Abstract
Background
Whether B-type natriuretic peptide (BNP) levels can be used to screen for ventricular dysfunction in patients at risk of heart failure but without overt symptoms is not known. We examined the characteristics of a BNP test for identifying systolic and diastolic dysfunction in outpatients with stable coronary disease.
Methods
In a cross-sectional study of 293 outpatients who had stable coronary disease and no history of heart failure, we compared elevations in plasma BNP levels with echocardiography for the diagnosis of systolic dysfunction (ejection fraction <55%) and diastolic dysfunction (diastolic dominant pulmonary vein flow with ejection fraction ≥55%).
Results
A total of 48 patients (16%) had systolic dysfunction, and among the remaining 245 with preserved systolic function, 31 (13%) had diastolic dysfunction. At the standard cutpoint of >100 pg/mL, an elevated BNP level was 38% sensitive (80% specific) for systolic dysfunction and 55% sensitive (85% specific) for diastolic dysfunction. Negative likelihood ratios were 0.8 (95% confidence interval [CI]: 0.6 to 1.0) for systolic dysfunction and 0.5 (95% CI: 0.4 to 0.8) for diastolic dysfunction. Positive likelihood ratios were 1.9 (95% CI: 1.2 to 2.9) for systolic dysfunction and 3.8 (95% CI: 2.4 to 5.9) for diastolic dysfunction. Areas under the receiver operating characteristic curves were 0.59 (95% CI: 0.49 to 0.69) for systolic dysfunction and 0.79 (95% CI: 0.71 to 0.87) for diastolic dysfunction.
Conclusion
These data suggest that BNP is not a useful screening test for asymptomatic ventricular dysfunction in patients with stable coronary disease.
B-type natriuretic peptide (BNP) is a neurohormone that is secreted from the ventricle in response to elevated volume and filling pressures (1–3). Elevated BNP levels are highly sensitive and specific for distinguishing heart failure from other causes of dyspnea in symptomatic patients (4–7), and strongly predict systolic and diastolic dysfunction in patients referred for echocardiography or cardiac catheterization for evaluation of symptoms of heart failure (8–14).
The ease and low cost associated with a plasma test for heart failure has led to the suggestion that BNP levels could also be used to screen for ventricular dysfunction among patients without overt symptoms of heart failure (15–17); furthermore, there are data supporting the early initiation of medical therapy for systolic dysfunction even before the onset of symptoms (18,19). Based in part on this evidence, the current American Heart Association guidelines recommend initiation of both angiotensin-converting enzyme inhibitors and beta-blockers in patients with asymptomatic left ventricular systolic dysfunction (20).
Evaluating the potential use of BNP measurement as a screening test for ventricular dysfunction requires examination of BNP levels in patients without dyspnea because the differing prevalence and spectrum of disease in patients with or without overt symptoms of heart failure may result in different sensitivities and specificities for ventricular dysfunction. To determine whether BNP levels can be used to identify patients with asymptomatic systolic or diastolic dysfunction, we investigated the association between plasma BNP levels and ventricular dysfunction in patients with stable coronary disease. Since patients with coronary disease are at high risk of heart failure (21), this group represents a relevant target population in which a BNP screening strategy might reasonably be adopted.
Methods
Study Participants
The Heart and Soul Study is a prospective cohort study of how psychosocial factors influence the outcomes of patients with coronary disease. We recruited patients with coronary disease who were identified through administrative databases from two Department of Veterans Affairs Medical Centers (San Francisco and Palo Alto, California) and one university medical center (University of California, San Francisco). Eligible participants had at least one of the following criteria: a history of myocardial infarction; angiographic evidence of ≥50% stenosis in one or more coronary vessels; evidence of exercise-induced ischemia by treadmill electrocardiogram or stress nuclear perfusion imaging; a history of coronary revascularization; or a clinical diagnosis of coronary disease as documented by an internist or a cardiologist.
Eligible patients were invited by mail to attend a baseline study appointment, and a total of 510 participants were enrolled between September 2000 and December 2001. Patients were excluded if they were unable to walk one block or were planning to move out of the local area within 3 years. For this cross-sectional study, we excluded participants for whom we could not obtain a blood sample (due to dislodged or thrombosed butterfly needle) after the 30-minute rest, leaving 368 participants. We excluded another 75 patients who reported a history of heart failure (as indicated by a “yes” answer to the question, “Has a doctor or nurse ever told you that you have congestive heart failure?”), leaving 293 patients for this analysis. The Institutional Review Board at each of the sites approved this protocol. All participants provided written informed consent.
