Abstract
Background
Depression leads to adverse outcomes in patients with coronary heart disease (CHD). Medication nonadherence is a potential mechanism for the increased risk of CHD events associated with depression, but it is not known whether depression is associated with medication nonadherence in outpatients with stable CHD.
Methods
We examined the association between current major depression (assessed using the Diagnostic Interview Schedule) and self-reported medication adherence in a cross-sectional study of 940 outpatients with stable CHD.
Results
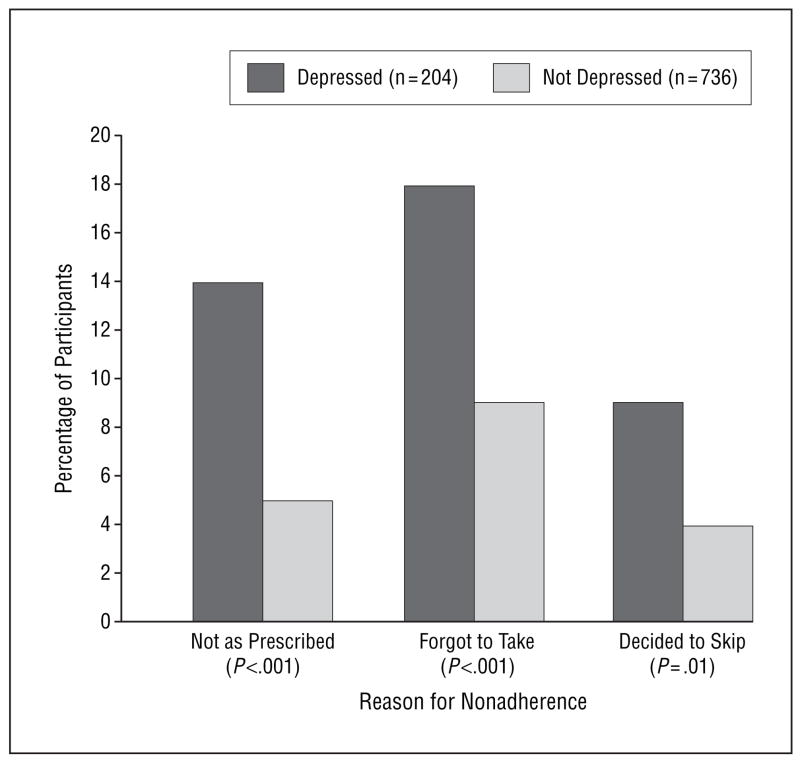
A total of 204 participants (22%) had major depression. Twenty-eight (14%) of 204 depressed participants reported not taking their medications as prescribed compared with 40 (5%) of 736 nondepressed participants (odds ratio [OR], 2.8; 95% confidence interval [CI], 1.7–4.7; P<.001). Twice as many depressed participants as nondepressed participants (18% vs 9%) reported forgetting to take their medications (OR, 2.4; 95% CI, 1.6–3.8; P<.001). Nine percent of depressed participants and 4% of nondepressed participants reported deciding to skip their medications (OR, 2.2; 95% CI, 1.2–4.2; P=.01). The relationship between depression and nonadherence persisted after adjustment for potential confounding variables, including age, ethnicity, education, social support, and measures of cardiac disease severity (OR, 2.2; 95% CI, 1.2–3.9; P=.009 for not taking medications as prescribed).
Conclusions
Depression is associated with medication nonadherence in outpatients with CHD. Medication nonadherence may contribute to adverse cardiovascular outcomes in depressed patients.
Major depression is an established risk factor for morbidity and mortality in patients with coronary heart disease (CHD).1–11 Recently, psychosocial factors, such as depression, have been identified as 1 of 9 modifiable risk factors, along with diabetes mellitus, smoking, and hypertension, that account for more than 90% of the risk of myocardial infarction worldwide.12 Several biological factors have been considered as potential mechanisms by which depression may lead to cardiac events, including increased sympathetic tone,13,14 increased catecholamine levels,15 increased cortisol levels,16 increased platelet activation, increased levels of inflammatory mediators,17 and antidepressant drug reactions.18 However, nonadherence to important cardiac prevention and treatment recommendations may also be associated with depression and could contribute to the increased risk of CHD events in depressed patients.19
Depression has been associated with medication nonadherence in the general population20 and in patients with human immunodeficiency virus,21,22 kidney transplants,23 hypertension,24,25 diabetes mellitus,26–30 and hyperlipidemia.31,32 Nonadherence to cardiac treatment recommendations has been linked to worse outcomes in cardiac patients.33 However, only 1 small study34 of 10 depressed and 45 nondepressed patients has examined the association between depression and medication adherence in patients with stable CHD, and no study, to our knowledge, has evaluated the relationship between depressive symptom severity and medication adherence.
To determine whether poor adherence is a mediator in the relationship between depression and CHD outcomes, we must first examine whether depression is associated with poor adherence in patients with stable CHD and whether this relationship differs by severity of depressive symptoms. We evaluated the association between depression and medication nonadherence in 940 outpatients with stable CHD from the Heart and Soul Study.
