hiv/topics/surveillance/resources/reports/index.htm.
- 24.Rowley D, Danel I, Berg C, Vinicor F. The reproductive years. In: Beckles GLA, Thompson-Reid R, editors. Diabetes and women’s health across the life stages. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Division of Diabetes Translation; 2001. [Google Scholar]
- 25.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111:1233–41. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 26.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: Findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–9. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 27.Silverberg MJ, Gore ME, French AL, Gandhi M, Glesby MJ, Kovacs A, et al. Prevalence of clinical symptoms associated with highly active antiretroviral therapy in the Women’s Interagency HIV Study. Clin Infect Dis. 2004;39:717–24. doi: 10.1086/423181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grinspoon SK. Metabolic syndrome and cardiovascular disease in patients with human immunodeficiency virus. Am J Med. 2005;118(Suppl 2):23S–8S. doi: 10.1016/j.amjmed.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 29.Grunfeld C, Tien P. Difficulties in understanding the metabolic complications of acquired immune deficiency syndrome. Clin Infect Dis. 2003;37(Suppl 2):S43–6. doi: 10.1086/375886. [DOI] [PubMed] [Google Scholar]
- 30.Kotler D. Challenges to diagnosis of HIV-associated wasting. J Acquir Immune Defic Syndr. 2004;37:S280–3. doi: 10.1097/01.qai.0000144383.55091.c1. [DOI] [PubMed] [Google Scholar]
- 31.Dube MP. Disorders of glucose metabolism in patients infected with human immunodeficiency virus. Clin Infect Dis. 2000;31:1467–75. doi: 10.1086/317491. [DOI] [PubMed] [Google Scholar]
- 32.Noor MA, Seneviratne T, Aweeka FT, Lo JC, Schwarz JM, Mulligan K, et al. Indinavir acutely inhibits insulin-stimulated glucose disposal in humans: A randomized, placebo-controlled study. AIDS. 2002;16:F1–8. doi: 10.1097/00002030-200203290-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Massip P, Marchou B, Bonnet E, Cuzin L, Montastruc JL. Lipodystrophia with protease inhibitors in HIV patients. Therapie. 1997;52:615. [PubMed] [Google Scholar]
- 34.Viraben R, Aquilina C. Indinavir-associated lipodystrophy. AIDS. 1998;12:F37–9. doi: 10.1097/00002030-199806000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Bacchetti P, Gripshover B, Grunfeld C, Heymsfield S, Mc-Creath H, Osmond D, et al. Fat distribution in men with HIV infection. J Acquir Immune Defic Syndr. 2005;40:121–31. doi: 10.1097/01.qai.0000182230.47819.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tien PC, Grunfeld C. What is HIV-associated lipodystrophy? Defining fat distribution changes in HIV infection. Curr Opin Infect Dis. 2004;17:27–32. doi: 10.1097/00001432-200402000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Study of Fat Redistribution and Metabolic Change in HIV Infection (FRAM) Fat distribution in women with HIV infection. J Acquir Immune Defic Syndr. 2006;42:562–71. doi: 10.1097/01.qai.0000229996.75116.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mulligan K, Anastos K, Justman J, Freeman R, Wichienkuer P, Robison E, et al. Fat distribution in HIV-infected women in the United States: DEXA substudy in the Women’s Interagency HIV Study. J Acquir Immune Defic Syndr. 2005;38:18–22. doi: 10.1097/00126334-200501010-00004. [DOI] [PubMed] [Google Scholar]
