Abstract
Properly apportioning the loads of metals in highway stormwater runoff to the appropriate sources requires accurate data on source composition, especially regarding constituents that help to distinguish among sources. Representative tire and brake samples were collected from privately owned vehicles and aqueous extracts were analyzed for twenty-eight elements. Correlation principal components analysis (PCA) revealed that tires were most influenced by Zn, Pb, and Cu, while brakes were best characterized by Na and Fe followed by Ba, Cu, Mg, Mn, and K; the latter three may be due to roadside soil contributions. Notably elevated Cd contributions were found in several brake samples. A targeted Cd-plated brake rotor was sampled, producing results consistent with the elevated levels found in the larger sample population. This enriched source of Cd is of particular concern due to high toxicity of Cd in aquatic ecosystems.
Keywords: metals, source apportionment, source characterization, principal components analysis, cadmium, brake rotors
INTRODUCTION
Stormwater runoff from roadways has been observed to contain many metals including zinc (Zn), cadmium (Cd), copper (Cu), nickel (Ni), lead (Pb), chromium (Cr), manganese (Mn), iron (Fe), vanadium (V), cobalt (Co), and aluminum (Al) (Sansalone and Buchberger, 1997; Westerlund and Viklander, 2006) and other constituents including arsenic (As), barium (Ba), beryllium (Be), chlorine (Cl), fluorine (F), potassium (K), magnesium (Mg), sodium (Na), antimony (Sb), selenium (Se), and calcium (Ca) (Dannecker et al., 1990). Important contributors to element loads on roadway surfaces include tires (Zn: Adachi and Tainosho, 2004; Davis et al., 2001), brakes (Cu, Pb, Zn, Sb: Davis et al., 2001; Legret and Pagotto, 1999; von Uexkull et al., 2005), yellow paint (Cr, Pb: Adachi and Tainosho, 2004), adjacent structures (Pb, Cu, Cd, and Zn: Davis et al., 2001), and atmospheric deposition (Cd: Davis et al., 2001).
Sorme and Lagerkvist (2002) completed a comprehensive budget for selected elements (Ni, Cu, Zn, Cd, Hg, and Pb) for a Swedish combined wastewater treatment plant. Transportation was estimated to be responsible for the following loads: 10–11% of total Zn, 9–11% of Pb, 5% of Cu, 1% of Cd, and less than 1% of Cr and Ni; they were unable to make a reasonable estimate for Hg. Car washes, which could represent vehicular elemental contributions to stormwater during a precipitation event, were a significant source of Pb, Cd, Zn, and Cr. Davis et al. (2001) considered roadway associated nonpoint sources in Maryland. They estimated that automobiles were responsible for 48% of Cu, 29% of Zn, 15% of Cd, and 2% of Pb. In a comprehensive review of long-term trends in metal contamination of San Francisco Bay (Flegal et al., 2005), Pb isotopes indicated leaded fuel as the primary source of Pb in the bay sediment and suspended solids. Additionally, Li (2006) confirmed Pb persistence in Canadian roadside soil well after the phase-out of leaded automotive fuels; elevated Pb concentrations were found in the top 0.3 m of roadside soil. As discussed by Barrett (2008) and statistically analyzed by Irish et al. (1998), spray from traffic on wet traditional pavement can “wash” the under-carriage of automobiles, resulting in an increase in constituents including copper and zinc.
Source characterization is a key step in understanding and controlling constituent loads to receiving waters. By characterizing nonpoint sources, their relative properties and contributions are revealed. Several studies have reported the characteristics of brake and tire wear particles, but sample collection and analysis methodologies have varied widely. Brake and tire sampling methods have typically sacrificed representativeness of tires and brakes within the current vehicle population for the sake of sample purity. Sample processing and analysis has been conducted on individual particles (Adachi and Tainosho, 2004), on bulk samples with x-ray diffraction (XRD) (von Uexkull et al., 2005) or proton induced x-ray emission (PIXE: Garg et al., 2000), and on dilution acid extracted or digested bulk samples with Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-AES), Graphite Furnace Atomic Adsorption Spectrometry (GFAAS), or Atomic Adsorption Spectrometry (AAS; Davis et al., 2001; Legret and Pagotto, 1999; von Uexkull et al., 2005; Westerlund, 2001; personal communication with Legret). This study aimed to characterize brake and tire associated constituents that would be produced under normal consumer usage conditions and that might be released to roadway surfaces while driving during dry weather or under storm conditions.
METHODOLOGY
Sample Collection
In previous studies, brake sampling methods have included: grinding up new brake pads (Legret and Pagotto, 1999; personal communication with Legret), washing parts with synthetic rain (Davis et al., 2001), employing a dynamometer (Committee, 2006; Garg et al., 2000; Sanders et al., 2003; von Uexkull et al., 2005), collection of dry debris with a spoon (von Uexkull et al., 2005) or tissue paper (Adachi and Tainosho, 2004; personal communication with Adachi), and collection of airborne particulate matter in driving tests in a wind tunnel and on public roads (Sanders et al., 2003). Tire sampling methods included abrading with a steel brush (Davis et al., 2001), chipping off small pieces with a blade (Legret and Pagotto, 1999; personal communication with Legret), and collecting street dust (Adachi and Tainosho, 2004).