METHODS
PARTICIPANTS
The Heart and Soul Study is a prospective cohort study of psychosocial factors and health outcomes in patients with CHD. Details regarding recruitment procedures have been published previously.35 Briefly, we used administrative databases to identify outpatients with documented CHD at 2 Veterans Affairs Medical Centers (San Francisco and Palo Alto, Calif), 1 university medical center (University of California at San Francisco), and 9 community health clinics in northern California. Patients were eligible to participate if they had at least 1 of the following: a history of myocardial infarction, angiographic evidence of at least 50% stenosis in 1 or more coronary vessels, previous evidence of exercise-induced ischemia by treadmill or nuclear testing, a history of coronary artery revascularization, or a diagnosis of CHD by an internist or a cardiologist. Patients were excluded only if they could not walk 1 block or were planning to move from the local area within 3 years.
Of 1024 individuals enrolled between September 11, 2000, and December 31, 2002, 84 were excluded from the present analysis because they were not taking a cardiac medication (β-blocker, renin-angiotensin system inhibitor, aspirin, or statin), leaving 940 participants for this cross-sectional study. All the participants completed a daylong study examination that included a comprehensive health interview, blood sample collection, an exercise treadmill test with stress echocardiography, a 6-minute walk, 24-hour Holter monitoring, and 24-hour urine sample collection. The protocol was approved by the appropriate institutional review boards, and all the participants provided written informed consent.
PREDICTOR VARIABLES
Our primary predictor variable was current (past month) major depression, defined according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, criteria.36 To assess major depression, we used the Computerized Diagnostic Interview Schedule-IV, a validated computerized version of the National Institute of Mental Health Diagnostic Interview Schedule.37–40 Trained research assistants administered the computerized interview. Participants found to have current depression were informed that they were experiencing depression, were instructed to discuss these symptoms with their primary care provider, and were provided with a list of local resources available for further evaluation and treatment.
Our secondary predictor variable was severity of depressive symptoms. To assess severity, we administered the 9-item Patient Health Questionnaire (PHQ).41,42 Based on this score, we categorized participants as having no symptoms to minimal depressive symptoms (PHQ score of 0–3), mild-to-moderate depressive symptoms (PHQ score of 4–9), or severe depressive symptoms (PHQ score ≥10).42
OUTCOME VARIABLES
We assessed overall medication adherence (not adherence to specific medications) using 3 questionnaire items based on those used in the CARDIA (Coronary Artery Risk Development in Young Adults) study43:
“In the past month, how often did you take your medications as the doctor prescribed?” Possible responses were all of the time (100%), nearly all of the time (90%), most of the time (75%), about half the time (50%), or less than half the time (<50%). Nonadherence was defined as 75% of the time or less.
“In the past month, how often did you forget to take 1 or more of your prescribed medications?” Possible responses were never, once in the past month, 2 to 3 times in the past month, once per week, several times per week, and nearly every day. We defined nonadherence as forgetting to take prescribed medications once per week or more.
“In the past month, how often did you decide to skip 1 or more of your prescribed medications?” Possible responses were the same as for question 2. We defined nonadherence as deciding to skip medications once per week or more.
We chose to measure self-reported medication adherence for several reasons. First, self-reported medication adherence has been validated as a reliable predictor of health outcomes, including blood pressure control,44 hospitalization for heart failure,45 serum drug concentrations,46 and response to anti-retroviral therapy.47 Second, in a study of hypertensive patients taking hydrochlorothiazide, self-reported medication adherence was more strongly correlated with qualitative urinary hydrochlorothiazide levels, changes in serum potassium levels, and decreases in blood pressure than was pill count adherence.48 Third, other methods of assessing adherence were not appropriate for our study. For example, pharmacy refills are often given for 90 days, so pharmacy data would not allow us to assess the association between current (past month) depression and current (past month) adherence. Finally, Medication Event Monitoring System caps are expensive and have their own limitations.49 For example, the number of cap openings does not necessarily reflect the number of pills ingested by the patient.
OTHER VARIABLES
Age, sex, ethnicity, education, income, marital status, living situation, medical history, smoking status, and alcohol use were determined by questionnaire. We assessed social support by asking participants, “Do you have as much contact as you would like with someone you feel close to, someone in whom you can trust and confide? (yes/no).”50
We determined angina frequency by asking participants, “During the past 4 weeks, on average, how many times have you had chest pain, chest tightness, or angina?”51 We measured weight and height and calculated body mass index. Participants were instructed to bring their medication bottles to the study appointment, and study personnel recorded all current medications. Patients were instructed not to take aspirin for 1 week before the examination to complete another part of the study protocol. We assessed left ventricular ejection fraction using a resting echocardiogram. Exercise capacity (metabolic equivalents achieved) was measured using a symptom-limited, graded exercise treadmill test according to a standard Bruce protocol.