- 39.Tien PC, Cole SR, Williams CM, Li R, Justman JE, Cohen MH, et al. Incidence of lipoatrophy and lipohypertrophy in the Women’s Interagency HIV study. J Acquir Immune Defic Syndr. 2003;34:461–6. doi: 10.1097/00126334-200312150-00003. [DOI] [PubMed] [Google Scholar]
- 40.Gripshover B, Tien P, Saag M, Osmond D, Bacchetti P, Grunfeld C. Lipoatrophy is the dominant feature of the lipodystrophy syndrome in HIV-infected men. 10th Conference on Retroviruses and Opportunistic Infections; Boston, MA. February 10–14, 2003. [Google Scholar]
- 41.Leow MK, Addy CL, Mantzoros CS. Clinical review 159: Human immunodeficiency virus/highly active antiretroviral therapy-associated metabolic syndrome: clinical presentation, pathophysiology, and therapeutic strategies. J Clin Endocrinol Metab. 2003;88:1961–76. doi: 10.1210/jc.2002-021704. [DOI] [PubMed] [Google Scholar]
- 42.Lichtenstein KA. Redefining lipodystrophy syndrome: Risks and impact on clinical decision making. J Acquir Immune Defic Syndr. 2005;39:395–400. doi: 10.1097/01.qai.0000167478.28051.3a. [DOI] [PubMed] [Google Scholar]
- 43.Grinspoon S. Insulin resistance in the HIV-lipodystrophy syndrome. Trends Endocrinol Metab. 2001;9:413–9. doi: 10.1016/s1043-2760(01)00472-6. [DOI] [PubMed] [Google Scholar]
- 44.Hadigan C, Meigs JB, Corcoran C, Rietschel P, Piecuch S, Basgoz N, et al. Metabolic abnormalities and cardiovascular disease risk factors in adults with human immunodeficiency virus infection and lipodystrophy. Clin Infect Dis. 2001;1:130–9. doi: 10.1086/317541. [DOI] [PubMed] [Google Scholar]
- 45.Meininger G, Hadigan C, Laposata M, Brown J, Rabe J, Louca J, et al. Elevated concentrations of free fatty acids are associated with increased insulin response to standard glucose challenge in human immunodeficiency virus-infected subjects with fat redistribution. Metabolism. 2002;51:260–6. doi: 10.1053/meta.2002.29999. [DOI] [PubMed] [Google Scholar]
- 46.Pittas AG, Joseph NA, Greenberg AS. Adipocytokines and insulin resistance. J Clin Endocrinol Metab. 2004;89:447–52. doi: 10.1210/jc.2003-031005. [DOI] [PubMed] [Google Scholar]
- 47.Kotler DP. HIV infection and lipodystrophy. Prog Cardiovasc Dis. 2003;45:269–84. doi: 10.1053/pcad.2003.2. [DOI] [PubMed] [Google Scholar]
- 48.Hommes MJ, Romijn JA, Endert E, Eeftinck Schattenkerk JK, Sauerwein HP. Insulin sensitivity and insulin clearance in human immunodeficiency virus-infected men. Metabolism. 1991;6:651–6. doi: 10.1016/0026-0495(91)90059-6. [DOI] [PubMed] [Google Scholar]
- 49.Saag M, Powderly W, Schambelan M, Benson C, Carr A, Currier J, et al. Switching antiretroviral drugs for treatment of metabolic complications in HIV-1 infection: Summary of selected trials. Topics HIV Med. 2002;10:47–51. [Google Scholar]
- 50.Drechsler H, Powderly WG. Switching effective antiretroviral therapy: A review. Clin Infect Dis. 2002;35:1219–30. doi: 10.1086/343050. [DOI] [PubMed] [Google Scholar]
- 51.Sherer R. HIV, HAART, and hyperlipidemia: Balancing the effects. J Acquir Immune Defic Syndr. 2003;34(Suppl 2):S123–9. doi: 10.1097/00126334-200310012-00005. [DOI] [PubMed] [Google Scholar]
- 52.Carr A, Samaras K, Chisholm DJ, Cooper DA. Pathogenesis of HIV-1-protease inhibitor-associated peripheral lipodystrophy, hyperlipidaemia, and insulin resistance. Lancet. 1998;351:1881–3. doi: 10.1016/S0140-6736(98)03391-1. [DOI] [PubMed] [Google Scholar]
