Abstract
CD73-deficient mice are valuable for evaluating the ability of CD73-generated adenosine to modulate adenosine receptor-mediated responses. Here we report the role of CD73 in regulating lymphocyte migration across two distinct barriers. In the first case, CD73-generated adenosine restricts the migration of lymphocytes across high endothelial venules (HEV) into draining lymph nodes after an inflammatory stimulus, apparently by triggering A2B receptors on HEV. Secondly, CD73 promotes the migration of pathogenic T cells into the central nervous system during experimental autoimmune encephalomyelitis. Experiments are in progress to determine whether this effect is also adenosine receptor-mediated and to identify the relevant adenosine receptor.
Keywords: CD73, ecto-5’-nucleotidase, leukocyte migration, experimental autoimmune encephalomyelitis
INTRODUCTION
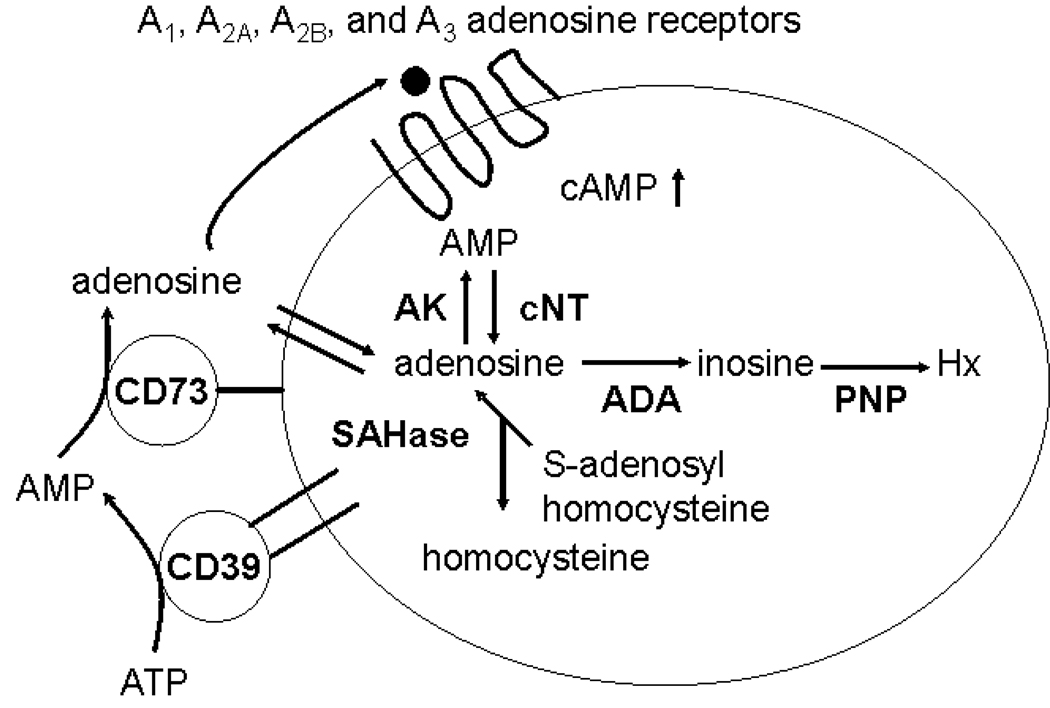
Adenosine, acting at cell surface adenosine receptors, regulates a number of important physiological responses including heart rate, vascular tone, neurotransmission, kidney function, and inflammation. There are four known seven transmembrane-spanning G protein-coupled adenosine receptors (ARs): A1, A2A, A2B, and A3.[1] The A2AR and A2BR are positively coupled to adenylate cyclase, while the A1R and A3R are negatively coupled. Adenosine to engage these receptors can be produced intracellularly from AMP by cytoplasmic nucleotidases or from S-adenosyl homocysteine via the action of S-adenosyl homocysteine hydrolase. Intracellular adenosine can leave the cell via an equilibrative nucleoside transporter. Alternatively, adenosine can be produced extracellularly from ATP via the combined action of ecto-apyrase (CD39) and ecto-5’-nucleotidase (CD73) (Figure 1). CD73-deficient mice are extremely valuable tools for evaluating the relative contributions of intracellularly- vs. extracellularly-produced adenosine in adenosine receptor-mediated responses (reviewed in [2]). For example, CD73-generated adenosine is essential for the anti-inflammatory action of methotrexate in the carageenan-induced air pouch model of inflammation and in the phenomenon of ischemic preconditioning. CD73-generated adenosine also plays an important role in modulating hypoxia-induced vascular leak and neutrophil accumulation into tissues. Here we present evidence that CD73-generated adenosine regulates the migration of lymphocytes across at least two distinct types of physiological barriers, but with opposite consequences. In the first case, CD73-generated adenosine restricts the migration of lymphocytes across high endothelial venules (HEV) into draining lymph nodes after an inflammatory stimulus. In contrast, CD73-generated adenosine promotes the entrance of pathogenic T cells into the central nervous system (CNS) in experimental autoimmune encephalomyelitis (EAE), a model for multiple sclerosis.