Measurements
B-type natriuretic peptide
Prior to the study appointment, subjects completed an overnight fast, except for taking their regularly prescribed medications. After a 30-minute supine rest, blood samples were drawn into chilled ethylenediaminetetraacetic acid tubes, mixed with aprotinin, then aliquoted and stored at −70°C for up to 9 months. We used the Triage BNP fluorescence immunoassay (Biosite Diagnostics, La Jolla, California) to measure BNP levels in frozen plasma samples thawed to room temperature. The lowest detectable measurement for this assay was 5 pg/mL. The interassay coefficient of variation was 10.1% for 28.8 pg/mL, 12.4% for 586 pg/mL, and 16.2% for 1180 pg/mL. The laboratory technician who measured BNP levels was at a different site and blinded to the results of the echocardiogram.
Systolic and diastolic dysfunction
After plasma was drawn and frozen, participants underwent resting echocardiography using an Acuson Sequoia Ultrasound System (Mountain View, California) with a 3.5-MHz transducer. A complete resting two-dimensional echocardiogram, including imaging and Doppler in all standard views and subcostal imaging of the inferior vena cava, was performed. We obtained standard two-dimensional parasternal short-axis and apical two- and four-chamber views during held inspiration; these were planimetered using a computerized digitization system to determine end-diastolic and end-systolic left ventricular volume and ejection fraction. One author (NBS) interpreted all of the echocardiograms, blinded to the results of the BNP assay.
For the primary analysis, we considered patients with an ejection fraction <55% to have systolic dysfunction. As a secondary analysis, we also evaluated the association between BNP levels and more severe systolic dysfunction, using a lower cutpoint (ejection fraction <45%). We categorized patients as having diastolic dysfunction if they had an ejection fraction ≥55% and the velocity time integral in their pulmonary vein was greater during diastole than during systole (22).
Other measurements
Participants underwent full exercise treadmill testing using a standard Bruce protocol with continuous 12-lead electrocardiographic monitoring. An echocardiogram was performed immediately before and following exercise. Inducible ischemia was defined as the presence of new wall motion abnormalities at peak exercise or electrocardiographic evidence of ischemia during exercise. Self-reported age, sex, ethnicity, medical history, and smoking status were determined by questionnaire. Alcohol use was measured using the AUDIT-C questionnaire (23), with a score of ≥4 indicating regular alcohol use. Self-reported physical activity was determined using the question, “Which of the following statements best describes how physically active you have been during the last month, that is, done activities such as 15–20 minutes of brisk walking, swimming, general conditioning, or recreational sports?” Participants who answered “fairly”, “quite”, “very”, or “extremely active” (vs. “not at all” or “a little active”) were considered physically active. We measured angina symptoms using the angina subscale of the Seattle Angina Questionnaire (24), and defined current angina as a score of <75 on the 100-point scale. We also measured weight and height, and calculated body mass index (kg/m2).
Medication use was assessed with a detailed interview. Subjects were instructed to bring their medication bottles to the study appointment during which study personnel recorded all medications. Levels of creatinine, total cholesterol, and high-density lipoprotein and low-density lipoprotein cholesterol were measured from sera after the overnight fast. We calculated creatinine clearance from 24-hour urine collections.
Data Analysis
We divided subjects into those with plasma BNP levels ≤100 pg/mL and those with levels >100 pg/mL, based on a cutpoint used in prior studies (6,7). Differences in characteristics were compared using the Student t test and chi-squared test, as appropriate.
We compared BNP values in subjects with and without ventricular dysfunction using a Wilcoxon rank sum test. To determine the association of BNP levels with systolic and diastolic dysfunction, we used logistic regression analyses with levels >100 pg/mL as the independent variable and ventricular dysfunction as the dependent variable. To obtain adjusted risk estimates, we entered all variables associated with elevated BNP levels (at P <0.10) into multivariable models. For the logistic regression analyses, we report odds ratios with 95% confidence intervals. Since beta-blockers, renin-angiotensin system inhibitors, and diuretics are important medications in the treatment of heart failure, we tested for interactions between BNP values and use of these medications. We also tested for interactions between BNP level and age based on reports from previous studies (25).
We calculated sensitivity, specificity, and likelihood ratios (with exact binomial 95% confidence intervals) using standard formulas (26). In addition, we examined test characteristics of BNP levels using a lower cutpoint (>30 pg/mL). We used continuous BNP measurements to generate receiver operating characteristic (ROC) curves, and calculated areas under the ROC curves by the trapezoidal rule (27). All analyses were performed using Stata, version 7 (College Station, Texas).
Results
Of the 293 subjects, 67 (23%) had plasma BNP levels >100 pg/mL (Table 1). Compared with those with lower BNP levels, these subjects were older, had lower total serum cholesterol levels, had lower creatinine clearance, and were also more likely to be taking renin-angiotensin system inhibitors.
Table 1.
