Abstract
Background
Elevated levels of C-reactive protein (CRP) are associated with an increased risk of coronary events, but whether inflammation is associated with inducible ischemia in patients with stable coronary disease is unknown.
Methods and Results
We recruited patients with known coronary disease from 2 VA Medical Centers and 1 University-based medical center for the Heart and Soul Study. We measured CRP levels in 118 participants who had exercise-induced ischemia and in 111 who did not have inducible ischemia, as determined by stress echocardiography. We used logistic regression to examine the risk of exercise-induced ischemia associated with elevated CRP. We found that 75% (39/52) of participants in the highest CRP category (>0.38 mg/dL) had inducible ischemia, compared with 45% (79/177) in the lower 4 categories combined (adjusted odds ratio 4.2; 95% confidence interval 1.6 to 11; P=0.004). However, this association differed in users and nonusers of β-blockers and statins. Among 89 participants who did not use β-blockers, 93% in the highest CRP category had exercise-induced ischemia, compared with 42% in the lower 4 categories (P=0.03). Among 67 participants who did not use statins, 94% in the highest CRP category had exercise-induced ischemia, compared with 44% in the lower 4 categories (P=0.009). We did not observe a significant association between CRP and ischemia among participants who were treated with either of these medications.
Conclusion
Elevated CRP levels are associated with inducible ischemia in patients with stable coronary disease, particularly among those not treated with β-blockers or statins.
Keywords: coronary disease, ischemia, risk factors, inflammation, epidemiology
Inflammation is an important factor in the pathogenesis of atherosclerosis,1,2 and several markers of inflammation have been associated with an increased risk of cardiovascular events.3–6 Of these, C-reactive protein (CRP) has emerged as one of the strongest predictors of ischemic events, independent of other known risk factors for coronary disease.7–14 Some have proposed that CRP may eventually be useful as a diagnostic test for identifying patients at high risk for future events.15 However, the pathophysiology by which inflammatory markers such as CRP become elevated in patients at risk for future coronary events is not well understood.
Most studies that have demonstrated an association between CRP and coronary disease have examined the relation between CRP and risk of future coronary events, such as those characterized by plaque instability or thrombus formation. Only a few studies have examined the association between CRP and anatomic measures of coronary disease. Two have compared CRP concentrations with coronary calcification as measured by electron beam computed tomography,16,17 but neither found any association. Five studies have compared CRP levels with results from coronary angiography, 18–21 but their results were conflicting; 3 found an association between CRP and degree of coronary stenosis,18–20 and 2 found no or minimal association.14,21
Because anatomic findings do not necessarily correlate with severity of ischemia or risk of acute coronary syndromes,22 electron beam computed tomography and angiography may be less accurate than functional studies, such as exercise electrocardiography or stress echocardiography, in identifying patients who have myocardium at risk for future events. To determine whether CRP is associated with inducible ischemia and to explore the potential role of β-blockers and statins in modifying this association, we examined the association between CRP, exercise-induced ischemia, and use of preventive medications in a cohort of patients with known coronary disease.
Methods
Participants
The Heart and Soul study is an ongoing prospective cohort study designed to determine how psychosocial factors influence disease progression in patients with coronary disease. Between September 2000 and October 2001, 425 participants were recruited from 2 VA Medical Centers (San Francisco VA Medical Center and the VA Palo Alto Health Care System, California) and 1 University-based medical center (University of California, San Francisco). Patients were eligible to participate if they had a history of any of the following: myocardial infarction, coronary revascularization, angiographic evidence of ≥50% stenosis in one or more coronary vessels, evidence of exercise-induced ischemia by treadmill or nuclear testing, or a diagnosis of coronary disease that was documented by an internist or cardiologist. Patients were excluded if they were unable to walk 1 block or were planning to move out of the local area within 3 years.
Study participants were instructed not to take aspirin for 1 week, not to eat for 12 hours (except for medications, which they were instructed to take with water), and not to smoke for 5 hours before their study appointment. On the morning of their appointment, serum samples were obtained and frozen at −70°C. Later the same day, after serum was drawn and stored for measurement of CRP, all study participants completed a full exercise treadmill test with a stress echocardiogram.