Figure 1. Pathways of adenosine formation.
Adenosine can be generated intracellularly by the action of cytoplasmic 5’-nucleotidase (cNT) or S-adenosyl homocysteine hydrolase (SAHase), or extracellularly through the combined action of CD39 (ecto-apyrase) and ecto-5’-nucleotidase (CD73). AK, adenosine kinase; ADA, adenosine deaminase; PNP, purine nucleoside phosphorylase; Hx, hypoxanthine.
METHODS
Mice
CD73−/− mice have been previously described.[3] All procedures involving mice were approved by the IACUC of either the Oklahoma Medical Foundation or Cornell University College of Veterinary Medicine.
Lymphocyte migration
Wild type and cd73−/− mice were injected with 1 µg LPS in the left rear footpad and with PBS in the right rear footpad. Twenty-four hours later, they received 107 fluorescently labeled wild type splenocytes i.v. One hour later, the mice were euthanized and the draining lymph nodes were harvested. Single cell suspensions were made. Cell counts were performed and the percentages of fluorescent lymphocytes were determined by flow cytometry.
EAE induction and evaluation
Wild type and cd73−/− mice were immunized with a peptide from myelin oligodendrocyte protein (MOG35–55 peptide) in complete Freund’s adjuvant s.c. in each flank and subsequently treated with Pertussis toxin i.v. using standard protocols. They were then evaluated daily for disease activity using the following scale: 0 – no sign of disease; 1 – limp tail; 2 – limp tail and partial hind limb paralysis; 3 – total hind limb paralysis; 4 – fore and hindlimb paralysis; 5 – death.
RESULTS AND DISCUSSION
To determine whether CD73-generated adenosine can regulate lymphocyte migration across endothelial barriers in addition to neutrophil migration, we assessed rates of lymphocyte migration into draining lymph nodes of wild type and CD73-deficient mice after injection of the inflammatory stimulus LPS into the rear footpad. Although the sizes of popliteal lymph nodes of untreated wild type and cd73−/− mice were similar, the draining popliteal lymph nodes of LPS-treated cd73−/− mice were approximately 1.5- to 2-fold larger than those of LPS-treated wild type mice (Table 1). To more directly assess rates of lymphocyte migration, wild type and cd73−/− mice were injected with 107 fluorescent wild type splenocytes i.v. 24 hours after LPS treatment. Flow cytometry of lymph node cells harvested one hour later revealed that cd73−/− mice had approximately 2- to 2.5-fold higher rates of lymphocyte migration (Table 1). There was no preference for T cells or B cells or CD73+ vs. CD73− cells with respect to migration into draining lymph nodes. Therefore, we conclude that it is CD73 expression on endothelial cells rather than on lymphocytes that determines the rates of lymphocyte migration into draining lymph nodes.
Table 1.
CD73-deficient mice show increased rates of lymphocyte migration into draining lymph nodes after LPS injection.
| Total cells/lymph node (×105) | Fluorescent cells/lymph node | |||
|---|---|---|---|---|
| Strain/Treatmenta | PBS | LPS | PBS | LPS |
| Wild type (n=4) | 2.9 ± 1.5 | 10.4 ± 4.1 | 663 ± 44 | 840 ± 266 |
| CD73−/− (n=5) | 3.5 ± 2.0 | 17.8 ± 3.8 | 713 ± 332 | 1812 ± 299 |
| pb | N.S.b | 0.027 | N.S. | 0.0014 |
cd73+/+ and cd73−/− mice were injected with 1 µg LPS in the right rear footpad and with PBS in the left rear footpad. Twenty-four hours later, the mice were injected with 107 fluorescent wild type splenocytes. One hour later, the mice were euthanized and the draining lymph nodes removed. The cells were counted and the numbers of fluorescent lymphocytes were determined by flow cytometry.
The differences between wild type and cd73−/− mice were analyzed by a Student’s t test.
N.S. = not significant
To test the hypothesis that the role of CD73 was to produce adenosine for adenosine receptor signaling, we pre-treated LPS-injected wild type and cd73−/− mice with the general adenosine receptor agonist N-ethyl carboxamido adenosine (NECA) 6 hours prior to measuring rates of lymphocyte migration. NECA treatment normalized the high rate of lymphocyte migration previously observed in cd73−/− mice (data not shown). It is likely that the A2BR is regulating rates of lymphocyte migration across HEV, as it is the only adenosine receptor expressed in a cDNA library derived from primary murine HEV[4].