STATISTICAL ANALYSIS
Our goal was to examine the association between current depression and current medication adherence. Differences in characteristics between participants with and without current depression were compared using a standard 2-tailed t test for continuous variables and a χ2 test for dichotomous variables. We then used logistic regression to examine the association between current depression and medication adherence. To obtain adjusted risk estimates, we entered all Table 1 variables into backward-elimination logistic regression models. Variables that were associated with medication nonadherence (at P<.05) were retained in these models. To determine whether the association between depression and medication nonadherence differed by the use of specific medications, we tested for interactions between depression and the use of antidepressants, β-blockers, aspirin, statins, and renin-angiotensin system blockers. For these analyses, we report adjusted odds ratios (ORs) with 95% confidence intervals (CIs). All analyses were performed using statistical analysis software (SAS version 8; SAS Institute Inc, Cary, NC).
Table 1.
Characteristics of 940 Participants With Coronary Heart Disease by Depression Status*
| Characteristic | Not Depressed (n = 736) | Depressed (n = 204) | P Value |
|---|---|---|---|
| Demographics | |||
| Age, mean ± SD, y | 69 ± 10 | 62 ± 10 | <.001 |
| Female sex | 97 (13) | 55 (27) | <.001 |
| White race | 442 (60) | 124 (61) | .87 |
| High school graduate | 641 (87) | 180 (88) | .73 |
| Married | 347 (47) | 66 (33) | <.001 |
| Annual income <$20 000 | 324 (44) | 129 (63) | <.001 |
| Psychosocial and behavioral factors | |||
| Live alone | 242 (33) | 81 (40) | .06 |
| Poor social support | 193 (26) | 111 (55) | <.001 |
| Current smoking | 117 (16) | 62 (31) | <.001 |
| Regular alcohol use | 206 (28) | 63 (31) | .45 |
| BMI, mean ± SD | 28 ± 5 | 29 ± 6 | .01 |
| Medical history | |||
| Hypertension | 542 (74) | 149 (73) | .86 |
| Diabetes mellitus | 194 (26) | 67 (33) | .07 |
| Myocardial infarction | 416 (57) | 106 (52) | .25 |
| Congestive heart failure | 134 (18) | 39 (19) | .77 |
| Stroke | 109 (15) | 29 (14) | .83 |
| Revascularization | 476 (65) | 110 (54) | .005 |
| Renal insufficiency (≤60 mL/min) | 233 (29) | 59 (26) | .41 |
| Angina weekly or more | 119 (16) | 57 (28) | <.001 |
| Medication use | |||
| Cardiovascular medications, mean ± SD, No. | 2.8 ± 1.0 | 2.6 ± 1.0 | .18 |
| β-Blocker | 469 (64) | 125 (61) | .52 |
| Renin-angiotensin system blocker | 413 (56) | 110 (54) | .58 |
| Diuretic | 235 (32) | 58 (28) | .34 |
| Statin | 525 (71) | 130 (64) | .04 |
| Aspirin | 617 (84) | 175 (86) | .49 |
| Antidepressant | 76 (10) | 100 (49) | <.001 |
| Cardiac function | |||
| Left ventricular ejection fraction <50% | 93 (13) | 15 (7) | .03 |
| Poor exercise capacity (metabolic equivalents <5) | 166 (24) | 44 (24) | .93 |
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by the square of height in meters).
Data are given as number (percentage) except where indicated otherwise. Denominators may vary because of missing data.
RESULTS
Of the 940 participants, 204 (22%) had current depression based on the computerized version of the Diagnostic Interview Schedule. Compared with nondepressed participants, depressed participants were more likely to be younger, to be female, to be unmarried, and to have an annual income of less than $20 000 (Table 1). Depressed participants were more likely to have poor social support, to be current smokers, and to have a higher body mass index. They were less likely to have a history of coronary revascularization and more likely to report weekly angina. Participants with depression were less likely to be using statins and more likely to be using antidepressants. Compared with nondepressed participants, those with depression had worse cognitive function and higher ejection fractions. We observed no difference in the number of current cardiovascular medications between depressed and nondepressed participants.
Overall, 7% (68/940) of the participants reported not taking their medications as prescribed. Participants with depression were more likely than those without depression to report not taking their medications as prescribed, forgetting to take their medications, and deciding to skip their medications (Figure 1). After adjusting for potential confounding variables, depression remained associated with all 3 measures of medication non-adherence, although the association of depression with forgetting to take medications was no longer significant at the P<.05 level (Table 2).
Figure 1.
Proportion of participants with and without depression who were medication nonadherent.
Table 2.
Multivariable Association of Depression With Medication Nonadherence
| Reason for Nonadherence | Unadjusted OR (95% CI) | P Value | Adjusted OR (95% CI)* | P Value |
|---|---|---|---|---|
| Not taking medications as prescribed | 2.8 (1.7–4.7) | <.001 | 2.2 (1.2–3.9) | .009 |
| Forgot to take medications | 2.4 (1.6–3.8) | <.001 | 1.6 (1.0–2.7) | .06 |
| Decided to skip medications | 2.2 (1.2–4.2) | .01 | 2.1 (1.1–4.0) | .03 |
Abbreviations: CI, confidence interval; OR, odds ratio.