In this study, tire and brakes samples were collected from a tire service center located in Rancho Cordova, CA, which services roughly 1,000 vehicles per month and addresses only tire related issues. A metal-free buffered solution (pH~5), used to simulate water that washes the vehicle undercarriage during a rainstorm, conveying tire and brake constituents to stormwater runoff, was used for all sampling events. The metal-free buffer contained 0.02 M ammonium acetate (Ultra Pure Grade, ICN Biochemicals, Aurora, OH, USA; a commonly used liquid chromatography buffer) and 0.02 M glacial acetic acid (Baseline grade, Seastar Chemicals Inc, Sidney, BC, Canada) in double deionized water (Milli-Q A10, Millipore, Billerica, MA, USA). The resulting ionic strength was similar to the maximum value measured in California highway runoff (electrical conductivity was converted to ionic strength using the Russell approximation; Kayhanian et al., 2007). After mixing, the solution was solvent extracted with hexane (Optima Grade, Fisher Scientific, Fairlawn, NJ) to minimize organic content prior to sampling. Sample blanks were collected by pouring metal-free buffer directly into a clean amber glass bottle at the sampling location. All sample and blank bottles were sealed with Teflon lined caps and stored at 4°C. Sample summaries from the two collection trips are as follows: trip 1) 30 tire samples, 30 brake samples, and 7 blanks; trip 2) 26 tire samples, 23 brake samples, and 4 blanks.
Samples were collected from tires and brakes used by consumers under their own individual usage patterns which may have varied greatly. Samples were collected by extracting tire or brake surfaces with aqueous solutions; the methods employed in this study resulted in the collection and analysis of particle-bound and dissolved constituents. Consequently, the samples may have included constituents from a variety of non-vehicular sources, such as roadside soil or atmospheric particulate matter that were present on the tire/brake surfaces at the time of sampling. This approach was taken to allow the sampling of a diverse set of tire and brake samples under actual usage conditions and based on the idea that similarities among the brake or tire samples and differences between the categories could be discerned after analysis using appropriate statistical techniques discussed below.
Tires were sampled by removing portions of the tire’s exterior tread with a razor blade. The tire sample was transferred to an amber bottle and immediately submerged in metal-free buffer. Only tires that were slated for disposal were included in this study – tire wear varied and included tires with moderate to severe wear.
Following tire removal, a brake pad/rotor composite sample was collected from the disc brake assembly. No drum brakes were included in this study. Metal-free buffer was sprayed onto the brake pad/rotor interface and was then collected as it dripped from the brake assembly with a glass funnel and an amber bottle. Several of the brake samples had anomalous compositions. To further investigate this, a targeted sample of a Cd plated brake rotor was collected in a residential garage; the same sampling methodology was employed, however no blanks were collected. Due to the fact that this was a targeted sample, it was not included in further analysis for group results.
Sample Analysis
Samples were acidified to 1% (v/v) nitric acid (trace metal grade, Fisher Scientific) and were analyzed within six months with an Inductively Coupled Plasma Mass Spectrometer (ICP-MS; Agilent 7500i, Ar plasma at 1350W; EPA method 6020) to quantify twenty eight elements: Al, Sb, As, Ba, Be, Cd, Ca, Cs, Cr, Co, Cu, Ga, Fe, Pb, Li, Mg, Mn, Ni, K, Rb, Se, Ag, Na, Sr, Tl, U, V, and Zn. Prepared samples were introduced for processing using a peristaltic pump at 0.4 mL/min with 1.2 L/min carrier gas flow through a Babbington-style nebulizer into a Peltier-cooled double-pass spray-chamber at 2°C. Oxides were tuned to <0.4% CeO/Ce and double ions to 1.5% Ce++/Ce+. Sensitivity (cps/ppb) was 30k for Y and 20k for Li and Tl. External calibration standards are prepared using NIST-traceable standards from SpexCertiprep (Methuen, NJ) from 0.01 ppb to 100 ppb for minor elements, and up to 100,000 ppb for major elements.
RESULTS AND DISCUSSION
Elemental Composition of Brakes and Tires
Inter-sample elemental concentrations in the extracts, for both brake and tire samples, varied widely, which made direct concentration comparisons difficult. Elemental mass percents of ICP-MS measured elements (Mi/ΣM · 100%; where Mi is the mass of measured element i) were used to compare samples. In this study, all mass percentages are based on the total mass of ICP-MS measured elements, which does not include all elements. Table 1 summarizes the sample results, while comparisons to published values are shown in Table 2.
Table 1.