Characteristics of 293 Participants with Coronary Disease
| B-Type Natriuretic Peptide | |||
|---|---|---|---|
| Characteristic | ≤100 pg/mL (n = 226) |
>100 pg/mL (n = 67) |
P Value |
| Number (%) or Mean ± SD | |||
| Age | 67 ± 11 | 75 ± 9 | <0.001 |
| Male sex | 206 (91) | 64 (96) | 0.24 |
| White race | 140 (62) | 45 (67) | 0.44 |
| Hypertension | 146 (65) | 47 (70) | 0.45 |
| Chronic obstructive pulmonary disease | 38 (17) | 6 (9) | 0.11 |
| Diabetes | 46 (21) | 19 (28) | 0.18 |
| Prior myocardial infarction | 117 (52) | 42 (63) | 0.14 |
| Prior stroke | 26 (12) | 13 (19) | 0.10 |
| Prior revascularization | 146 (65) | 43 (64) | 0.88 |
| Current angina | 33 (15) | 9 (13) | 0.81 |
| Current smoking | 37 (16) | 7 (10) | 0.22 |
| Regular alcohol consumption | 68 (30) | 22 (33) | 0.72 |
| Minimal or no physical activity | 82 (37) | 25 (37) | 0.92 |
| Body mass index (kg/m2) | 28 ± 5 | 28 ± 4 | 0.29 |
| Inducible ischemia | 79 (36) | 31 (48) | 0.07 |
| Total cholesterol (mg/dL) | 183 ± 41 | 169 ± 42 | 0.02 |
| Low-density lipoprotein cholesterol (mg/dL) | 107 ± 34 | 99 ± 32 | 0.11 |
| High-density lipoprotein cholesterol (mg/dL) | 47 ± 15 | 44 ± 12 | 0.12 |
| Creatinine clearance (mL/min) | 85 ± 27 | 66 ± 28 | <0.001 |
| Current beta-blocker use | 124 (55) | 44 (66) | 0.12 |
| Current renin-angiotensin inhibitor use | 95 (42) | 43 (64) | 0.001 |
| Current aspirin use | 185 (82) | 51 (76) | 0.30 |
| Current diuretic use | 48 (21) | 21 (31) | 0.09 |
| Current statin use | 157 (69) | 47 (70) | 0.92 |
Systolic Dysfunction
Forty-eight subjects (16%) had systolic dysfunction (ejection fraction <55%), in whom BNP levels were higher than in those with normal ventricular function (median [interquartile range], 122.0 pg/mL [52.4 to 272.0 pg/mL] vs. 32.0 pg/mL [14.4 to 68.9 pg/mL], P = 0.05). Twenty-seven percent (18/67) of subjects with BNP levels >100 pg/mL had evidence of systolic dysfunction, compared with 13% (30/226) of those with lower levels (unadjusted odds ratio [OR] = 2.4; 95% confidence interval [CI]: 1.2 to 4.7; P = 0.01). In adjusted models, BNP levels >100 pg/mL were associated with a threefold increased odds of systolic dysfunction (Table 2). BNP levels >100 pg/mL were also strongly associated with lower ejection fraction (ejection fraction <45%). We did not observe any interaction between BNP values and use of beta blockers, renin-angiotensin system inhibitors, or diuretics.
Table 2.
Association of B-Type Natriuretic Peptide Levels >100 pg/mL with Systolic and Diastolic Dysfunction among 293 Subjects with Coronary Artery Disease
| Category of Ventricular Function | Proportion (N) with BNP Levels >100 pg/mL | Adjusted Odds Ratio (95% Confidence Interval)* | P Value |
|---|---|---|---|
| Systolic function | |||
| Normal systolic function (ejection fraction ≥55%) | 20% (49/245) | 1.0 | |
| Any systolic dysfunction (ejection fraction <55%) | 38% (18/48) | 3.0 (1.4–6.7) | 0.006 |
| Moderate systolic dysfunction (ejection fraction <45%) | 65% (11/17) | 6.4 (1.9–21) | 0.003 |
| Diastolic function | |||
| Normal diastolic function with ejection fraction ≥55% | 15% (31/213) | 1.0 | - |
| Diastolic dysfunction with ejection fraction ≥55% | 55% (17/31) | 5.6 (2.2–14.1) | <0.001 |
Adjusted for variables in Table 1 associated with BNP levels >100 pg/mL at P <0.1, including older age, presence of inducible ischemia, decreased creatinine clearance, lower cholesterol levels, use of renin-angiotensin inhibitors, and use of diuretics.
BNP = B-type natriuretic peptide.
Diastolic Dysfunction
Among the 245 subjects with preserved systolic function (ejection fraction ≥55%), 244 had an assessment of pulmonary vein flow. Of these 244 subjects, 31 (13%) had diastolic dysfunction, with higher BNP levels than in those with normal ventricular function (median [interquartile range], 104.0 pg/mL [38.0 to 170.0 pg/mL] vs. 28.0 pg/mL [13.2 to 49.4 pg/mL], P <0.001). Thirty-five percent (17/48) of subjects with BNP levels >100 pg/mL had evidence of diastolic dysfunction, compared with 7% (14/196) of subjects with levels ≤100 pg/mL (unadjusted OR = 7.1; 95% CI: 3.2 to 15.9; P <0.001). BNP levels >100 pg/mL were associated with a more than fivefold increased odds of diastolic dysfunction in adjusted analyses (Table 2). We did not observe any significant interactions between BNP values and use of beta blockers, renin-angiotensin system inhibitors, or diuretics.