All the variables from Table 1 were entered into a backward-elimination logistic regression model, with P<.05 for retention in the model. Other variables associated with not taking medications as prescribed were white race (OR, 0.4; 95% CI, 0.2–0.7), high school education (OR, 0.5; 95% CI, 0.3–0.9), weekly angina (OR, 2.1; 95% CI, 1.1–3.8), and number of cardiac medications (OR, 0.7; 95% CI, 0.5–0.9). Other variables associated with forgetting to take medications were Hispanic race (OR, 2.1; 95% CI, 1.1–4.1), poor social support (OR, 2.0; 95% CI, 1.2–3.2), weekly angina (OR, 2.3; 95% CI, 1.4–3.8), and use of a renin-angiotensin system blocker (OR, 0.5, 95% CI, 0.3–0.8). Other variables associated with deciding to skip medications were history of congestive heart failure (OR, 4.0; 95% CI, 1.9–8.4), history of myocardial infarction (OR, 0.5; 95% CI, 0.4–0.8), taking aspirin (OR, 15.3, 95% CI, 2.0–118), and number of cardiac medications (OR, 0.6, 95% CI, 0.4–0.8).
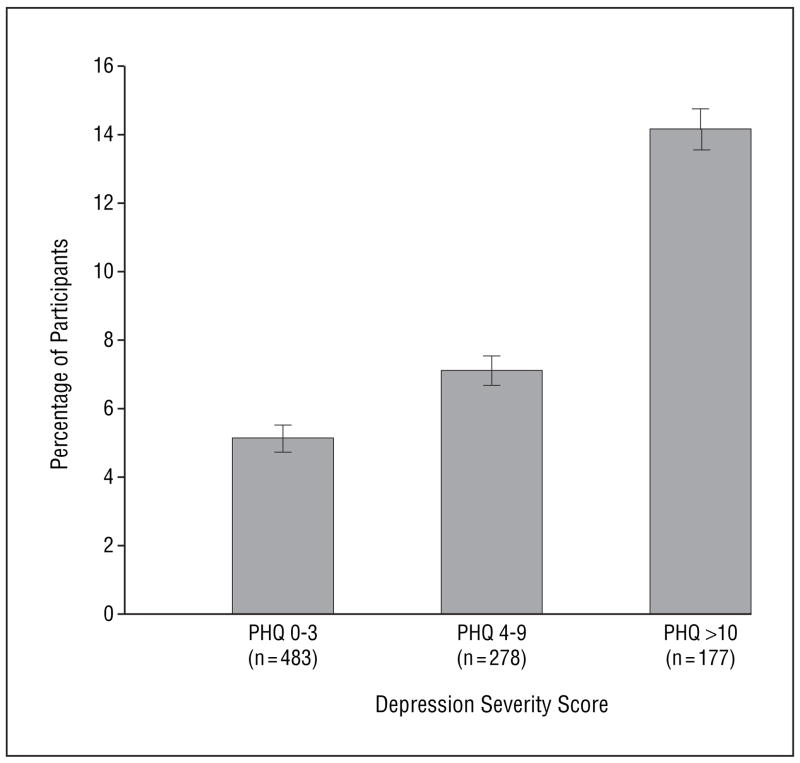
A total of 5% (24/483) of the participants with no symptoms to minimal depressive symptoms (PHQ score of 0–3) reported not taking medications as prescribed compared with 7% (19/278) of the participants with mild-to-moderate symptoms of depression (PHQ score of 4–9) and 14% (25/177) with severe symptoms of depression (PHQ score ≥10) (Figure 2). Compared with participants who had no symptoms to minimal depressive symptoms, those with severe depressive symptoms had 3-fold odds of not taking medications as prescribed (OR, 3.1; 95% CI, 1.7–5.7; P<.001). This association remained strong after adjustment for potential confounding variables (OR, 2.2; 95% CI, 1.1–4.2; P=.02).
Figure 2.
Proportion of participants who were medication nonadherent according to the depression severity score on the Patient Health Questionnaire (PHQ) (P=.003 for trend). Error bars represent SD.
We observed no difference in the association of depression with medication nonadherence in users and non-users of antidepressants, aspirin, statins, or renin-angiotensin system blockers (all P>.20 for interaction). However, the association between depression and medication nonadherence differed in users and nonusers of β-blockers (P<.001 for interaction). Among the 594 users of β-blockers, depression was associated with increased odds of not taking medications as prescribed (adjusted OR, 6.3; 95% CI, 2.9–13.7; P<.001), forgetting to take medications (OR, 2.0; 95% CI, 1.1–3.7; P=.02), and deciding to skip medications (OR, 2.5; 95% CI, 1.0–5.8; P=.03). Among the 346 nonusers of β-blockers, depression was not associated with not taking medications as prescribed (OR, 0.6; 95% CI, 0.2–1.5; P=.25), forgetting to take medications (OR, 1.5; 0.7–3.2; P=.35), or deciding to skip medications (OR, 1.7; 95% CI, 0.7–4.8; P=.26).