Summary statistics on mass percent composition of tires and brakes extracts and blank samples
| Mass Percent (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Tire Samples (n=56) | Brake Samples (n=53) | Sample Blanks (n=11) | |||||||
| Element Measured | Arithmetic Mean | Max | RSD% | Arithmetic Mean | Max | RSD% | Arithmetic Mean | Max | RSD% |
| Al | 2.6 | 6.7 | 49.0 | 9.7E-01 | 5.1 | 104.6 | 1.0 | 2.4 | 79.2 |
| Ba | 1.9 | 5.1 | 53.6 | 5.8 | 16.3 | 70.9 | 8.8E-01 | 4.9 | 158.2 |
| Ca | 23.9 | 75.7 | 51.4 | 6.7 | 22.0 | 64.1 | 12.1 | 21.4 | 51.5 |
| Fe | 9.0 | 21.7 | 57.6 | 47.4 | 85.1 | 44.0 | 1.7 | 5.3 | 80.4 |
| K | 3.5 | 6.4 | 34.6 | 5.7 | 41.3 | 119.1 | 2.5 | 4.9 | 48.8 |
| Mg | 3.7 | 18.2 | 63.4 | 7.6 | 47.7 | 115.5 | 1.3 | 3.3 | 76.3 |
| Na | 29.8 | 68.8 | 43.9 | 10.7 | 41.2 | 83.4 | 78.9 | 89.7 | 9.5 |
| Pb | 10.3 | 81.1 | 141.3 | 3.0E-01 | 3.5 | 220.8 | 4.6E-01 | 1.7 | 122.4 |
| Zn | 13.5 | 32.2 | 44.2 | 6.0 | 54.4 | 181.4 | 5.6E-01 | 1.5 | 67.7 |
| Ag | 2.2E-04 | 6.2E-04 | 69.8 | 2.4E-04 | 1.7E-03 | 137.2 | 1.4E-03 | 4.1E-03 | 122.8 |
| As | 5.3E-03 | 1.5E-02 | 69.0 | 8.1E-03 | 1.8E-01 | 308.1 | 4.5E-03 | 2.2E-02 | 166.9 |
| Be | 2.2E-04 | 4.9E-04 | 41.6 | 6.4E-05 | 5.6E-04 | 133.3 | 9.0E-04 | 2.6E-03 | 105.5 |
| Cd | 2.8E-02 | 1.0E-01 | 101.5 | 7.9E-01 | 39.4 | 683.7 | 2.3E-02 | 5.0E-02 | 63.6 |
| Co | 1.3E-02 | 6.6E-02 | 84.6 | 8.8E-03 | 3.1E-02 | 63.2 | 1.4E-03 | 2.6E-03 | 58.7 |
| Cr | 2.1E-01 | 2.3 | 241.7 | 1.0E-01 | 6.2E-01 | 110.5 | 5.8E-02 | 1.3E-01 | 53.9 |
| Cs | 3.7E-04 | 1.1E-03 | 63.6 | 2.9E-04 | 3.5E-03 | 173.1 | 1.6E-03 | 5.7E-03 | 120.3 |
| Cu | 8.6E-01 | 2.8 | 68.3 | 6.6 | 26.4 | 116.4 | 3.0E-01 | 1.3 | 126.2 |
| Ga | 1.5E-04 | 3.6E-04 | 51.5 | 5.1E-04 | 1.3E-03 | 68.6 | 7.0E-05 | 3.1E-04 | 131.2 |
| Li | 1.3E-02 | 8.2E-02 | 96.6 | 1.7E-02 | 4.3E-01 | 354.9 | 3.8E-03 | 6.7E-03 | 41.5 |
| Mn | 3.4E-01 | 6.0E-01 | 37.0 | 9.9E-01 | 2.9 | 62.5 | 2.3E-02 | 8.2E-02 | 102.7 |
| Ni | 8.9E-02 | 2.7E-01 | 59.3 | 5.1E-02 | 2.7E-01 | 91.5 | 1.2E-02 | 5.5E-02 | 119.0 |
| Rb | 4.6E-03 | 9.2E-03 | 32.7 | 9.5E-03 | 6.1E-02 | 126.9 | 3.1E-03 | 6.4E-03 | 66.6 |
| Sb | 6.0E-05 | 1.7E-04 | 92.5 | 1.0E-03 | 3.1E-02 | 461.9 | 2.1E-04 | 8.6E-04 | 127.8 |
| Se | 6.5E-03 | 3.0E-02 | 119.9 | 7.7E-04 | 4.7E-03 | 103.3 | 2.2E-02 | 8.1E-02 | 118.5 |
| Sr | 1.7E-01 | 4.3E-01 | 43.3 | 1.9E-01 | 1.8 | 128.6 | 5.1E-02 | 7.8E-02 | 36.0 |
| Tl | 8.7E-05 | 2.4E-04 | 77.3 | 6.4E-05 | 3.6E-04 | 125.2 | 1.2E-03 | 4.0E-03 | 130.4 |
| U | 3.1E-04 | 6.3E-04 | 43.9 | 1.1E-04 | 7.8E-04 | 133.7 | 1.9E-03 | 7.6E-03 | 133.7 |
| V | 8.6E-03 | 1.5E-02 | 35.5 | 9.6E-03 | 8.2E-02 | 138.6 | 7.6E-03 | 2.9E-02 | 122.5 |
Table 2.