For this cross-sectional analysis, we measured CRP in frozen serum samples from all 118 participants who had exercise-induced ischemia as measured by stress echocardiography, and from all 111 participants who did not have exercise-induced ischemia (negative stress echo and low-risk Duke Treadmill score ≥5).23 We did not measure CRP in the remaining 196 participants who had possible ischemia (negative stress echo, but moderate to high-risk Duke Treadmill score <5). This study was approved by the appropriate institutional review boards, and all participants provided written informed consent.
Measurements
C-Reactive Protein
We used the Roche Integra high-sensitivity assay to measure CRP. This immuno-turbidometric assay uses latex particles that are coated with monoclonal anti-CRP antibodies. CRP in each sample agglutinates with these particles and a precipitate is formed. The amount of precipitate is determined turbidimetrically at 522 nanometers. This assay has been standardized against the World Health Organization reference and compared with the Dade nephelometric method with a correlation coefficient of 0.997. The lowest detectable measurement with this assay was 0.025 mg/dL. The interassay coefficient of variation was 3.2%. The laboratory technicians who measured CRP were blinded to results of the stress echocardiogram.
Ischemia
Participants were instructed to fast for at least 4 hours before exercise, except for taking their usual medications as prescribed. We performed a symptom-limited, graded exercise treadmill test according to a Standard Bruce Protocol. Participants were asked to walk on a treadmill beginning at a workload of 20 to 30 watts and increasing by 20 to 30 watts every 3 minutes until reaching dyspnea, symptom-limited fatigue, chest discomfort, or electrocardiographic changes suggestive of ischemia. To achieve maximum heart rate, participants who were unable to continue the Standard Bruce protocol (for orthopedic or other reasons) were switched to slower settings on the treadmill and encouraged to exercise for as long as possible. Continuous, 12-lead electrocardiographic monitoring was performed throughout exercise, and total exercise capacity was estimated in metabolic equivalents (METs).
We performed resting and stress echocardiograms using an Acuson Sequoia Ultrasound System with a 3.5 MHz transducer. Just before exercise, standard 2-dimensional parasternal short-axis and apical 2- and 4-chamber views obtained during held inspiration were planimetered using a computerized digitization system to determine end-diastolic and end-systolic left-ventricular volume and left ventricular ejection fraction. At peak exercise, apical 2-chamber, 4-chamber, and precordial long-axis and short-axis views were obtained to detect the development of right or left ventricular dilatation or wall motion abnormalities during exercise. We defined exercise-induced ischemia as the presence of new wall motion abnormalities at peak exercise. Results from all echocardiograms were interpreted by an expert echocardiographer (N.B.S.) who was blinded to CRP results.
Other Measurements
Age, medical history, and smoking status were determined by questionnaire. We measured weight and height and calculated body mass index (kg/m2). Participants were instructed to bring their medication bottles to the study appointment, and study personnel recorded all current medications. We considered participants users of β-blockers, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins), angiotensin-converting enzyme inhibitors, or angiotensin-receptor blockers if they reported taking these medications daily. We considered participants users of aspirin or estrogen if they reported taking these medications weekly or more.
We determined frequency of angina by asking, “Over the past four weeks, on average, how many times have you had chest pain, chest tightness, or angina?”24 Participants who answered “once or more per week” were considered to have current angina. Serum samples were obtained for measurement of creatinine, total cholesterol, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) after a 12-hour fast. We estimated creatinine clearance using 24-hour urine collections.
Statistical Analysis
We divided participants into the following 5 prespecified CRP categories on the basis of quintiles used in prior studies25: CRP ≤0.07 mg/dL; >0.07 to ≤0.11 mg/dL; >0.11 to ≤0.19 mg/dL; >0.19 to ≤0.38 mg/dL; and >0.38 mg/dL. Differences in baseline characteristics were compared using ANOVA (or non-parametric equivalent) for continuous variables and χ2 tests (or Fisher's exact test if fewer than 5 observations in any cell) for dichotomous variables. We compared the unadjusted frequency of exercise-induced ischemia among participants in the 5 CRP categories using a χ2 test for trend.