COMMENT
We found that depression was associated with medication nonadherence in 940 outpatients with CHD. Although our study participants reported high adherence overall, those with depression were more likely to report not taking their medications as prescribed, forgetting to take their medications, and deciding to skip their medications. In addition, participants with more severe depression were more likely to be nonadherent. This association between depression and nonadherence persisted after adjusting for other factors that may affect medication adherence, such as age, ethnicity, education, social support, and measures of cardiac disease severity.
These findings confirm those from Carney et al,34 who found that depression was associated with nonadherence to prescribed aspirin in 10 depressed and 45 non-depressed patients. Our results also support a study by Ziegelstein et al32 that found decreased adherence to recommended behavior and lifestyle changes, including stopping smoking, exercising regularly, and following a low-fat diet, in 31 depressed vs 173 nondepressed patients after acute myocardial infarction. Ziegelstein et al32 reported that depression was associated with taking less prescription medication, but it was unclear whether the frequency of “taking prescription medication” was measuring adherence or the number and frequency of medications prescribed. To our knowledge, ours is the first large study to examine the association between depression and medication nonadherence in patients with stable CHD and to demonstrate that more severe depression is associated with greater nonadherence.
There are several reasons why depression may be associated with medication nonadherence. First, expectations of the benefit of treatment recommendations are an essential component of patient adherence.20 Depressed patients often have an outlook of hopelessness, which may compromise any confidence in the benefits of therapy. Second, depression can be accompanied by a lack of the energy and focus required to follow through with treatment recommendations. Third, depressed patients may be more sensitive to medication adverse effects and thus more likely to discontinue medication use. Alternatively, it may be that medication nonadherence leads to depression.
Although one might expect that patients with angina symptoms and a history of revascularization would be more cognizant of their cardiac disease and thus more likely to be adherent to medications, we found that depression was associated with medication nonadherence even after correction for differences in angina frequency and history of revascularization between depressed and nondepressed participants. Because our results are cross-sectional, it is unclear whether medication nonadherence led to greater angina frequency in the depressed participants or whether depressed patients were less likely to take their medications because they felt sicker.
We found that the association between depression and nonadherence differed in users and nonusers of β-blockers. Depression seemed to be associated with medication nonadherence in participants who were using β-blockers but not in those who were not using β-blockers. It is possible that depressed patients taking β-blockers may be less likely to adhere because they believe that the β-blockers are causing depressive symptoms or because they are more sensitive to the adverse effects of β-blockers. However, we did not measure adherence to β-blockers specifically, and given the low overall rate of medication nonadherence in our study, these post hoc observations may also be due to chance.
Previous studies1–11 have found that patients who are depressed have a 70% increased rate of CHD events, including nonfatal myocardial infarction and CHD death, compared with those who are not depressed. Aspirin, β-blockers, renin-angiotensin system blockers, and lipid-lowering medications reduce mortality in patients who have CHD.52–54 If our findings are replicated and depression is associated with a 2-fold odds of not taking medications as prescribed, then depressed patients may not be receiving the benefits of these medications.
The participants in this study had a high overall rate of adherence. Because our subjects were health-conscious, motivated volunteers in a clinical research study, their adherence was likely greater than in the general population of patients with CHD. Thus, the 2-fold odds of nonadherence associated with depression would likely have a greater effect in a less adherent population outside of a clinical study. It is possible that interventions aimed at improving medication adherence in patients with heart disease, such as greater use of medication organizers, pharmacist checks, and nurse follow-up, would decrease the adverse cardiovascular events associated with depression.
The strengths of this study include its large sample size, interview diagnosis of depression, and comprehensive measurement of potential confounding variables. Several limitations must also be considered in interpreting our results. First, we measured overall medication adherence rather than adherence to CHD treatment–specific medications. However, it is unlikely that any discrepancy in overall vs CHD treatment adherence would differ by depression status, particularly because the issue of cost is not a concern to participants recruited from the Veterans Affairs system, who represented most of the participants in this study. Moreover, it has been demonstrated that poor adherence to specific cardiac medications is associated with poor adherence in general.55
Second, we assessed medication adherence by self-report. However, other methods of adherence, such as electronic monitoring, pill counts, pharmacy refill records, and biological assays, are also fraught with problems.56 With many of these other measures of adherence, the patient is given previous warning of monitoring, which has a tendency to overestimate adherence.49,57 In contrast, self-reported adherence provides a current or short-term estimate of medication adherence, which may be more accurate than pill counts or prescription records.48 Furthermore, self-reported adherence has been validated as are liable predict or of clinical outcomes44,47 and serum drug concentrations.46 Third, the remarkably low prevalence of nonadherence in this study may have limited the power to perform subanalyses of the overall findings. Fourth, the causal direction between depression and medication nonadherence cannot be determined by a cross-sectional study. Finally, most of the participants were older men, so our results may not be generalizable to women.