Test Characteristics
The areas under the ROC curves for BNP and the detection of systolic and diastolic dysfunction were all under 0.80 (Figures 1 to 3). At the cutpoint of <100 pg/mL, BNP levels had poor sensitivity for both systolic dysfunction and diastolic dysfunction (Table 3). Although a lower cutpoint of <30 pg/mL improved the sensitivity of BNP measurements for both forms of ventricular dysfunction, the overall test characteristics remained poor (Table 4).
Figure 1.
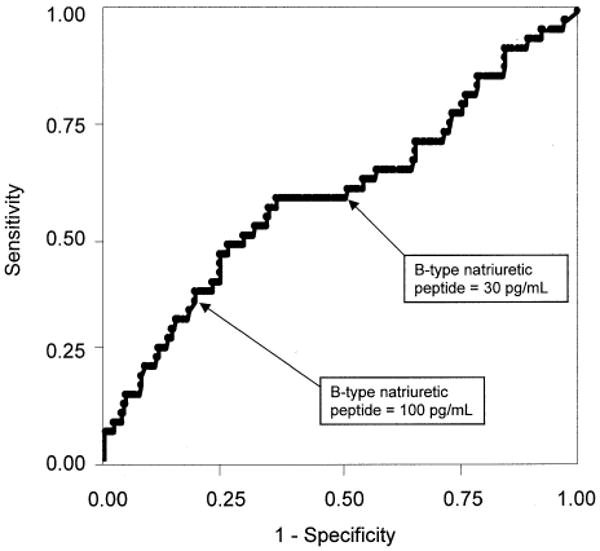
Receiver operating characteristic curve for B-type natriuretic peptide and ejection fraction <55% in 293 patients with coronary artery disease. Area under the curve = 0.59 (95% confidence interval: 0.49 to 0.69).
Figure 3.
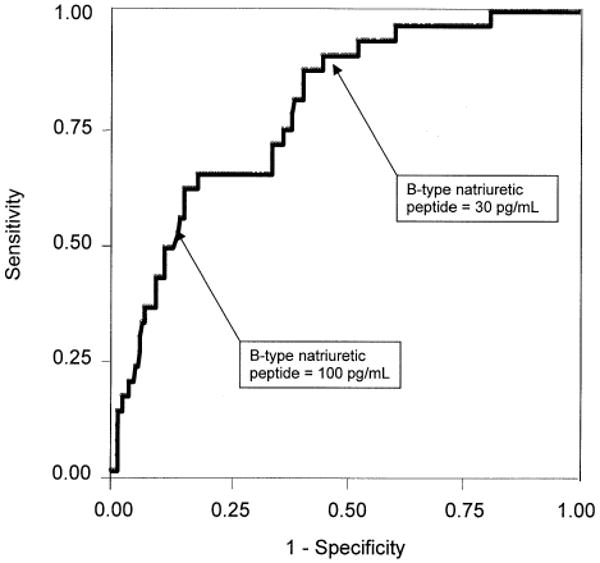
Receiver operating characteristic curve for B-type natriuretic peptide and diastolic dysfunction in 244 patients with coronary artery disease and ejection fraction ≥55%. Area under the curve = 0.79 (95% confidence interval: 0.71 to 0.87).
Table 3.
Test Characteristics of B-Type Natriuretic Peptide >100 pg/mL for Identifying Ventricular Dysfunction in 293 Participants with Coronary Heart Disease
| Ventricular Function | Sensitivity (%) (n/N)* | Specificity (%) (n/N)† | Likelihood Ratio (95% Confidence Interval) |
|
|---|---|---|---|---|
| Positive | Negative | |||
| Systolic Function | ||||
| Ejection fraction <55% | 38 (18/48) | 80 (196/245) | 1.9 (1.2–2.9) | 0.8 (0.6–1.0) |
| Ejection fraction <45% | 65 (11/17) | 80 (220/276) | 3.2 (2.1–4.9) | 0.4 (0.2–0.8) |
| Diastolic Function | ||||
| Diastolic dysfunction with ejection fraction ≥55% | 55 (17/31) | 85 (182/213) | 3.8 (2.4–5.9) | 0.5 (0.4–0.8) |
True positives/all with diagnosis.
True negatives/all without diagnosis.
Table 4.