To determine the adjusted association of CRP and exercise-induced ischemia, we used logistic regression analyses with CRP category as the independent variable and ischemia as the dependent variable. To obtain adjusted risk estimates, we entered all variables from Table 1 into logistic regression models that included the 5 CRP categories as dummy variables. Variables that were associated with exercise-induced ischemia (at P<0.1) were retained in these models. To explore potential modifying effects of cardiac medications, we tested for statistical interactions between CRP and each medication, and performed analyses stratified by use of any medication that had a significant interaction with CRP. For all of these analyses, we report odds ratios (ORs) with 95% confidence intervals (CIs). Analyses were performed using the Statistical Analysis Software (version 8, SAS Institute, Inc).
TABLE 1. Characteristics of 229 Participants With Coronary Disease, Including 118 With Exercise-Induced Ischemia and 111 Without Inducible Ischemia.
| Variable | Ischemia (n=118) |
No Ischemia (n=111) |
P Value |
|---|---|---|---|
| Age, y | 71.9±9.2 | 66.9±10.9 | <0.001 |
| Male sex, % | 95 | 95 | 0.91 |
| White race, % | 67 | 58 | 0.15 |
| History, % | |||
| Hypertension | 71 | 70 | 0.84 |
| Myocardial infarction | 65 | 51 | 0.03 |
| Congestive heart failure | 26 | 7 | <0.001 |
| Stroke | 13 | 11 | 0.66 |
| Diabetes | 32 | 21 | 0.07 |
| Coronary artery bypass grafting | 47 | 47 | 0.92 |
| Angioplasty | 32 | 49 | 0.009 |
| Current smoking, % | 18 | 19 | 0.80 |
| Body mass index, kg/m2 | 27.8±5.0 | 28.0±4.4 | 0.77 |
| Exercise capacity, METs | 5.9±2.5 | 9.0±2.6 | <0.001 |
| Left ventricular ejection fraction | 0.61±0.11 | 0.64±0.09 | 0.006 |
| Current angina, % | 39 | 32 | 0.24 |
| Medication, % | |||
| β-Blocker | 62 | 60 | 0.82 |
| Statin | 68 | 74 | 0.31 |
| ACE inhibitor | 55 | 39 | 0.01 |
| Aspirin | 86 | 79 | 0.21 |
| Estrogen (in women) | 17 | 50 | 0.55 |
| Labs | |||
| LDL cholesterol | 105.1±34.3 | 107.1±32.9 | 0.67 |
| HDL cholesterol | 45.7±16.7 | 47.5±12.4 | 0.36 |
| Total/HDL cholesterol | 4.2±1.4 | 4.0±1.2 | 0.24 |
| Creatinine clearance | 70.9±23.3 | 86.0±28.2 | <0.001 |
Values are expressed as mean±SD or percent. ACE indicate angiotensin-converting enzyme.
Results
Compared with participants who did not have inducible ischemia, those with inducible ischemia were older, more likely to have a history of myocardial infarction, CHF, or diabetes, and were less likely to have had an angioplasty. Participants with inducible ischemia also had decreased exercise capacity, ejection fraction, and creatinine clearance, and were more likely to be prescribed an angiotensin-converting enzyme inhibitor (Table 1).
Of the 229 participants, 52 (23%) had CRP levels in the highest category (>0.38 mg/dL). The median CRP level in the highest category was 0.73 mg/dL (interquartile range 0.42 to 1.04 mg/dL). Seventy five percent of participants in the highest CRP category had inducible ischemia, as compared with 38% of those in the lowest category (OR 4.8, 95% CI, 2.1 to 11; P<0.001). This association remained strong after adjusting for all Table 1 variables in a forced entry model (OR 7.2, 95% CI, 1.8 to 29; P=0.005), and after entering these variables into a backward elimination model (OR 5.3, 95% CI, 1.6 to 18; P=0.008) (Table 2). We also compared participants in the highest CRP category with those in the lower 4 categories combined and found that elevated CRP was associated with greater than 4-fold increased odds of exercise-induced ischemia (OR 4.2, 95% CI 1.6 to 11; P=0.004) adjusted for age, history of angioplasty, body mass index, exercise capacity, LDL, HDL, and total/HDL ratio.