In summary, we found that depression is associated with medication nonadherence in a large population of outpatients with CHD. Our findings should direct attention toward medication nonadherence as a possible mechanism by which depression may lead to cardiac events and as a potential target for efforts to improve cardiovascular outcomes in depressed patients.
Acknowledgments
Funding/Support: This work was supported by grants from the Department of Veterans Affairs (Epidemiology Merit Review Program), Washington, DC; the Robert Wood Johnson Foundation (Generalist Physician Faculty Scholars Program), Princeton, NJ; the American Federation for Aging Research (Paul Beeson Faculty Scholars in Aging Research Program), New York, NY; and the Nancy Kirwan Heart Research Fund, San Francisco, Calif.
Role of the Sponsor: None of the funding sources had any role in the collection of data, the interpretation of results, or the preparation of this manuscript.
Footnotes
Financial Disclosure: None.
References
- 1.Barefoot JC, Brummett BH, Helms MJ, Mark DB, Siegler IC, Williams RB. Depressive symptoms and survival of patients with coronary artery disease. Psychosom Med. 2000;62:790–795. doi: 10.1097/00006842-200011000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Blumenthal JA, Lett HS, Babyak MA, et al. Depression as a risk factor for mortality after coronary artery bypass surgery. Lancet. 2003;362:604–609. doi: 10.1016/S0140-6736(03)14190-6. [DOI] [PubMed] [Google Scholar]
- 3.Burg MM, Benedetto MC, Soufer R. Depressive symptoms and mortality two years after coronary artery bypass graft surgery (CABG) in men. Psychosom Med. 2003;65:508–510. doi: 10.1097/01.psy.0000077509.39465.79. [DOI] [PubMed] [Google Scholar]
- 4.Bush DE, Ziegelstein RC, Tayback M, et al. Even minimal symptoms of depression increase mortality risk after acute myocardial infarction. Am J Cardiol. 2001;88:337–341. doi: 10.1016/s0002-9149(01)01675-7. [DOI] [PubMed] [Google Scholar]
- 5.Carney RM, Blumenthal JA, Catellier D, et al. Depression as a risk factor for mortality after acute myocardial infarction. Am J Cardiol. 2003;92:1277–1281. doi: 10.1016/j.amjcard.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Frasure-Smith N, Lesperance F, Talajic M. Depression following myocardial infarction: impact on 6-month survival. JAMA. 1993;270:1819–1825. [PubMed] [Google Scholar]
- 7.Frasure-Smith N, Lesperance F. Depression and other psychological risks following myocardial infarction. Arch Gen Psychiatry. 2003;60:627–636. doi: 10.1001/archpsyc.60.6.627. [DOI] [PubMed] [Google Scholar]
- 8.Kaufmann MW, Fitzgibbons JP, Sussman EJ, et al. Relation between myocardial infarction, depression, hostility, and death. Am Heart J. 1999;138:549–554. doi: 10.1016/s0002-8703(99)70159-6. [DOI] [PubMed] [Google Scholar]
- 9.Ladwig KH, Kieser M, Konig J, Breithardt G, Borggrefe M. Affective disorders and survival after acute myocardial infarction: results from the post-infarction late potential study. Eur Heart J. 1991;12:959–964. [PubMed] [Google Scholar]
- 10.Lesperance F, Frasure-Smith N, Juneau M, Theroux P. Depression and 1-year prognosis in unstable angina. Arch Intern Med. 2000;160:1354–1360. doi: 10.1001/archinte.160.9.1354. [DOI] [PubMed] [Google Scholar]
- 11.Lesperance F, Frasure-Smith N, Talajic M, Bourassa MG. Five-year risk of cardiac mortality in relation to initial severity and one-year changes in depression symptoms after myocardial infarction. Circulation. 2002;105:1049–1053. doi: 10.1161/hc0902.104707. [DOI] [PubMed] [Google Scholar]
- 12.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 13.Carney RM, Blumenthal JA, Stein PK, et al. Depression, heart rate variability, and acute myocardial infarction. Circulation. 2001;104:2024–2028. doi: 10.1161/hc4201.097834. [DOI] [PubMed] [Google Scholar]
- 14.Gehi AK, Rumsfeld JS, Liu H, Schiller NB, Whooley MA. Relation of self-reported angina pectoris to inducible myocardial ischemia in patients with known coronary artery disease: the Heart and Soul Study. Am J Cardiol. 2003;92:705–707. doi: 10.1016/s0002-9149(03)00831-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Otte C, Neylan TC, Pipkin S, Browner WS, Whooley MA. Depressive symptoms and 24-hour urinary norepinephrine in patients with coronary disease: findings from the Heart and Soul Study. Am J Psychiatry. doi: 10.1176/appi.ajp.162.11.2139. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Otte C, Marmar CR, Pipkin SS, Moos R, Browner WS, Whooley MA. Depression and 24-hour urinary cortisol in medical outpatients with coronary heart disease: the Heart and Soul Study. Biol Psychiatry. 2004;56:241–247. doi: 10.1016/j.biopsych.2004.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lesperance F, Frasure-Smith N, Theroux P, Irwin M. The association between major depression and levels of soluble intercellular adhesion molecule 1, interleukin-6, and C-reactive protein in patients with recent acute coronary syndromes. Am J Psychiatry. 2004;161:271–277. doi: 10.1176/appi.ajp.161.2.271. [DOI] [PubMed] [Google Scholar]