Comparison of reported brake and tire elemental compositions; in parentheses, brake Zn-normalized (X/Zn) and tire Cu-normalized (X/Cu) values are shown
| Source | Units | Zn | Cu | Pb | Cd | Cr | Ni | Sb | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brakes | |||||||||||||||
| Davis et al. (2001)a, h | mg/L | 0.33 | (−) | 0.28 | (0.85) | 0.011 | (0.03) | 1.9E-3 | (0.01) | n/a | (−) | n/a | (−) | n/a | (−) |
| This studyh | mg/L | 630 | (−) | 740 | (1.2) | 2.9 | (<0.01) | 1.8g | (<0.01) | 42 | (0.07) | 10 | (0.02) | 3.7E-04 | (<0.01) |
| Legret and Pagotto (1999)a, i | mg/kg | 21,800 | (−) | 142,000 | (6.5) | 3,900 | (0.18) | 2.7 | (<0.01) | n/a | (−) | n/a | (−) | n/a | (−) |
| Westerlund (2001)b, j | mg/kg | 23,830 | (−) | 117,941 | (5.0) | 9,052 | (0.38) | 11.6 | (<0.01) | 137 | (0.01) | 141 | (0.01) | n/a | (−) |
| von Uexkull et al. (2005)c, j | mg/kg | 20,000 | (−) | 20,000 | (1.0) | 510 | (0.03) | 57 | (<0.01) | 8,700 | (0.44) | 190 | (0.01) | 43,000 | (2.2) |
| Adachi and Tainosho (2004)d, e | mg/kg | 1,000 | (−) | 12,000 | (12) | 3,000 | (3.0) | n/a | (−) | 0 | (0) | n/a | (−) | 9,000 | (9.0) |
| This studye, h | mg/kg | 60,000 | (−) | 66,000 | (1.1) | 3,041 | (0.05) | 86g | (<0.01) | 1,000 | (0.02) | 510 | (0.01) | 10 | (<0.01) |
| Tires | |||||||||||||||
| Davis et al. (2001)a, h | mg/kg | 3,400 | (680) | 5 | (−) | 17 | (3.4) | 1 | (0.20) | n/a | (−) | n/a | (−) | n/a | (−) |
| Legret and Pagotto (1999)a, i | mg/kg | 10,250 | (5,700) | 1.8 | (−) | 6.3 | (3.5) | 2.6 | (1.4) | n/a | (−) | n/a | (−) | n/a | (−) |
| This studye, h | mg/kg | 1.3E5 | (15) | 8,600 | (−) | 1.0E5 | (12) | 276 | (0.03) | 2,090 | (0.24) | 891 | (0.10) | 0.60 | (<0.01) |
| Roadside/background soil | |||||||||||||||
| Hjortenkrans et al. (2006)a, j | mg/kg | 48 | 8.3 | 13 | 0.16 | 10 | 4.4 | 0.46 | |||||||
| Bradford et al. (1996)f, j | mg/kg | 149 | 29 | 24 | 0.36 | 122 | 57 | 0.60 | |||||||
By GFAAS or AAS
Front Linings by ICP-MS
Disk brake pads by X-ray fluorescence or ICP-AES
Individual particles by Scanning Electron Microscope with Energy Dispersive Spectrometry
mg/kg calculated from measured elements, not total mass
CA average; Inductively Coupled Plasma Optical Emission Spectroscopy and ICP-MS
Does not include elevated population
Bulk samples – synthetic rainwater or metal-free buffer, then dilute acid extracted
Bulk sample – ashed and digested
Bulk sample – digested (various methods)
Particle-bound and dissolved constituent contribution from the service center would be indicated in the blank analysis, however if the constituents were deposited/sorbed prior to entry into the garage, then they would not be included in blank analysis. For an element to be considered statistically significant, the sample populations (brake or tire) had to be statistically distinguishable from the blank population. Bonferroni mean contrasts, completed using R on the mass percent data, were restricted to tire-blank and brake-blank (g=2; Kutner et al., 2005). At the α=0.05 family-wise level, Li, V, Cr, As, Cd, and Sb were determined to be statistically insignificant.
Outliers for each statistically insignificant element were evaluated in R using DFFITS, a form of studentized deleted residuals; a point was considered an outlier if the DFFITSi > 2*(1/n)1/2 where n is the number of sample points (Kutner et al, 2005). Thirteen samples were identified as outliers: 0 out of 56 tire samples, 3 out of 11 blanks, and 10 out of 53 brake samples. The following are the number of outliers per element: Li (1), V (2), Cr (0), As (1), Cd (5), and Sb (7). All contrasts remained statistically insignificant when the outliers were excluded. Three brake samples were outliers for both Cd and Sb; no linear relationship was observed between Cd and Sb when either all samples or just brake samples were considered. Unless otherwise noted, all sample extracts were included in further analysis.
Elemental concentrations in the brake samples were more variable than either the tire samples or the blanks. Sanders et al. (2003) provided background on the variability in manufacturer brake pad composition and Westerlund (2001) observed wide variability in metal contributions from different brake pads. Brake arithmetic mean and median values differ by more than a factor of five for more than half of the elements analyzed and differ by factors of 53 and 117 for the Pb and Cd respectively.
Comparisons among the references in Table 2 were made more difficult by differences in sampling and analysis techniques; to provide the broadest bases for comparisons among results, both aqueous (mg/L) and solid (mg/kg) concentrations have been included in the table. Furthermore, the mass fractions presented in this study were the mass fraction of elements measured by ICP-MS and not the mass fraction of the total sample mass; this is also true for the work of Adachi and Tainosho (2004). In this study, the sum of the 28 included elements represented only 16.9% of averaged California background soil (Bradford et al., 1996), for example. Elemental ratios across the different studies should be more consistent because this might account for variations in extraction/digestion efficiency or particle-solution ratios. Zn was used as the brake normalizer (X/Zn) and Cu as the tire normalizer (X/Cu). Zn and Cu were selected because they had no sample outliers in this data set, indicating greater consistency, were included in all previous studies considered in Table 2, and are not major contributors to brakes and tires, respectively. Additionally, as discussed by Hwang et al. (2006), differences in extraction/digestion procedures can significantly influence resulting concentrations. Among the studies compared in Table 2, results from studies that employed dilute acid extraction were not systematically different from those employing various digestion methods.