TABLE 2. Association Between CRP and Exercise-Induced Ischemia in 229 Participants With Coronary Disease.
| CRP Category | Proportion With Ischemia | Unadjusted OR (95% CI) |
Adjusted OR (95% CI)* |
|---|---|---|---|
| I (≤0.07 mg/dL) | 38% (20/52) | 1.0 | 1.0 |
| II (>0.07 to 0.11) | 55% (18/33) | 1.9 (0.8–4.6) | 1.4 (0.4–4.6) |
| III (>0.11 to ≤0.19) | 46% (22/48) | 1.4 (0.6–3.0) | 1.4 (0.5–4.1) |
| IV (>0.19 to ≤0.38) | 43% (19/44) | 1.2 (0.5–2.8) | 1.1 (0.4–3.2) |
| V (>0.38) | 75% (39/52) | 4.8 (2.1–11) | 5.3 (1.6–18) |
All variables from Table 1 were entered into a backwards elimination logistic regression model including the 5 CRP categories. The variables associated with exercise-induced ischemia (at P<0.10) in the multivariable model were age, history of angioplasty, body mass index, exercise capacity, LDL, HDL, and total cholesterol/HDL.
In stratified analyses, we observed similar associations between high CRP (>0.38 mg/dL) and inducible ischemia in participants with or without a history of myocardial infarction, with or without a history of diabetes mellitus, and with or without symptoms of angina (data not shown). We found no evidence of an interaction between CRP and use of aspirin (P for interaction=0.89) or angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers (P for interaction=0.38).
The relation between high CRP and ischemia, however, seemed to differ among users and nonusers of β-blockers (P for interaction=0.06) and users and nonusers of statins (P for interaction=0.06). We observed a strong association between CRP and ischemia among participants who were not treated with β-blockers or statins, and a markedly diminished association between CRP and ischemia among participants who were treated with these medications (Table 3, Figure 1 and Figure 2). Among the 111 participants treated with both statins and β-blockers, 58% (15/26) in the highest CRP category had exercise-induced ischemia, as compared with 45% (38/85) in the lower 4 categories combined (P=0.26).
TABLE 3. Association Between Exercise-Induced Ischemia and Elevated CRP Levels Stratified by Use of β-Blockers and Statins.
| CRP Category | Proportion With Ischemia | Adjusted OR (95% CI)* |
P Value |
|---|---|---|---|
| Participants using β-blockers (n=140) | |||
| I–IV | 47% (48/103) | 1.0 | |
| V | 68% (25/37) | 2.7 (0.9–8.1) | 0.07 |
| Participants not using β-blockers (n=89) | |||
| I–IV | 42% (31/74) | 1.0 | |
| V | 93% (14/15) | 13 (1.2–130) | 0.03 |
| Participants using statins (n=162) | |||
| I–IV | 45% (57/127) | 1.0 | |
| V | 66% (23/35) | 2.7 (0.9–8.0) | 0.07 |
| Participants not using statins (n=67) | |||
| I–IV | 44% (22/50) | 1.0 | |
| V | 94% (16/17) | 120 (3.3–>999) | 0.009 |
Adjusted for age, history of angioplasty, body mass index, exercise capacity, LDL, HDL, total cholesterol/HDL, and concurrent use of statins (for β-blocker analysis) or β-blockers (for statin analysis).
Figure 1.