EAE is an experimental model for multiple sclerosis in which MOG-specific T cells enter the CNS and initiate an inflammatory response that eventually results in demyelination and paralysis. To determine whether CD73-generated adenosine might also restrict the entrance of pathogenic T cells into the CNS, we evaluated the severity of EAE in wild type and cd73−/− mice. Much to our surprise, CD73-deficient mice were highly resistant to EAE (Figure 2A). This was not, however, due to the failure of cd73−/− T cells to become activated after MOG35–55 immunization, as T cells derived from immunized cd73−/− mice caused much more severe disease than T cells derived from immunized wild type mice when transferred into T cell-deficient hosts (i.e., TCRα−/− mice) (Figure 2B). Rather, our data suggest that CD73 is needed for pathogenic T cells to enter the CNS. This conclusion is supported by immunohistochemistry showing very low numbers of leukocytes in the brain tissue of cd73−/− mice immunized to develop EAE compared to the number of leukocytes in the brains of wild type mice (data not shown). We hypothesize that adenosine receptor signaling triggered by CD73-generated adenosine plays a role in allowing migration of leukocytes into the CNS. The specific adenosine receptor subtype that might be involved has not yet been identified. However, since CD73 has also been reported to function as an adhesion molecule in the interaction between lymphocytes and endothelial cells,[5] this possibility cannot be eliminated by the available data.
Figure 2. EAE severity in intact and recipient mice.
A. cd73+/+ (CD73 WT) and cd73−/− (CD73 KO) mice were immunized to develop EAE. The average maximal disease activity score is shown. B. CD73 WT and CD73 KO mice were induced to develop EAE. On day 12, 2.5 × 106 CD4+ T cells were purified and adoptively transferred to TCRα−/− mice. The recipients were then immunized to develop EAE. The average maximal disease activity score is shown.
There are several potential mechanisms by which adenosine receptor signaling could regulate lymphocyte migration across different types of endothelial barriers. For example, in the case of HEV where barrier function is maintained through adherens junctions involving VE-cadherin and catenins, cell-cell contacts are stabilized by Rap1, a small GTPase that utilizes Epac, a cAMP-activated guanidine exchange factor as its effector.[6] In the case of tight junctions, it is known that cAMP can regulate permeability in a PKA-dependent manner by activating myosin light chain phosphatase. This decreases the ability of myosin light chain to interact with actin filaments to form stress fibers and results in increased barrier function. However, in other circumstances, cAMP can also cause the delocalization of VE-cadherin from junctional adhesion complexes, leading to increased permeability.[7] Clearly, more experimental work needs to be done in order to understand the role of CD73 and adenosine receptor signaling in regulating the migration of lymphocytes across different types of endothelial (and epithelial) barriers. Insight gained from such experiments may lead to the development of adenosine-based therapeutics for the treatment of a variety of conditions including vascular leak and inflammation.
ACKNOWLEDGMENTS
This work was supported by NIH grants AI18220 (L.F.T.) and AI57854 (M.S.B.). L.F.T. holds the Putnam City Distinguished Chair in Cancer Research.
References
- 1.Palmer TM, Stiles GL. Review: Neurotransmitter receptors VII: adenosine receptors. Neuropharm. 1995;34:683–694. doi: 10.1016/0028-3908(95)00044-7. [DOI] [PubMed] [Google Scholar]
- 2.Takedachi M, Colgan SP, Thompson LF. The role of CD73 in the generation of extracellular adenosine for adenosine receptor signaling. In: Hasko G, Cronstein BN, Szabo C, editors. Adenosine Receptors: Therapeutic Aspects for Inflammatory and Immune Diseases. 2006. [Google Scholar]
- 3.Thompson LF, Eltzschig HK, Ibla JC, Van De Wiele CJ, Resta R, Morote-Garcia JC, Colgan SP. Crucial role for ecto-5'-nucleotidase (CD73) in vascular leakage during hypoxia. J. Exp. Med. 2004;200:1395–1405. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Airas L, Niemela J, Jalkanen S. CD73 engagement promotes lymphocyte binding to endothelial cells via a lymphocyte function-associated antigen-1-dependent mechanism. J. Immunol. 2000;165:5411–5417. doi: 10.4049/jimmunol.165.10.5411. [DOI] [PubMed] [Google Scholar]
- 5.Umemoto E, Tanaka T, Kanda H, Jin S, Tohya K, Otani K, Matsutani K, Matsumoto M, Ebisuno Y, Jang MH, Fukuda M, Hirata T, Miyasaka M. Nepmucin, a novel HEV sialomucin, mediates L-selectin-dependent lymphocyte rolling and promotes lymphocyte adhesion under flow. J. Exp. Med. 2006;203:1603–1614. doi: 10.1084/jem.20052543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orlova VV, Economopoulou M, Lupu F, Santoso S, Chavakis T. Junctional adhesion molecule-C regulates vascular endothelial permeability by modulating VE-cadherin-mediated cell-cell contacts. J. Exp. Med. 2006;203:2703–2714. doi: 10.1084/jem.20051730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bindewald K, Gunduz D, Hartel F, Peters SC, Rodewald C, Nau S, Schafer M, Neumann J, Piper HM, Noll T. Opposite effect of cAMP signaling in endothelial barriers of different origin. Am. J. Physiol. Cell. Physiol. 2004;287:C1246–C1255. doi: 10.1152/ajpcell.00132.2004. [DOI] [PubMed] [Google Scholar]