Brake comparisons in Table 2 are restricted to: Zn, Cu, Pb, Cd, Cr, Ni, and Sb. Most likely due to the SEM-EDS analytical technique, which probes a defined volume of an individual particle, ratios from Adachi and Tainosho’s work (2004) are significantly different than all other presented values. In most studies, Cu was the most abundant element measured in brakes and was enriched over Zn (Cu/Zn > 1). Zn was often the second most abundant. Cd was commonly the least abundant of the metals considered. Pb, Cd, Cr, and Ni were all consistently less abundant than Zn (X/Zn<1); Sb/Zn spanned seven orders of magnitude, including values both greater than and less than unity. Ni/Zn brake ratios were the most consistent, all on the order of ~ 0.01 which was consistently less than the soil Ni/Zn ratio.
Comparisons among elemental ratios (X/Cu) for tires were possible only for Zn, Pb, and Cd. Zn was the most abundant in all cases presented; Pb was also always enriched compared to Cu (Pb/Cu>1), while Cd was found to be enriched by Legret and Pagotto (1999), but depleted compared to Cu by Davis et al. (2001) and in this study. All values for Zn, Pb, and Cd were noticeably different from the soil ratios, as were this study’s ratios for Cr, Ni, and Sb. The soil ratios (X/Cu) from Hjortenkrans et al. (2006) and Bradford et al. (1996) show reasonable agreement for most elements, except Cr and Ni; in these cases, Hjortenkrans et al. found them both to be depleted compared to Cu, while Bradford et al. found them both to be enriched.
Principal Components Analysis
Correlation principal components analysis (PCA) is a statistical method that transforms a multi-dimensional data set into a new vector space with orthogonal axes. Orthogonality eliminates redundancy and maximizes variance along the principal factors or axes. PCA has previously been used for source apportionment (Larsen and Baker, 2003; Topalian et al., 1999), however here the primary sources are known, and so it is used to elucidate elemental relationships and source markers.
PCA was applied using two separate input data transformations: the mass fraction data and the data normalized to Al (Xi/Ali for element X in sample i); only the elements that were considered statistically significant were included. PCA performed directly on the mass fraction data revealed inherent variance in the data. In contrast, normalizing to an abundant crustal element reduced the variance contributed by crustal materials; Al was selected due to its substantial crustal abundance (California average soil background Al is 7.3 % (Bradford et al., 1996)) and its minimal contribution to overall un-normalized variance according to the mass fraction PCA. The principal components are shown in Table 3 and the samples, transformed to fit the new orthogonal axes, are shown in Figure 1. Factors that contributed to the first 85% of the cumulative variance were included. In the following discussion, factors will be referred to as F1A, F2A, and F3A for the first, second, and third factors of the mass fraction PCA and similarly F1B and F2B for the respective Al normalized PCA factors.
Table 3.
Correlation principal components; only non-zero values included
| X or X/Al | Na | Mg | Al | K | Ca | Mn | Fe | Cu | Zn | Ba | Pb | Variance (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mass Fraction (Un-Normalized) Principal Components | ||||||||||||
| F1A | −0.60 | 0.05 | −0.02 | 0.01 | −0.19 | 0.01 | 0.77 | 0.05 | −0.06 | 0.05 | −0.07 | 58.1 |
| F2A | −0.70 | 0.03 | 0.02 | 0.04 | 0.39 | 0.00 | −0.40 | 0.03 | 0.26 | 0.01 | 0.35 | 20.6 |
| F3A | 0.09 | −0.07 | −0.03 | −0.09 | −0.22 | 0.00 | 0.08 | −0.14 | −0.41 | −0.06 | 0.86 | 6.7 |
| Al Normalized Principal Components | ||||||||||||
| F1B | −0.01 | −0.05 | (−) | −0.01 | −0.01 | −0.02 | −1.00 | −0.01 | −0.03 | −0.04 | 0.00 | 71.7 |
| F2B | 0.98 | 0.01 | (−) | 0.02 | 0.19 | 0.00 | −0.01 | −0.01 | −0.02 | 0.00 | −0.01 | 22.3 |
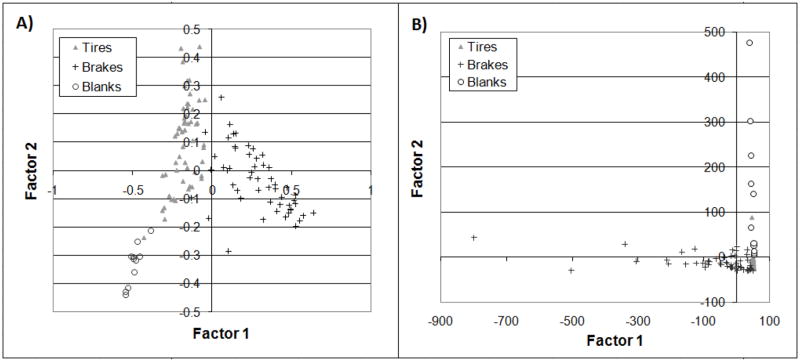
Figure 1.
Correlation PCA transformed sample data. A) Mass fraction data. B) Al normalized data.
F1A was characterized by strong influences of Fe (+) and Na (−), and less significantly by Mg, Cu, Ba, Mn and K (+) and Ca, Pb, Zn, and Al (−). F2A groups Fe and Na were strongly negative, while Ca, Pb, and Zn were strongly positive, and K, Cu, Mg, Al, and Ba were slightly positive. Again in F3A, Na and Fe were together (slightly positive) and Pb was strongly positive; Zn and Ca were strongly negative with Cu, K, Mg, Ba, and Al slightly negative.