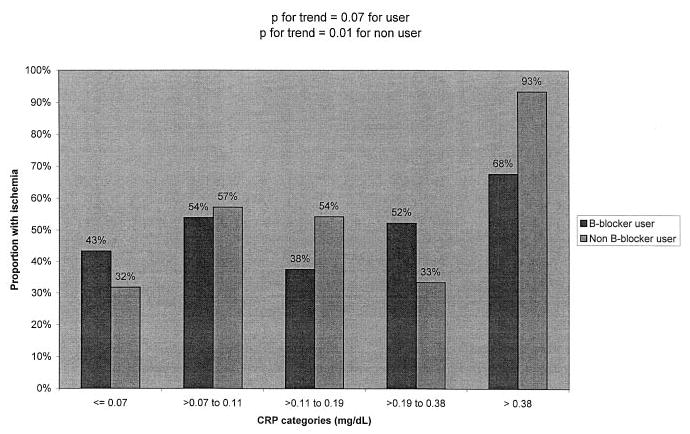
Proportion with ischemia by CRP category and β-blocker use.
Figure 2.
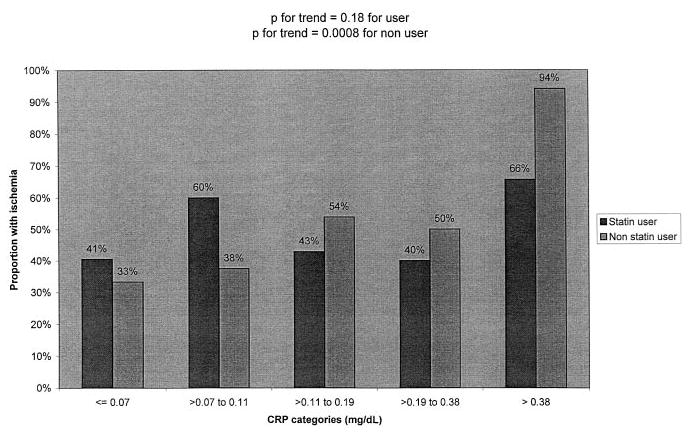
Proportion with ischemia by CRP category and statin use.
To determine whether CRP levels differed by use of β-blockers or statins, we compared the median CRP and the proportion of patients who had high CRP among users and nonusers of these medications. We did not find a significant difference in median CRP levels between users and nonusers of statins (0.15 versus 0.18 mg/dL, P=0.6), or users and nonusers of β-blockers (0.15 versus 0.17, P=0.6).
Discussion
We found that elevated levels of CRP (>0.38 mg/dL) were associated with greater than 4-fold increased odds of inducible ischemia in older adults with coronary disease. Elevated CRP was a stronger predictor of exercise-induced ischemia than symptoms of angina or any of the traditional cardiac risk factors, particularly among patients who were untreated with β-blockers or statins. Treatment with β-blockers or statins appeared to uncouple the strong association between CRP and inducible ischemia. Our cross-sectional study cannot determine whether CRP is a marker of an inflammatory process leading to ischemia, whether it is part of an inflammatory reaction to ischemia, or whether the causal pathways are bi-directional. The strong association we observed between CRP and ischemia, however, offers insight into potential mechanisms for the association between inflammatory markers and coronary disease.
Inflammation has been implicated as a mediator in all stages of atherosclerotic disease,1,2 and CRP, which reacts with the C-polysaccharide of the pneumococcus, can be used to detect levels of inflammation.26 Because CRP is an acute-phase reactant that increases with any tissue injury or infection, it does not discriminate between cardiac and noncardiac processes.26 However, chronic inflammation, as indicated by high concentrations of C-reactive protein, is associated with an increased risk of future coronary events. Likewise, elevations in inflammatory markers predict worse outcomes in patients with acute coronary syndromes, independent of the extent of myocardial injury.3,4,7–12,18 Thus, elevated concentrations of CRP may reflect a greater degree of atherosclerotic burden or faster progression to flow-limiting stenosis. The combination of greater atherosclerotic burden and increased plaque inflammation could lead to inducible ischemia through reduced perfusion and decreased regional blood flow.