- 53.Tebas P, Yarasheski K, Henry K, Claxton S, Kane E, Bordenave B, et al. Evaluation of the virological and metabolic effects of switching protease inhibitor combination antiretroviral therapy to nevirapine-based therapy for the treatment of HIV infection. AIDS Res Hum Retroviruses. 2004;20:589–94. doi: 10.1089/0889222041217374. [DOI] [PubMed] [Google Scholar]
- 54.ter Hofstede HJ, Willems HL, Koopmans PP. Serum L-lactate and pyruvate in HIV-infected patients with and without presumed NRTI-related adverse events compared to healthy volunteers. J Clin Virol. 2004;29:44–50. doi: 10.1016/s1386-6532(03)00085-4. [DOI] [PubMed] [Google Scholar]
- 55.Grunfeld C, Kotler DP, Shigenaga JK, Doerrler W, Tierney A, Wang J, et al. Circulating interferon-alpha levels and hypertriglyceridemia in the acquired immunodeficiency syndrome. Am J Med. 1991;90:154–62. [PubMed] [Google Scholar]
- 56.Purnell JQ, Zambon A, Knopp RH, Pizzuti DJ, Achari R, Leonard JM, et al. Effect of ritonavir on lipids and post-heparin lipase activities in normal subjects. AIDS. 2000;14:51–7. doi: 10.1097/00002030-200001070-00006. [DOI] [PubMed] [Google Scholar]
- 57.van der Valk M, Kastelein JJ, Murphy RL, van Leth F, Katlama C, Horban A, et al. Nevirapine-containing antiretroviral therapy in HIV-1 infected patients results in an anti-atherogenic lipid profile. AIDS. 2001;15:2407–14. doi: 10.1097/00002030-200112070-00008. [DOI] [PubMed] [Google Scholar]
- 58.Negredo E, Ribalta J, Ferre R, Salazar J, Rey-Joly C, Sirera G, et al. Efavirenz induces a striking and generalized increase of HDL-cholesterol in HIV-infected patients. AIDS. 2004;18:819–21. doi: 10.1097/00002030-200403260-00017. [DOI] [PubMed] [Google Scholar]
- 59.Amiel C, Ostertag A, Slama L, Baudoin C, N’Guyen T, Lajeunie E, et al. BMD is reduced in HIV-infected men irrespective of treatment. J Bone Miner Res. 2004;19:402–9. doi: 10.1359/JBMR.0301246. [DOI] [PubMed] [Google Scholar]
- 60.Mondy K, Tebas P. Emerging bone problems in patients infected with human immunodeficiency virus. Clin Infect Dis. 2003;36(Suppl 2):S101–5. doi: 10.1086/367566. [DOI] [PubMed] [Google Scholar]
- 61.National Osteoporosis Foundation Web site. [Accessed November 26, 2007];Fast facts on osteoporosis. Available from: www.nof.org/osteoporosis/disease-facts.htm.
- 62.McComsey G, Huang J, Woolley I, Young B, Sax P, Gerber M, et al. Fragility fractures in HIV-infected subjects, an area for improvement. 11th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA. February 8–11, 2004. [Google Scholar]
- 63.Kuhne CA, Heufelder AE, Hofbauer LC. Bone and mineral metabolism in human immunodeficiency virus infection. J Bone Miner Res. 2001;16:2–9. doi: 10.1359/jbmr.2001.16.1.2. [DOI] [PubMed] [Google Scholar]
- 64.Mondy K, Yarasheski K, Powderly WG, Whyte M, Claxton S, DeMarco D, et al. Longitudinal evolution of bone mineral density and bone markers in human immunodeficiency virus-infected individuals. Clin Infect Dis. 2003;36:482–90. doi: 10.1086/367569. [DOI] [PubMed] [Google Scholar]
- 65.Carr A, Miller J, Eisman JA, Cooper DA. Osteopenia in HIV-infected men: Association with asymptomatic lactic acidemia and lower weight pre-antiretroviral therapy. AIDS. 2001;15:703–9. doi: 10.1097/00002030-200104130-00005. [DOI] [PubMed] [Google Scholar]
- 66.Hsue PY, Lo JC, Franklin A, Bolger AF, Martin JN, Deeks SG, et al. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation. 2004;109:1603–8. doi: 10.1161/01.CIR.0000124480.32233.8A. [DOI] [PubMed] [Google Scholar]