Test Characteristics of B-Type Natriuretic Peptide >30 pg/mL for Identifying Ventricular Dysfunction in 293 Participants with Coronary Heart Disease
| Ventricular Function | Sensitivity (%) (n/N)* | Specificity (%) (n/N)† | Likelihood Ratio (95% Confidence Interval) |
|
|---|---|---|---|---|
| Positive | Negative | |||
| Systolic Function | ||||
| Ejection fraction <55% | 60 (29/48) | 47 (116/245) | 1.2 (0.9–1.5) | 0.8 (0.6–1.0) |
| Ejection fraction <45% | 76 (13/17) | 48 (131/276) | 1.5 (1.1–1.9) | 0.5 (0.2–1.2) |
| Diastolic Function | ||||
| Diastolic dysfunction with ejection fraction ≥55% | 90 (28/31) | 53 (113/213) | 1.9 (1.6–2.3) | 0.2 (0.1–0.5) |
True positives/all with diagnosis.
True negatives/all without diagnosis.
The test characteristics of BNP measurements varied by age. The area under the ROC curve of BNP values for the detection of ejection fraction <55% was 0.53 (95% CI: 0.38 to 0.67) in subjects younger than 65 years, 0.60 (95% CI: 0.39 to 0.81) in those aged 65 to 75 years, and 0.75 (95% CI: 0.62 to 0.88) in those older than 75 years. Similar variations by age were observed with test characteristics for BNP and the detection of diastolic dysfunction, with an area under the ROC curve of 0.63 (95% CI: 0.47 to 0.80) in subjects younger than 65 years, 0.85 (95% CI: 0.75 to 0.95) in those 65 to 75 years of age, and 0.83 (95% CI: 0.72 to 0.94) in those older than 75 years.
Discussion
We found that elevated plasma BNP levels (>100 pg/mL) were associated with both systolic dysfunction and diastolic dysfunction among outpatients with stable coronary disease. However, strong epidemiological associations do not necessarily yield good diagnostic tests, and the overall test characteristics of BNP measurements were poor. The ability of BNP levels to detect ventricular dysfunction was greatest for more severe forms of systolic dysfunction and among patients older than 75 years, but even in these subgroups the area under the ROC curve was ≤0.85. Thus, although BNP assays may be important diagnostic tools in symptomatic cohorts (4–14,28), their utility in detecting asymptomatic ventricular dysfunction in patients with stable coronary disease appears limited.
The poor test characteristics of BNP levels for the detection of systolic dysfunction that we demonstrated are similar to those found in other studies (29–33), including one that (as in our study) measured BNP levels in patients with known coronary disease (32). However, several studies have demonstrated excellent sensitivity and specificity for BNP screening in unselected patients (34–36). One potential explanation for these conflicting results is that BNP levels may be more accurate for detecting systolic dysfunction in unselected patients than in patients with coronary disease. The poor test characteristics in patients with coronary disease may reflect a greater prevalence of competing diagnoses, including ischemia (37,38), left ventricular hypertrophy (30,39,40), and diastolic dysfunction (8), which may also cause elevations in BNP levels.
Another potential reason for the differences in results is that previous studies used lower ejection fraction thresholds (14,34–36,40) that may have yielded better test characteristics. However, none of these studies excluded patients with a history of heart failure, and test characteristics are usually enhanced with greater disease prevalence and severity. Thus, a more likely explanation for the better BNP test characteristics in unselected patients is that patients with symptomatic heart failure were included in such studies.
Our results also differ from those of studies involving patients with overt symptoms of dyspnea (4–14,28), likely owing to the differing prevalence and spectrum of disease. Patients with dyspnea are likely to have more severe disease than patients in a screening cohort, since symptoms such as dyspnea are highly correlated with the pressure and volume overload that result in BNP elevation. Thus, although BNP measurement may be a useful tool in patients with heart failure symptoms, it has limited utility in detecting ventricular dysfunction in patients without overt symptoms.
The ease and relative low cost of BNP assays have led to suggestions that BNP measurement might be a useful first step in a multiphasic screening approach, in which all patients undergo BNP screening but only those with positive test results undergo echocardiography (29,35,41). The primary goal of using BNP measurements as a diagnostic test would be to identify as many patients as possible who would benefit from a diagnostic echocardiogram. To accomplish this goal, we would choose a low BNP cutpoint that maximizes sensitivity (and minimizes false negatives). Since false-negative tests would result in missed cases of systolic dysfunction, such patients would not only fail to benefit from the early initiation of medical therapies, they would also be subject to future delays in diagnosis if clinicians viewed the negative test as definitive.
Consider BNP testing in a sample of 1000 patients with stable coronary disease. Assuming a 16.4% prevalence of an ejection fraction <55% (as in our study), referring those with BNP levels >30 pg/mL for echocardiography would result in 98 new diagnoses of systolic dysfunction. However, 40% (66/164) of cases would be missed, and 443 patients would undergo unnecessary echocardiograms. Assuming a 7.0% prevalence of an ejection fraction <45%, a BNP cutpoint of 30 pg/mL would identify 53 patients with systolic dysfunction. However, 930 patients would receive unnecessary BNP tests, and 484 patients would require unnecessary echocardiograms.