It is also plausible that elevations in CRP are part of an inflammatory reaction to chronic myocardial ischemia. Because exercise-induced ischemia is strongly associated with chronic ischemia,27 study participants who had inducible ischemia were probably also experiencing myocardial hypoperfusion in their daily lives. Thus, an alternative explanation for the association between CRP and ischemia is that decreased perfusion of myocardial tissue may lead to an inflammatory reaction that involves CRP. Such an explanation would imply that CRP is a risk factor for future coronary events because it is a marker of ischemia-induced inflammation. This hypothesis is consistent with the 2- to 4-fold increase in CRP levels observed in patients with acute coronary syndromes as compared with stable angina,18 suggesting that levels of acute-phase reactants, such as CRP, may be higher in patients with greater degrees of ischemia.
In exploring whether use of cardiac medications modulates the relation between CRP and ischemia, we observed a strong association between CRP and ischemia among nonusers of β-blockers and statins. However, this association was markedly reduced among participants who were treated with these medications. Depending on whether we assume that inflammatory processes cause ischemia or the reverse, our results can be interpreted in 1 of 3 ways. β-Blockers and statins may reduce the ischemic response to inflammation, decrease the inflammatory response to ischemia, or both.
Previous studies have found that statins reduce rates of coronary events below that expected from lipid-lowering alone and lead to reductions in CRP levels over time, implying that statins have antiinflammatory properties.28–30 Likewise, β-blockers have been shown to reduce inflammation through decreases in sympathetic tone.31 Although we did not observe a statistically significant difference in CRP levels between users and nonusers of statins, the effect sizes were similar in magnitude to those found in other larger studies of statin use.29,30 Assuming CRP is a marker of an inflammatory process that causes ischemia, use of β-blockers and statins may prevent this inflammatory process from promoting atherogenesis and ischemia. If, on the other hand, inflammation and CRP are part of a reaction to ischemia, then β-blockers and statins may decrease this inflammatory response by reducing the severity of ischemia.
Several limitations should be considered in interpreting our results. First, our study participants were mostly men with known coronary heart disease, and thus we are unable to determine whether our results generalize to women or to patients without known coronary heart disease. The CRP values in our study population are similar to those in other outpatient studies,29,30 however, and they correlate with the quintiles suggested for determining risk of future cardiac events in various populations.25 Second, stress echocardiography may have misclassified some ischemic participants as nonischemic. Misclassification usually results in weaker, not stronger associations, however, so it is unlikely that this explains our findings. Third, our study design was cross-sectional. Because no study has determined whether CRP is associated with current inducible ischemia in patients with stable coronary disease, the strong association we demonstrated between CRP and ischemia provides valuable insight into the biological mechanisms linking elevated CRP with future events.
Finally, our study did not include anatomic measures of coronary disease, and thus we were not able to determine whether CRP is associated with ischemia because of greater underlying disease burden or through another mechanism. Because anatomic findings do not necessarily correlate with severity of ischemia or risk of acute coronary syndromes, however, anatomic studies may be less accurate than functional studies, such as exercise echocardiography, in identifying patients who have myocardium at risk for future events. Therefore, we believe that demonstrating a relation between CRP and functional ischemia provides an important advance in our understanding of the relation between inflammation and coronary disease, whether or not this association is independent of atherosclerotic burden.
In summary, high CRP (>0.38 mg/dL) seems to be strongly and independently associated with inducible ischemia in patients with stable coronary disease, particularly among those who are untreated with β-blockers or statins. The mechanisms underlying the association between inflammation and ischemia, as well as potential interventions to prevent ischemia associated with inflammatory processes, deserve further study.
Acknowledgments
We thank James C. Ritchie, PhD, FACB, DABCC, for performing the CRP assays. This study was supported by grants from the Department of Veterans Affairs, the American Federation for Aging Research (Paul Beeson Scholars Program), the Robert Wood Johnson Foundation (Faculty Scholars Program), and the Ischemia Research and Education Foundation. Drs Shlipak and Whooley are supported by Research Career Development Awards from the Department of Veterans Affairs Health Services Research and Development Service.