Commonalities in the mass fraction factor loadings indicate correlations in elemental data and variances that could implicate common sources; factor signs were arbitrary. Among the three un-normalized components, Zn and Ca exhibited similar loadings in all three factors. Mg, Cu, Ba, and K also exhibited similar loadings, however the loadings were less significant (closer to 0). Interestingly, Na and Fe were on opposite ends of the spectrum in the first factor, but were grouped in the second and third factors, which may indicate multiple sources of those ubiquitous elements. For example, Na in the highway environment may come from crustal materials, atmospheric transport and deposition of sea salt aerosol, roadway de-icing salt or evaporation of rainfall containing Na.
Tire samples were most clearly defined by negative F1A and positive F1B. Negative F1A was characterized by Na, Ca, Pb, Zn, and Al, while F2A was dominated by Ca, Pb, and Zn with minor contributions from K, Cu, Mg, Al, and Ba. Positive F1B was characterized only by small Pb factor loadings (0.005), but negative F2B included Zn, Fe, Cu, and Pb. Tire samples had strong commonalities with Zn and Pb followed by Cu. The influence of Zn can be explained by its use as a major component of tire production (~1 % by mass; Councell et al., 2004). Sorme and Lagerkvist (2002) estimated that tires were the most significant source of roadway associated Zn and Davis et al. (2001) estimated that tires were responsible for 25% of the Zn in urban runoff. Pb and Cu have both been previously associated with tire wear (Davis et al., 2001; Legret and Pagotto, 1999), however as in these results, their mass contributions, as well as their significance in identifying tire samples, was considerably less than that of Zn. Pb may also be a signature of roadside particles that were deposited on the tire prior to entering the garage. In spite of being phased out of gasoline in the United States starting in 1973, Pb has continued to be found at elevated levels in roadside particles (Preciado and Li, 2006).
The brake samples were most clearly aligned with the positive F1A and the negative F1B axes, both of which indicated Fe, Mg, Cu, Ba, Mn, and K influences. F1B also included Zn, Ca, and Na. Fe has been previously identified as a significant brake pad component (Adachi and Tainosho, 2004; Garg et al., 2000; Hur et al., 2003). The presence of Cu has been noted in many publications (Adachi and Tainosho, 2004; Davis et al., 2001; Garg et al., 2000; Hur et al., 2003). For example, brake pads are usually composed of binder materials, fibers (often containing Cu), filler, and frictional additives (Garg et al., 2000). Cu was found in more than ten different components including copper fiber, copper powder, brass fiber, bronze powder, and copper fiber with copper sulfide and organic content (Committee, 2006). Cu is used extensively in the production of brake pads as a friction and binding agent and it is currently believed that these materials may be a major contributor to the copper loads in stormwater in many areas including ecologically sensitive areas like San Francisco Bay (Committee, 2006). By contrast, the presence of Ba has received little attention and was only reported previously by Sternbeck et al. (2002). Fe, Mg, Mn, and K are all crustal elements and may be indicative of soil particles adhering to brake assembly components and being collected with the brake sample.
The blanks were most clearly defined by the −F1A/−F2A and the +F1B/+F2B regions which jointly were strongly characterized by Na and Ca. Additionally −F1A/−F2A included Fe, Pb, Zn, and Al and +F2B included K and Mg. Each of the blank elements, present in the tire center background, was found in the tires and/or brakes. Na, Ca, Fe, Al, K, and Mg are all important crustal elements. The blanks/tires fell on the same side of F1, opposite the brakes samples, in both PCA approaches. This indicated that blanks and tires share more characteristics than either does with brakes. From the un-normalized data (A), blanks can be distinguished from tire samples by their elevated Na and Fe mass fractions; similarly, the normalized blanks (B) can be distinguished by the Na contributions. Arithmetic mean mass fractions for Na and Fe were as follows: Nablanks = 0.789, Feblanks = 0.017; Natires = 0.298, Fetires = 0.090.
Cadmium Lined Brake Parts
The elemental analysis performed on the brake wear samples yielded a significant statistical anomaly with regards to highly elevated cadmium concentrations; occasional brake samples with elevated Cd content were also noted by Westerlund (2001).
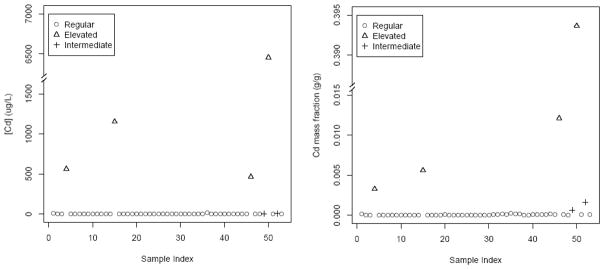
Samples were deemed to be part of a separate elevated population if they contained greater than C̄d+5* σCd. On a concentration basis, four samples were considered elevated, while on a mass fraction basis, the same four plus two others were considered elevated. The four samples that were included on both bases are listed as “elevated,” the two that were only considered elevated on a mass fraction basis are shown as “intermediate,” and all others are considered “regular.” Based on inspection of Figure 2 the intermediates were included in the regular population, and the four samples that were identified in both bases were considered the elevated population. Of these four, two came from sample trip 1 and two came from sample trip 2.
Figure 2.