- 67.Solages A, Vita JA, Thornton DJ, Murray J, Heeren T, Craven DE, et al. Endothelial function in HIV-infected persons. Clin Infect Dis. 2006;42:1325–32. doi: 10.1086/503261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Godsland IF. Oestrogens and insulin secretion. Diabetologia. 2005;48:2213–20. doi: 10.1007/s00125-005-1930-0. [DOI] [PubMed] [Google Scholar]
- 69.Gaspard U, Scheen A, Endrikat J, Buicu C, Lefebvre P, Gerlinger C, et al. A randomized study over 13 cycles to assess the influence of oral contraceptives containing ethinylestradiol combined with drospirenone or desogestrel on carbohydrate metabolism. Contraception. 2003;67:423–9. doi: 10.1016/s0010-7824(02)00537-1. [DOI] [PubMed] [Google Scholar]
- 70.Spellacy WN. Carbohydrate metabolism during treatment with estrogen, progestogen, and low-dose oral contraceptives. Am J Obstet Gynecol. 1982;142(6 Pt 2):732–4. doi: 10.1016/s0002-9378(16)32479-6. [DOI] [PubMed] [Google Scholar]
- 71.Ludicke F, Gaspard UJ, Demeyer F, Scheen A, Lefebvre P. Randomized controlled study of the influence of two low estrogen dose oral contraceptives containing gestodene or desogestrel on carbohydrate metabolism. Contraception. 2002;66:411–5. doi: 10.1016/s0010-7824(02)00415-8. [DOI] [PubMed] [Google Scholar]
- 72.Godsland IF, Crook D. Update on the metabolic effects of steroidal contraceptives and their relationship to cardiovascular disease risk. Am J Obstet Gynecol. 1994;170(5 Pt 2):1528–36. doi: 10.1016/s0002-9378(94)05015-5. [DOI] [PubMed] [Google Scholar]
- 73.Livingstone C, Collison M. Sex steroids and insulin resistance. Clin Sci (Lond) 2002;102:151–66. doi: 10.1042/cs1020151. [DOI] [PubMed] [Google Scholar]
- 74.Garza-Flores J, de la Cruz DL, Valles de Bourges V, Sanches-Nincio R, Martinez M, Fuziwara JL, Perez-Palacios G. Long-term effects of depot-medroxyprogesterone acetate on lipoprotein metabolism. Contraception. 1991;44:61–71. doi: 10.1016/0010-7824(91)90106-p. [DOI] [PubMed] [Google Scholar]
- 75.Knopp RH, LaRosa JC, Burkman RT., Jr Contraception and dyslipidemia. Am J Obstet Gynecol. 1993;168(6 Pt 2):1994–2005. doi: 10.1016/s0002-9378(12)90941-2. [DOI] [PubMed] [Google Scholar]
- 76.Bass KM, Newschaffer CJ, Klag MJ, Bush TL. Plasma lipoprotein levels as predictors of cardiovascular death in women. Arch Intern Med. 1993;153:2209–16. [PubMed] [Google Scholar]
- 77.Vessey M, Mant J, Painter R. Oral contraception and other factors in relation to hospital referral for fracture. Findings in a large cohort study. Contraception. 1998;57:231–5. doi: 10.1016/s0010-7824(98)00026-2. [DOI] [PubMed] [Google Scholar]
- 78.Bahamondes L, Juliato CT, Villarreal M, Sobreira-Lima B, Simoes JA, dos Santos Fernandes AM. Bone mineral density in users of two kinds of once-a-month combined injectable contraceptives. Contraception. 2006;74:259–63. doi: 10.1016/j.contraception.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 79.Cobb KL, Kelsey JL, Sidney S, Ettinger B, Lewis CE. Oral contraceptives and bone mineral density in white and black women in CARDIA. Coronary Risk Development in Young Adults. Osteoporos Int. 2002;13:893–900. doi: 10.1007/s001980200123. [DOI] [PubMed] [Google Scholar]
- 80.Federal Drug Agency Web site. [Accessed April 9, 2007];Physician information, 2004. Available from: www.fda.gov/medwatch/SAFETY/2004/DepoProvera_Label.pdf.