References
- 1.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Hennekens CH, Buring JE, et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 4.Liuzzo G, Biasucci LM, Gallimore JR, et al. The prognostic value of C-reactive protein and serum amyloid a protein in severe unstable angina. N Engl J Med. 1994;331:417–424. doi: 10.1056/NEJM199408183310701. [DOI] [PubMed] [Google Scholar]
- 5.Zhang R, Brennan ML, Fu X, et al. Association between myeloperoxidase levels and risk of coronary artery disease. JAMA. 2001;286:2136–2142. doi: 10.1001/jama.286.17.2136. [DOI] [PubMed] [Google Scholar]
- 6.Lindmark E, Diderholm E, Wallentin L, et al. Relationship between interleukin 6 and mortality in patients with unstable coronary artery disease. JAMA. 2001;286:2107–113. doi: 10.1001/jama.286.17.2107. [DOI] [PubMed] [Google Scholar]
- 7.Haverkate F, Thompson SG, Pyke SD, et al. Production of C-reactive protein and risk of coronary events in stable and unstable angina. European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group. Lancet. 1997;349:462–466. doi: 10.1016/s0140-6736(96)07591-5. [DOI] [PubMed] [Google Scholar]
- 8.Ridker PM, Cushman M, Stampfer MJ, et al. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 9.Koenig W, Sund M, Frohlich M, et al. C-Reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA Augsburg Cohort Study, 1984 to 1992. Circulation. 1999;99:237–242. doi: 10.1161/01.cir.99.2.237. [DOI] [PubMed] [Google Scholar]
- 10.Kuller LH, Tracy RP, Shaten J, et al. Relation of C-reactive protein and coronary heart disease in the MRFIT nested case-control study. Am J Epidemiol. 1996;144:537–547. doi: 10.1093/oxfordjournals.aje.a008963. [DOI] [PubMed] [Google Scholar]
- 11.Tracy RP, Lemaitre RN, Psaty BM, et al. Relationship of C-reactive protein to risk of cardiovascular disease in the elderly: results from the Cardiovascular Health Study and the Rural Health Promotion Project. Arterioscler Thromb Vasc Biol. 1997;17:1121–1127. doi: 10.1161/01.atv.17.6.1121. [DOI] [PubMed] [Google Scholar]
- 12.Ridker PM, Buring JE, Shih J, et al. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98:731–733. doi: 10.1161/01.cir.98.8.731. [DOI] [PubMed] [Google Scholar]
- 13.Rossi E, Biasucci LM, Citterio F, et al. Risk of myocardial infarction and angina in patients with severe peripheral vascular disease: predictive role of C-reactive protein. Circulation. 2002;105:800–803. doi: 10.1161/hc0702.104126. [DOI] [PubMed] [Google Scholar]
- 14.Zebrack JS, Muhlestein JB, Horne BD, et al. C-reactive protein and angiographic coronary artery disease: independent and additive predictors of risk in subjects with angina. J Am Coll Cardiol. 2002;39:632–637. doi: 10.1016/s0735-1097(01)01804-6. [DOI] [PubMed] [Google Scholar]
- 15.Ridker PM. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation. 2001;103:1813–1818. doi: 10.1161/01.cir.103.13.1813. [DOI] [PubMed] [Google Scholar]
- 16.Hunt ME, O'Malley PG, Vernalis MN, et al. C-reactive protein is not associated with the presence or extent of calcified subclinical atherosclerosis. Am Heart J. 2001;141:206–210. doi: 10.1067/mhj.2001.112488. [DOI] [PubMed] [Google Scholar]
- 17.Redberg RF, Rifai N, Gee L, et al. Lack of association of C-reactive protein and coronary calcium by electron beam computed tomography in postmenopausal women: implications for coronary artery disease screening. J Am Coll Cardiol. 2000;36:39–43. doi: 10.1016/s0735-1097(00)00680-x. [DOI] [PubMed] [Google Scholar]