Cd concentrations and mass fractions
The regular population had a mean extract Cd mass fraction=4.36e-5 g g−1 measured (1.8 μg L−1) and the elevated population had a mean Cd mass fraction=7.16e-2 g g−1 measured (2159 μg L−1). Using either concentration or mass fraction as a basis, the elevated population mean was >1200 times the regular population extract Cd mean. The mean mass fraction for all Cd data points was ~180 times greater than the segregated regular population. In spite of defining two populations, mass fraction coefficients of variation were reduced but remained high for both populations: CVAll = 684%, CVReg = 139%, and CVElev = 228%. Clearly, variance among the elevated population remained high; one sample contained ~40% Cd, which far exceeded all other samples.
It was hypothesized that the significantly elevated Cd concentrations were due to Cd plated brake parts, especially rotors. Cadmium lined brake parts have been manufactured and marketed as far back as the 1928 Ford Model A (Ford Motor Co., 1927) as an aesthetic solution to prevent brake corrosion; cadmium brake plating is now employed to minimize squeaking while braking. The practice of plating brake parts, especially brake rotors, continues today with a wide variety of aftermarket racing rotors available with cadmium, zinc, or nickel plating. Zn and Ni, also used in brake plating, contained outliers on both mass fraction and concentration bases, but none of them were as extreme as those observed in the Cd data; Zn contained outliers to a greater degree than did Ni.
A Power Slot brand rotor, manufactured by Power Performance Group, Inc. (Chatsworth, CA), was sampled (same sample collection technique; no blanks) in a residential garage. At the time of the sample trip the rotors were nearing the end of their useful life and were scheduled to be replaced. The measured sampled Cd concentration was 19,890 μg L−1 Cd; of the 28 measured elements, the sample extract contained 62.1% Cd. While this sample was considerably more enriched in Cd than were any of the previous samples, it confirmed that the elevated Cd concentrations observed in samples from both sampling trips 1 and 2 are not likely to have been random outliers, but instead were drawn from a different population. These samples, presumably all from Cd plated brakes, also possibly constitute a noteworthy, mobile Cd source. Due to the significant wear already present on the rotors, it can be assumed that elevated Cd releases are dispersed and perhaps increase over the rotor lifespan.
Given the elevated toxicity of Cd in relation to Cu, it is important that it and any other unknown contaminants are identified and addressed while evaluating nonpoint sources. Among traffic related airborne particulates in Ireland and the U.K., Cd was found to be predominantly associated with the smallest size-resolved fraction (dp<0.5 μm), and across all analyzed size fractions (dp<0.5 μm to 7.2 μm), Cd was among the most soluble at ~35–50% soluble (Birmili et al, 2006). In California coastal marshes, sediment Cd was attributed to anthropogenic sources (Hwang et al., 2006). The elevated Cd findings emphasize that identifying nonpoint source characteristics is a key step toward reducing stormwater contaminant loads. This need was clearly demonstrated by the unexpected Cd concentrations discovered while examining the brake wear samples in this study, and the lack of similar results from previous studies despite the numerous evaluations of contaminants from brake sources in recent years.
CONCLUSION
Considerable variability was present among the samples, but clear grouping became apparent after applying principal components analysis. Intra-set variability is probably largely due to differences in manufacturer processes and practices. Brake samples were found to be characterized by Fe, Cu, and Ba, while tires were characterized by Zn, Pb, and Cu.
Several brake samples were found to contain significantly elevated Cd concentrations (>1200 times normal concentrations), which have been attributed to Cd plated brake rotors. Plated brakes could represent a significant nonpoint source for Cd, and potentially Zn and Ni, although significantly elevated concentrations were not observed in this study.
Acknowledgments
This research was conducted with financial support from the California Department of Transportation (contract number 43A0168) and from the National Institute of Environmental Health Sciences (NIEHS), NIH (grant number 5 P42 ES004699). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the sponsoring agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Erica R. McKenzie, Email: ermckenzie@ucdavis.edu.
Jon E. Money, Email: moneyj@SacCounty.NET.
Peter G. Green, Email: pggreen@ucdavis.edu.
References
- Adachi K, Tainosho Y. Characterization of heavy metal particles embedded in tire dust. Environ Int. 2004;30:1009–1017. doi: 10.1016/j.envint.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Barrett ME. Effects of a permeable friction course on highway runoff. J Irrig Drain E-Asce. 2008;134:646–651. [Google Scholar]
- Birmili W, Allen AG, Bary F, Harrison RM. Trace metal concentrations and water solubility in size-fractionated atmospheric particles and influence of road traffic. Environ Sci Technol. 2006;40:1144–1153. doi: 10.1021/es0486925. [DOI] [PubMed] [Google Scholar]
- Bradford GR, Chang AC, Page AL, Bakhtar D, Frampton JA, Wright H. Background Concentrations of Trace and Major Elements in California Soils. Kearney Foundation of Soil Science. 1996 [Google Scholar]
- Committee BMCPE. Generating a Representative Sample of Brake Pad Wear Debris. Brake Pad Partnership. 2006:12. < http://www.suscon.org/brakepad/pdfs/CompilationofReviewers%27CommentsonGeneratingaSampleofBPWDdraftReport11-17-05.pdf>.