Although performing 484 unnecessary echocardiograms would be more efficient than sending the 1000 patients for echocardiography, this strategy would still miss 24% (17/70) of patients with an ejection fraction <45%. Thus, it is unclear whether using BNP testing to identify patients who would benefit from echocardiography is any better than performing echocardiography in all 1000 patients with coronary disease, especially since echocardiography provides other information besides ejection fraction.
We observed a strong association between BNP levels and diastolic dysfunction, as has been observed in a previous study (8). Currently, there are no guidelines for the management of patients with asymptomatic diastolic dysfunction, and detection of diastolic dysfunction is unlikely to change current management patterns. However, the association between BNP elevations and asymptomatic diastolic dysfunction highlights the high prevalence of other diagnoses in high-risk patients, which may cause “falsely” elevated plasma BNP levels and thus limit the ability of elevated BNP levels to detect systolic dysfunction (for which guidelines do exist).
Our study has several limitations. The sample was predominantly male. Some subjects may have had symptoms of heart failure and thus would not have met the criteria for a screening cohort. However, since we excluded patients who reported a history of heart failure, and all of the participants completed an intensive daylong examination that included an exercise treadmill test, it is unlikely that any of them had overt symptoms of heart failure. We used an echocardiographic definition of diastolic dysfunction, but the exact association of these abnormal contractile properties with the clinical entity of heart failure is still uncertain (42).
In summary, we found that elevated BNP levels are associated with systolic and diastolic dysfunction in outpatients with stable coronary disease, but the poor test characteristics of this assay do not warrant its routine use as a screening tool for asymptomatic ventricular dysfunction in patients with coronary heart disease.
Figure 2.
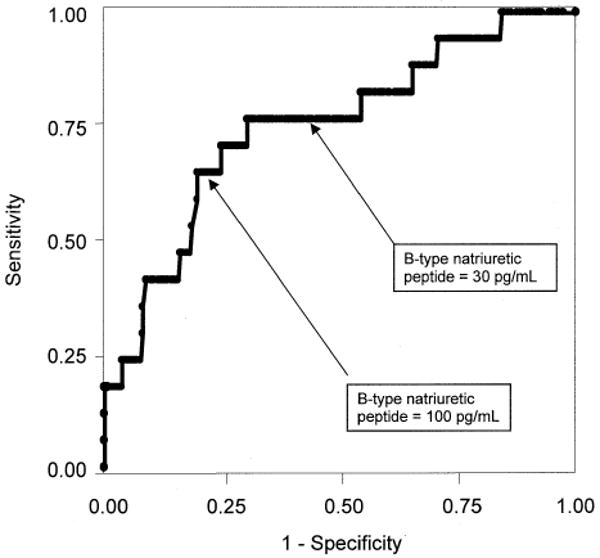
Receiver operating characteristic curve for B-type natriuretic peptide and ejection fraction <45% in 293 patients with coronary artery disease. Area under the curve = 0.75 (95% confidence interval: 0.62 to 0.88).
Acknowledgments
This work was supported by the Department of Veterans Affairs (Epidemiology Merit Review Program), the Robert Wood Johnson Foundation (Generalist Physician Faculty Scholars Program), the American Federation for Aging Research (Paul Beeson Faculty Scholars in Aging Research Program), the Ischemia Research and Education Foundation, and the University of California, San Francisco (Hellman Family Award). Dr. Whooley is supported by an Advanced Research Career Development Award from the Department of Veterans Affairs Health Services Research and Development Service.