- 18.Carney RM, Freedland KE, Miller GE, Jaffe AS. Depression as a risk factor for cardiac mortality and morbidity: a review of potential mechanisms. J Psychosom Res. 2002;53:897–902. doi: 10.1016/s0022-3999(02)00311-2. [DOI] [PubMed] [Google Scholar]
- 19.Ziegelstein RC. Depression in patients recovering from a myocardial infarction. JAMA. 2001;286:1621–1627. doi: 10.1001/jama.286.13.1621. [DOI] [PubMed] [Google Scholar]
- 20.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160:2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 21.Ammassari A, Trotta MP, Murri R, et al. Correlates and predictors of adherence to highly active antiretroviral therapy: overview of published literature. J Acquir Immune Defic Syndr. 2002;31(suppl 3):S123–S127. doi: 10.1097/00126334-200212153-00007. [DOI] [PubMed] [Google Scholar]
- 22.Ammassari A, Antinori A, Aloisi MS, et al. Depressive symptoms, neurocognitive impairment, and adherence to highly active antiretroviral therapy among HIV-infected persons. Psychosomatics. 2004;45:394–402. doi: 10.1176/appi.psy.45.5.394. [DOI] [PubMed] [Google Scholar]
- 23.Jindel RM, Joseph JT, Morris MC, Santella RN, Baines LS. Noncompliance after kidney transplantation: a systematic review. Transplant Proc. 2003;35:2868–2872. doi: 10.1016/j.transproceed.2003.10.052. [DOI] [PubMed] [Google Scholar]
- 24.Wang PS, Bohn RL, Knight E, Glynn RJ, Mogun H, Avorn J. Noncompliance with antihypertensive medications: the impact of depressive symptoms and psychosocial factors. J Gen Intern Med. 2002;17:504–511. doi: 10.1046/j.1525-1497.2002.00406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim MT, Han HR, Hill MN, Rose L, Roary M. Depression, substance use, adherence behaviors, and blood pressure in urban hypertensive black men. Ann Behav Med. 2003;26:24–31. doi: 10.1207/S15324796ABM2601_04. [DOI] [PubMed] [Google Scholar]
- 26.Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med. 2000;160:3278–3285. doi: 10.1001/archinte.160.21.3278. [DOI] [PubMed] [Google Scholar]
- 27.Ciechanowski PS, Katon WJ, Russo JE, Hirsch IB. The relationship of depressive symptoms to symptom reporting, self-care and glucose control in diabetes. Gen Hosp Psychiatry. 2003;25:246–252. doi: 10.1016/s0163-8343(03)00055-0. [DOI] [PubMed] [Google Scholar]
- 28.Katon W, Von Korff M, Ciechanowski P, et al. Behavioral and clinical factors associated with depression among individuals with diabetes. Diabetes Care. 2004;27:914–920. doi: 10.2337/diacare.27.4.914. [DOI] [PubMed] [Google Scholar]
- 29.Lin EH, Katon W, Von Korff M, et al. Relationship of depression and diabetes self-care, medication adherence, and preventive care. Diabetes Care. 2004;27:2154–2160. doi: 10.2337/diacare.27.9.2154. [DOI] [PubMed] [Google Scholar]
- 30.Musselman DL, Betan E, Larsen H, Phillips LS. Relationship of depression to diabetes types 1 and 2: epidemiology, biology, and treatment. Biol Psychiatry. 2003;54:317–329. doi: 10.1016/s0006-3223(03)00569-9. [DOI] [PubMed] [Google Scholar]
- 31.Benner JS, Glynn RJ, Mogun H, Neumann PJ, Weinstein MC, Avorn J. Long-term persistence in use of statin therapy in elderly patients. JAMA. 2002;288:455–461. doi: 10.1001/jama.288.4.455. [DOI] [PubMed] [Google Scholar]
- 32.Ziegelstein RC, Fauerbach JA, Stevens SS, Romanelli J, Richter DP, Bush DE. Patients with depression are less likely to follow recommendations to reduce cardiac risk during recovery from a myocardial infarction. Arch Intern Med. 2000;160:1818–1823. doi: 10.1001/archinte.160.12.1818. [DOI] [PubMed] [Google Scholar]
- 33.Horwitz RI, Viscoli CM, Berkman L, et al. Treatment adherence and risk of death after a myocardial infarction. Lancet. 1990;336:542–545. doi: 10.1016/0140-6736(90)92095-y. [DOI] [PubMed] [Google Scholar]
- 34.Carney RM, Freedland KE, Eisen SA, Rich MW, Jaffe AS. Major depression and medication adherence in elderly patients with coronary artery disease. Health Psychol. 1995;14:88–90. doi: 10.1037//0278-6133.14.1.88. [DOI] [PubMed] [Google Scholar]