- Councell TB, Duckenfield KU, Landa ER, Callender E. Tire-wear particles as a source of zinc to the environment. Environ Sci Technol. 2004;38:4206–4214. doi: 10.1021/es034631f. [DOI] [PubMed] [Google Scholar]
- Dannecker W, Au M, Stechmann H. Substance Load in Rainwater Runoff from Different Streets in Hamburg. Sci Total Environ. 1990;93:385–392. doi: 10.1016/0048-9697(90)90129-i. [DOI] [PubMed] [Google Scholar]
- Davis AP, Shokouhian M, Ni SB. Loading estimates of lead, copper, cadmium, and zinc in urban runoff from specific sources. Chemosphere. 2001;44:997–1009. doi: 10.1016/s0045-6535(00)00561-0. [DOI] [PubMed] [Google Scholar]
- Flegal AR, Conaway CH, Scelfo GM, Hibdon SA, Sanudo-Wilhelmy SA. A review of factors influencing measurements of decadal variations in metal contamination in San Francisco Bay, California. Ecotoxicology. 2005;14:645–660. doi: 10.1007/s10646-005-0016-6. [DOI] [PubMed] [Google Scholar]
- Ford Motor Company. Display Ad 23 – The New Ford Car will sell at a Surprisingly Low Price Complete details of the new car which will be officially announced this Friday. Wall St J (1889-Current file) 1927 December 1;:7. [Google Scholar]
- Garg BD, Cadle SH, Mulawa PA, Groblicki PJ, Laroo C, Parr GA. Brake wear particulate matter emissions. Environ Sci Technol. 2000;34:4463–4469. [Google Scholar]
- Hjortenkrans D, Bergback B, Haggerud A. New metal emission patterns in road traffic environments. Environ Monit Assess. 2006;117:85–98. doi: 10.1007/s10661-006-7706-2. [DOI] [PubMed] [Google Scholar]
- Hur J, Yim S, Schlautman MA. Copper leaching from brake wear debris in standard extraction solutions. J Environ Monit. 2003;5:837–843. doi: 10.1039/b303820c. [DOI] [PubMed] [Google Scholar]
- Hwang HM, Green PG, Higashi RM, Young TM. Tidal salt marsh sediment in California, USA. Part 2: Occurrence and anthropogenic input of trace metals. Chemosphere. 2006;54:1899–1909. doi: 10.1016/j.chemosphere.2006.01.053. [DOI] [PubMed] [Google Scholar]
- Irish LB, Barrett ME, Malina JF, Charbeneau RJ. Use of regression models for analyzing highway storm-water loads. J Environ Eng-Asce. 1998;124:987–993. [Google Scholar]
- Kutner M, Nachtsheim C, Li W, Neter J. Applied linear statistical models: ASA. 2005 [Google Scholar]
- Larsen RK, Baker JE. Source apportionment of polycyclic aromatic hydrocarbons in the urban atmosphere: A comparison of three methods. Environ Sci Technol. 2003;37:1873–1881. doi: 10.1021/es0206184. [DOI] [PubMed] [Google Scholar]
- Kayhanian M, Suverkropp C, Ruby A, Tsay K. Characterization and prediction of highway runoff constituent event mean concentration. J Environ Manag. 2007;85:279–295. doi: 10.1016/j.jenvman.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Legret M, Pagotto C. Evaluation of pollutant loadings in the runoff waters from a major rural highway. Sci Total Environ. 1999;235:143–150. doi: 10.1016/s0048-9697(99)00207-7. [DOI] [PubMed] [Google Scholar]
- Li LY. Retention capacity and environmental mobility of Pb in soils along highway corridor. Water Air Soil Pollut. 2006;170:211–227. [Google Scholar]
- Preciado HF, Li LY. Evaluation of metal loadings and bioavailability in air, water and soil along two highways of British Columbia, Canada. Water Air Soil Pollut. 2006;172:81–108. [Google Scholar]
- Sanders PG, Xu N, Dalka TM, Maricq MM. Airborne brake wear debris: Size distributions, composition, and a comparison of dynamometer and vehicle tests. Environ Sci Technol. 2003;37:4060–4069. doi: 10.1021/es034145s. [DOI] [PubMed] [Google Scholar]
- Sansalone JJ, Buchberger SG. Partitioning and first flush of metals in urban roadway storm water. J Environ Eng-Asce. 1997;123:134–143. [Google Scholar]
- Sorme L, Lagerkvist R. Sources of heavy metals in urban wastewater in Stockholm. Sci Total Environ. 2002;298:131–145. doi: 10.1016/s0048-9697(02)00197-3. [DOI] [PubMed] [Google Scholar]
- Sternbeck J, Sjodin A, Andreasson K. Metal emissions from road traffic and the influence of resuspension - results from two tunnel studies. Atmos Environ. 2002;36:4735–4744. [Google Scholar]
- Topalian ML, Castane PM, Rovedatti MG, Salibian A. Principal component analysis of dissolved heavy metals in water of the Reconquista river (Buenos Aires, Argentina) Bull Environ Contam Toxicol. 1999;63:484–490. doi: 10.1007/s001289901006. [DOI] [PubMed] [Google Scholar]
- von Uexkull O, Skerfving S, Doyle R, Braungart M. Antimony in brake pads - a carcinogenic component? J Clean Prod. 2005;13:19–31. [Google Scholar]
- Westerlund C, Viklander M. Particles and associated metals in road runoff during snowmelt and rainfall. Sci Total Environ. 2006;362:143–156. doi: 10.1016/j.scitotenv.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Westerlund KG. Metal emissions from Stockholm traffic–wear of brake linings. The Stockholm Environ Health Prot Admin. 2001;100:64. [Google Scholar]