References
- 1.Yoshimura M, Yasue H, Okumura K, et al. Different secretion patterns of atrial natriuretic peptide and brain natriuretic peptide in patients with congestive heart failure. Circulation. 1993;87:464–469. doi: 10.1161/01.cir.87.2.464. [DOI] [PubMed] [Google Scholar]
- 2.Nakagawa O, Ogawa Y, Itoh H, et al. Rapid transcriptional activation and early mRNA turnover of brain natriuretic peptide in cardiocyte hypertrophy. J Clin Invest. 1995;96:1280–1287. doi: 10.1172/JCI118162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maeda K, Tsutamoto T, Wada A, et al. Plasma brain natriuretic peptide as a biochemical marker of high left ventricular end-diastolic pressure in patients with symptomatic left ventricular dysfunction. Am Heart J. 1998;135:825–832. doi: 10.1016/s0002-8703(98)70041-9. [DOI] [PubMed] [Google Scholar]
- 4.Cabanes L, Richaud-Thiriez B, Fulla Y, et al. Brain natriuretic peptide blood levels in the differential diagnosis of dyspnea. Chest. 2001;120:2047–2050. doi: 10.1378/chest.120.6.2047. [DOI] [PubMed] [Google Scholar]
- 5.Dao Q, Krishnaswamy P, Kazanegra R, et al. Utility of B-type natriuretic peptide in the diagnosis of congestive heart failure in an urgent-care setting. J Am Coll Cardiol. 2001;37:379–385. doi: 10.1016/s0735-1097(00)01156-6. [DOI] [PubMed] [Google Scholar]
- 6.Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161–167. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 7.McCullough PA, Nowak RM, McCord J, et al. B-type natriuretic peptide and clinical judgment in emergency diagnosis of heart failure: analysis from Breathing Not Properly (BNP) Multinational Study. Circulation. 2002;106:416–422. doi: 10.1161/01.cir.0000025242.79963.4c. [DOI] [PubMed] [Google Scholar]
- 8.Cowie MR, Struthers AD, Wood DA, et al. Value of natriuretic peptides in assessment of patients with possible new heart failure in primary care. Lancet. 1997;350:1349–1353. doi: 10.1016/S0140-6736(97)06031-5. [DOI] [PubMed] [Google Scholar]
- 9.Lubien E, DeMaria A, Krishnaswamy P, et al. Utility of B-natriuretic peptide in detecting diastolic dysfunction: comparison with Doppler velocity recordings. Circulation. 2002;105:595–601. doi: 10.1161/hc0502.103010. [DOI] [PubMed] [Google Scholar]
- 10.Maisel AS, Koon J, Krishnaswamy P, et al. Utility of B-natriuretic peptide as a rapid, point-of-care test for screening patients undergoing echocardiography to determine left ventricular dysfunction. Am Heart J. 2001;141:367–374. doi: 10.1067/mhj.2001.113215. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto K, Burnett JC, Jr, Jougasaki M, et al. Superiority of brain natriuretic peptide as a hormonal marker of ventricular systolic and diastolic dysfunction and ventricular hypertrophy. Hypertension. 1996;28:988–994. doi: 10.1161/01.hyp.28.6.988. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto K, Burnett JC, Jr, Bermudez EA, et al. Clinical criteria and biochemical markers for the detection of systolic dysfunction. J Card Fail. 2000;6:194–200. doi: 10.1054/jcaf.2000.9676. [DOI] [PubMed] [Google Scholar]
- 13.Omland T, Aakvaag A, Bonarjee VV, et al. Plasma brain natriuretic peptide as an indicator of left ventricular systolic function and long-term survival after acute myocardial infarction. Circulation. 1996;93:1963–1969. doi: 10.1161/01.cir.93.11.1963. [DOI] [PubMed] [Google Scholar]
- 14.Valli N, Georges A, Corcuff JB, et al. Assessment of brain natriuretic peptide in patients with suspected heart failure: comparison with radionuclide ventriculography data. Clin Chim Acta. 2001;306:19–26. doi: 10.1016/s0009-8981(01)00388-6. [DOI] [PubMed] [Google Scholar]
- 15.McDonagh TA. Asymptomatic left ventricular dysfunction in the community. Curr Cardiol Rep. 2000;2:470–474. doi: 10.1007/s11886-000-0062-x. [DOI] [PubMed] [Google Scholar]
- 16.McMurray JV, McDonagh TA, Davie AP, et al. Should we screen for asymptomatic left ventricular dysfunction to prevent heart failure? Eur Heart J. 1998;19:842–846. doi: 10.1093/eurheartj/19.6.842. [DOI] [PubMed] [Google Scholar]
- 17.Struthers AD, Morris AD. Screening for and treating left-ventricular abnormalities in diabetes mellitus: a new way of reducing cardiac deaths? Lancet. 2002;359:1430–1432. doi: 10.1016/S0140-6736(02)08358-7. [DOI] [PubMed] [Google Scholar]
- 18.Konstam MA, Kronenberg MW, Rousseau MF, et al. Effects of the angiotensin converting enzyme inhibitor enalapril on the long-term progression of left ventricular dilatation in patients with asymptomatic systolic dysfunction? Circulation. 1993;88:2277–2283. doi: 10.1161/01.cir.88.5.2277. [DOI] [PubMed] [Google Scholar]
- 19.Pfeffer MA, Braunwald E, Moye LA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction? N Engl J Med. 1992;327:669–677. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- 20.Hunt SA, Baker DW, Chin MH, et al. ACC/AHA Guidelines for the Evaluation and Management of Chronic Heart Failure in the Adult? Circulation. 2001;104:2996–3007. doi: 10.1161/hc4901.102568. [DOI] [PubMed] [Google Scholar]