- 81.Kaunitz AM. Depo-Provera’s black box: Time to reconsider? Contraception. 2005;72:165–7. doi: 10.1016/j.contraception.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 82.Cundy T, Cornish J, Roberts H, Reid IR. Menopausal bone loss in long-term users of depot medroxyprogesterone acetate contraception. Am J Obstet Gynecol. 2002;186:978–83. doi: 10.1067/mob.2002.122420. [DOI] [PubMed] [Google Scholar]
- 83.Albertazzi P, Bottazzi M, Steel SA. Bone mineral density and depot medroxyprogesterone acetate. Contraception. 2006;73:577–83. doi: 10.1016/j.contraception.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 84.Ott SM, Scholes D, LaCroix AZ, Ichikawa LE, Yoshida CK, Barlow WE. Effects of contraceptive use on bone biochemical markers in young women. J Clin Endocrinol Metab. 2001;86:179–85. doi: 10.1210/jcem.86.1.7118. [DOI] [PubMed] [Google Scholar]
- 85.Orr-Walker BJ, Evans MC, Ames RW, Clearwater JM, Cundy T, Reid IR. The effect of past use of the injectable contraceptive depot medroxyprogesterone acetate on bone mineral density in normal post-menopausal women. Clin Endocrinol (Oxf) 1998;49:615–8. doi: 10.1046/j.1365-2265.1998.00582.x. [DOI] [PubMed] [Google Scholar]
- 86.Lara-Torre E, Edwards CP, Perlman S, Hertweck SP. Bone mineral density in adolescent females using depot medroxyprogesterone acetate. J Pediatr Adolesc Gynecol. 2004;17:17–21. doi: 10.1016/j.jpag.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 87.Ouellet D, Hsu A, Qian J, Locke CS, Eason CJ, Cavanaugh JH, et al. Effect of ritonavir on the pharmacokinetics of ethinyl oestradiol in healthy female volunteers. Br J Clin Pharmacol. 1998;46:111–6. doi: 10.1046/j.1365-2125.1998.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mildvan D, Yarrish R, Marshak A, Hutman HW, McDonough M, Lamson M, et al. Pharmacokinetic interaction between nevirapine and ethinyl estradiol/norethindrone when administered concurrently to HIV-infected women. J Acquir Immune Defic Syndr. 2002;29:471–7. doi: 10.1097/00126334-200204150-00007. [DOI] [PubMed] [Google Scholar]
- 89.Lamson M, MacGregor T, Riska P, Erickson D, Maxfield P, Rowland L, et al. Nevirapine induces both CYP3A4 and CYP2B6 metabolic pathways. Clin Pharmacol Ther. 1999;65:137. [Google Scholar]
- 90.Cohn SE, Park JG, Watts DH, Stek A, Hitti J, Clax PA, et al. Depo-medroxyprogesterone in women on antiretroviral therapy: Effective contraception and lack of clinically significant interactions. Clin Pharmacol Ther. 2007;81:222–7. doi: 10.1038/sj.clpt.6100040. [DOI] [PubMed] [Google Scholar]
- 91.Crawford P. Interactions between antiepileptic drugs and hormonal contraception. CNS Drugs. 2002;16:263–72. doi: 10.2165/00023210-200216040-00005. [DOI] [PubMed] [Google Scholar]
- 92.McCann MF, Potter LS. Progestin-only oral contraception: A comprehensive review. Contraception. 1994;50(6 Suppl 1):S1–195. [PubMed] [Google Scholar]
- 93.Backman T. Benefit-risk assessment of the levonorgestrel intrauterine system in contraception. Drug Saf. 2004;27:1185–204. doi: 10.2165/00002018-200427150-00003. [DOI] [PubMed] [Google Scholar]
- 94.Kayikcioglu F, Gunes M, Ozdegirmenci O, Haberal A. Effects of levonorgestrel-releasing intrauterine system on glucose and lipid metabolism: A 1-year follow-up study. Contraception. 2006;73:528–31. doi: 10.1016/j.contraception.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 95.Delvaux T, Nostlinger C. Reproductive choice for women and men living with HIV: Contraception, abortion and fertility. Reprod Health Matters. 2007;15(29 Suppl):46–66. doi: 10.1016/S0968-8080(07)29031-7. [DOI] [PubMed] [Google Scholar]
- 96.Speroff L, Fritz M. Clinical gynecologic endocrinology and infertility. 7. Philadelphia: Lippincott Williams & Wilkins; 2005. [Google Scholar]