- 18.Bazzino O, Ferreiros ER, Pizarro R, et al. C-reactive protein and the stress tests for the risk stratification of patients recovering from unstable angina pectoris. Am J Cardiol. 2001;87:1235–1239. doi: 10.1016/s0002-9149(01)01511-9. [DOI] [PubMed] [Google Scholar]
- 19.Anderson JL, Carlquist JF, Muhlestein JB, et al. Evaluation of C-reactive protein, an inflammatory marker, and infectious serology as risk factors for coronary artery disease and myocardial infarction. J Am Coll Cardiol. 1998;32:35–41. doi: 10.1016/s0735-1097(98)00203-4. [DOI] [PubMed] [Google Scholar]
- 20.Haidari M, Javadi E, Sadeghi B, et al. Evaluation of C-reactive protein, a sensitive marker of inflammation, as a risk factor for stable coronary artery disease. Clin Biochem. 2001;34:309–315. doi: 10.1016/s0009-9120(01)00227-2. [DOI] [PubMed] [Google Scholar]
- 21.Azar RR, Aoun G, Fram DB, et al. Relation of C-reactive protein to extent and severity of coronary narrowing in patients with stable angina pectoris or abnormal exercise tests. Am J Cardiol. 2000;86:205–207. doi: 10.1016/s0002-9149(00)00856-0. [DOI] [PubMed] [Google Scholar]
- 22.Ambrose JA, Tannenbaum MA, Alexopoulos D, et al. Angiographic progression of coronary artery disease and the development of myocardial infarction. J Am Coll Cardiol. 1988;12:56–62. doi: 10.1016/0735-1097(88)90356-7. [DOI] [PubMed] [Google Scholar]
- 23.Shaw LJ, Peterson ED, Shaw LK, et al. Use of a prognostic treadmill score in identifying diagnostic coronary disease subgroups. Circulation. 1998;98:1622–1630. doi: 10.1161/01.cir.98.16.1622. [DOI] [PubMed] [Google Scholar]
- 24.Spertus JA, Winder JA, Dewhurst TA, et al. Development and evaluation of the Seattle Angina Questionnaire. J Am Coll Cardiol. 1995;25:333–341. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]
- 25.Rifai N, Ridker PM. Proposed cardiovascular risk assessment algorithm using high-sensitivity C-reactive protein and lipid screening. Clin Chem. 2001;47:28–30. [PubMed] [Google Scholar]
- 26.Levinson SS, Elin RJ. What is C-reactive protein telling us about coronary artery disease? Arch Intern Med. 2002;162:389–392. doi: 10.1001/archinte.162.4.389. [DOI] [PubMed] [Google Scholar]
- 27.Deedwania PC, Carbajal EV. Exercise test predictors of ambulatory silent ischemia during daily life in stable angina pectoris. Am J Cardiol. 1990;66:1151–1156. doi: 10.1016/0002-9149(90)91090-s. [DOI] [PubMed] [Google Scholar]
- 28.Horne BD, Muhlestein JB, Carlquist JF, et al. Statin therapy, lipid levels, C-reactive protein and the survival of patients with angiographically severe coronary artery disease. J Am Coll Cardiol. 2000;36:1774–1780. doi: 10.1016/s0735-1097(00)00950-5. [DOI] [PubMed] [Google Scholar]
- 29.Albert MA, Danielson E, Rifai N, et al. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE) study. JAMA. 2001;286:64–70. doi: 10.1001/jama.286.1.64. [DOI] [PubMed] [Google Scholar]
- 30.Ridker PM, Rifai N, Clearfield M, et al. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med. 2001;344:1959–1965. doi: 10.1056/NEJM200106283442601. [DOI] [PubMed] [Google Scholar]
- 31.Ohtsuka T, Hamada M, Hiasa G, et al. Effect of beta-blockers on circulating levels of inflammatory and anti-inflammatory cytokines in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2001;37:412–417. doi: 10.1016/s0735-1097(00)01121-9. [DOI] [PubMed] [Google Scholar]