- 21.Gheorghiade M, Bonow RO. Chronic heart failure in the United States: a manifestation of coronary artery disease? Circulation. 1998;97:282–289. doi: 10.1161/01.cir.97.3.282. [DOI] [PubMed] [Google Scholar]
- 22.Angeja BG, Grossman W. Evaluation and management of diastolic heart failure. Circulation. 2003;107:659–663. doi: 10.1161/01.cir.0000053948.10914.49. [DOI] [PubMed] [Google Scholar]
- 23.Bush K, Kivlahan DR, McDonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking? Arch Intern Med. 1998;158:1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 24.Spertus JA, Winder JA, Dewhurst TA, et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease? J Am Coll Cardiol. 1995;25:333–341. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]
- 25.Redfield MM, Rodeheffer RJ, Jacobsen SJ, et al. Plasma brain natriuretic peptide concentration: impact of age and gender? J Am Coll Cardiol. 2002;40:976–982. doi: 10.1016/s0735-1097(02)02059-4. [DOI] [PubMed] [Google Scholar]
- 26.Sackett D, Haynes R, Guyatt G, et al. Clinical Epidemiology: A Basic Science For Clinical Medicine. Boston, Massachusetts: Little, Brown and Company; 1991. [Google Scholar]
- 27.STATA-Corporation. STATA-7 Reference Guide. College Station, Texas: Stata Press; 2001. [Google Scholar]
- 28.Landray MJ, Lehman R, Arnold I. Measuring brain natriuretic peptide in suspected left ventricular systolic dysfunction in general practice: cross-sectional study? BMJ. 2000;320:985–986. doi: 10.1136/bmj.320.7240.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith H, Pickering RM, Struthers A, et al. Biochemical diagnosis of ventricular dysfunction in elderly patients in general practice? BMJ. 2000;320:906–908. doi: 10.1136/bmj.320.7239.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vasan RS, Benjamin EJ, Larson MG, et al. Plasma natriuretic peptides for community screening for left ventricular hypertrophy and systolic dysfunction? JAMA. 2002;288:1252–1259. doi: 10.1001/jama.288.10.1252. [DOI] [PubMed] [Google Scholar]
- 31.Hetmanski DJ, Sparrow NJ, Curtis S, Cowley AJ. Failure of plasma brain natriuretic peptide to identify left ventricular systolic dysfunction in the community? Heart. 2000;84:440–441. doi: 10.1136/heart.84.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McClure SJ, Caruana L, Davie AP, et al. Cohort study of plasma natriuretic peptides for identifying left ventricular systolic dysfunction in primary care? BMJ. 1998;317:516–519. doi: 10.1136/bmj.317.7157.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Omland T, Aakvaag A, Vik-Mo H. Plasma cardiac natriuretic peptide determination as a screening test for the detection of patients with mild left ventricular impairment? Heart. 1996;76:232–237. doi: 10.1136/hrt.76.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDonagh TA, Robb SD, Murdoch DR, et al. Biochemical detection of left-ventricular systolic dysfunction? Lancet. 1998;351:9–13. doi: 10.1016/s0140-6736(97)03034-1. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura M, Endo H, Nasu M, et al. Value of plasma B type natriuretic peptide measurement for heart disease screening in a Japanese population? Heart. 2002;87:131–135. doi: 10.1136/heart.87.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hobbs FD, Davis RC, Roalfe AK, et al. Reliability of N-terminal pro-brain natriuretic peptide assay in diagnosis of heart failure: cohort study in representative and high risk community populations. BMJ. 2002;324:1498. doi: 10.1136/bmj.324.7352.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Lemos JA, Morrow DA, Bentley JH, et al. The prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes? N Engl J Med. 2001;345:1014–1021. doi: 10.1056/NEJMoa011053. [DOI] [PubMed] [Google Scholar]
- 38.Bibbins-Domingo K, Ansari M, Schiller NB, Massie B, Whooley MA. BNP and ischemia in patients with stable coronary disease: Data from the Heart and Soul Study? Circulation. 2003;108:2987–2992. doi: 10.1161/01.CIR.0000103681.04726.9C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kohno M, Horio T, Yokokawa K, et al. Brain natriuretic peptide as a marker for hypertensive left ventricular hypertrophy: changes during 1-year antihypertensive therapy with angiotensin-converting enzyme inhibitor? Am J Med. 1995;98:257–265. doi: 10.1016/S0002-9343(99)80372-6. [DOI] [PubMed] [Google Scholar]
- 40.Luchner A, Burnett JC, Jr, Jougasaki M, et al. Evaluation of brain natriuretic peptide as marker of left ventricular dysfunction and hypertrophy in the population? J Hypertens. 2000;18:1121–1128. doi: 10.1097/00004872-200018080-00018. [DOI] [PubMed] [Google Scholar]
- 41.Niinuma H, Nakamura M, Hiramori K. Plasma B-type natriuretic peptide measurement in a multiphasic health screening program? Cardiology. 1998;90:89–94. doi: 10.1159/000006825. [DOI] [PubMed] [Google Scholar]
- 42.Grodecki PV, Klein AL. Pitfalls in the echo-Doppler assessment of diastolic dysfunction? Echocardiography. 1993;10:213–234. doi: 10.1111/j.1540-8175.1993.tb00032.x. [DOI] [PubMed] [Google Scholar]