- 35.Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health-related quality of life: the Heart and Soul Study. JAMA. 2003;290:215–221. doi: 10.1001/jama.290.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 37.Levitan RD, Blouin AG, Navarro JR, Hill J. Validity of the computerized DIS for diagnosing psychiatric inpatients. Can J Psychiatry. 1991;36:728–731. [PubMed] [Google Scholar]
- 38.Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID), I: history, rationale, and description. Arch Gen Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 39.Williams JB, Gibbon M, First MB, et al. The Structured Clinical Interview for DSM-III-R (SCID), II: multisite test-retest reliability. Arch Gen Psychiatry. 1992;49:630–636. doi: 10.1001/archpsyc.1992.01820080038006. [DOI] [PubMed] [Google Scholar]
- 40.Robins LN, Helzer JE, Croughan J, Ratcliff KS. National Institute of Mental Health Diagnostic Interview Schedule: its history, characteristics, and validity. Arch Gen Psychiatry. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- 41.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study: Primary Care Evaluation of Mental Disorders. JAMA. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 42.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cutter GR, Burke GL, Dyer AR, et al. Cardiovascular risk factors in young adults: the CARDIA baseline monograph. Control Clin Trials. 1991;12:1S–77S. doi: 10.1016/0197-2456(91)90002-4. [DOI] [PubMed] [Google Scholar]
- 44.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 45.Ghali JK, Kadakia S, Cooper R, Ferlinz J. Precipitating factors leading to decompensation of heart failure: traits among urban blacks. Arch Intern Med. 1988;148:2013–2016. [PubMed] [Google Scholar]
- 46.Blumberg EJ, Hovell MF, Kelley NJ, Vera AY, Sipan CL, Berg JP. Self-report INH adherence measures were reliable and valid in Latino adolescents with latent tuberculosis infection. J Clin Epidemiol. 2005;58:645–648. doi: 10.1016/j.jclinepi.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 47.Godin G, Gagne C, Naccache H. Validation of a self-reported questionnaire assessing adherence to antiretroviral medication. AIDS Patient Care STDS. 2003;17:325–332. doi: 10.1089/108729103322231268. [DOI] [PubMed] [Google Scholar]
- 48.Haynes RB, Taylor DW, Sackett DL, Gibson ES, Bernholz CD, Mukherjee J. Can simple clinical measurements detect patient noncompliance? Hypertension. 1980;2:757–764. doi: 10.1161/01.hyp.2.6.757. [DOI] [PubMed] [Google Scholar]
- 49.Matsui D, Hermann C, Klein J, Berkovitch M, Olivieri N, Koren G. Critical comparison of novel and existing methods of compliance assessment during a clinical trial of an oral iron chelator. J Clin Pharmacol. 1994;34:944–949. doi: 10.1002/j.1552-4604.1994.tb04009.x. [DOI] [PubMed] [Google Scholar]
- 50.Williams RB, Barefoot JC, Califf RM, et al. Prognostic importance of social and economic resources among medically treated patients with angiographically documented coronary artery disease [published correction appears in JAMA. 1992;268:2652] JAMA. 1992;267:520–524. [PubMed] [Google Scholar]
- 51.Spertus JA, Winder JA, Dewhurst TA, et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–341. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]
- 52.May GS, Eberlein KA, Furberg CD, Passamani ER, DeMets DL. Secondary prevention after myocardial infarction: a review of long-term trials. Prog Cardiovasc Dis. 1982;24:331–352. doi: 10.1016/0033-0620(82)90010-x. [DOI] [PubMed] [Google Scholar]
- 53.May GS, Furberg CD, Eberlein KA, Geraci BJ. Secondary prevention after myocardial infarction: a review of short-term acute phase trials. Prog Cardiovasc Dis. 1983;25:335–359. doi: 10.1016/0033-0620(83)90013-0. [DOI] [PubMed] [Google Scholar]
- 54.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G Heart Outcomes Prevention Evaluation Study Investigators. Effects of an angiotensin-converting–enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients [published corrections appear in N Engl J Med. 2000;342:748 and 2000;342:1376] N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 55.Irvine J, Baker B, Smith J, et al. Poor adherence to placebo or amiodarone therapy predicts mortality: results from the CAMIAT study. Psychosom Med. 1999;61:566–575. doi: 10.1097/00006842-199907000-00023. [DOI] [PubMed] [Google Scholar]
- 56.Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47:555–567. doi: 10.1016/s0022-3999(99)00057-4. [DOI] [PubMed] [Google Scholar]
- 57.Kruse W, Rampmaier J, Ullrich G, Weber E. Patterns of drug compliance with medications to be taken once and twice daily assessed by continuous electronic monitoring in primary care. Int J Clin Pharmacol Ther. 1994;32:452–457. [PubMed] [Google Scholar]