Abstract
Objectives
To assess the effects of rivastigmine on the core domains of Alzheimer’s disease.
Design
Prospective, randomised, multicentre, double blind, placebo controlled, parallel group trial. Patients received either placebo, 1-4 mg/day (lower dose) rivastigmine, or 6-12 mg/day (higher dose) rivastigmine. Doses were increased in one of two fixed dose ranges (1-4 mg/day or 6-12 mg/day) over the first 12 weeks with a subsequent assessment period of 14 weeks.
Setting
45 centres in Europe and North America.
Participants
725 patients with mild to moderately severe probable Alzheimer’s disease diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, fourth edition, and the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association.
Outcome measures
Cognitive subscale of the Alzheimer’s disease assessment scale, rating on the clinician interview based impression of change incorporating caregiver information scale, and the progressive deterioration scale.
Results
At the end of the study cognitive function had deteriorated among those in the placebo group. Scores on the Alzheimer’s disease assessment scale improved in patients in the higher dose group when compared with patients taking placebo (P<0.05). Significantly more patients in the higher dose group had improved by 4 points or more than had improved in the placebo group (24% (57/242) v 16% (39/238)). Global function as rated by the clinician interview scale had significantly improved among those in the higher dose group compared with those taking placebo (P<0.001), and significantly more patients in the higher dose group showed improvement than did in the placebo group (37% (80/219) v 20% (46/230)). Mean scores on the progressive deterioration scale improved from baseline in patients in the higher dose group but fell in the placebo group. Adverse events were predominantly gastrointestinal, of mild to moderate severity, transient, and occurred mainly during escalation of the dose. 23% (55/242) of those in the higher dose group, 7% (18/242) of those in the lower dose group, and 7% (16/239) of those in the placebo group discontinued treatment because of adverse events.
Conclusions
Rivastigmine is well tolerated and effective. It improves cognition, participation in activities of daily living, and global evaluation ratings in patients with mild to moderately severe Alzheimer’s disease. This is the first treatment to show compelling evidence of efficacy in a predominantly European population.
Key messages
In a 6 month trial rivastigmine was effective in treating the core cognitive and functional symptoms of patients with mild to moderate Alzheimer’s disease
Rivastigmine at doses of 6-12 mg/day produces clinically relevant and statistically significant improvements in cognitive and global assessments, and in activities of daily living
The effects of rivastigmine are dose dependent
Rivastigmine was well tolerated in this population of elderly patients
Introduction
One of the most successful treatments for Alzheimer’s disease has been the use of acetylcholinesterase inhibitors to enhance surviving cholinergic neurotransmission by inhibiting the breakdown of released acetylcholine. The first of these drugs approved for treating Alzheimer’s disease, tacrine, is effective but can cause an increase in liver enzyme concentrations; in some countries, such as in the United Kingdom, this has prevented it from being licensed.1–3 More recently, a second acetylcholinesterase inhibitor, donepezil (a piperidine derivative) has become available.4,5 Clinical trials have reported benefits on cognition and global evaluations.4,5 Rivastigmine is a novel, “pseudo-irreversible,” brain selective inhibitor of acetylcholinesterases, the metabolism of which is almost totally independent of the hepatic cytochrome P450 system.6–8
The aim of this study was to evaluate the safety and efficacy of two doses of rivastigmine (1-4 mg/day and 6-12 mg/day) compared with a placebo over 26 weeks in patients with probable Alzheimer’s disease. The study was carried out predominantly in European centres using a design similar to that employed in a parallel study in North America9 as part of a global evaluation programme (Alzheimer’s disease treatment with ENA-713)10 This programme is the largest programme of clinical trials conducted to date for treatment for dementia; it consists of four trials with over 3300 patients at 111 centres in 10 countries.
Participants and methods
Patients
To be enrolled in the study patients had to be 50-85 years of age and not able to bear children (older or younger patients could enter the study with the approval of the medical expert (MG or AC-S). All patients met criteria for Alzheimer’s type dementia as described in the Diagnostic and Statistical Manual of Mental Disorders, fourth edition11 and criteria for probable Alzheimer’s disease according to criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association.12 Participants also had to have scores of 10-26 on the mini-mental state examination.13 Most patients were recruited from the community either through their general practitioners or directly, and each patient had a responsible caregiver and, along with their caregiver, provided written informed consent. Patients with concomitant diseases such as hypertension, non-insulin dependent diabetes, and arthritis were included. Only those with severe and unstable cardiac disease, severe obstructive pulmonary disease, or other life threatening conditions (such as rapidly progressing malignancies) were excluded. Patients taking drugs for coexistent diseases were included except for those taking anticholinergic drugs, health food supplements containing acetylcholine precursors, putative memory enhancers, insulin, and psychotropic drugs (the use of small doses of short acting benzodiazepines, chloral hydrate, or haloperidol was allowed). The trial procedures were in accord with the ethical standards of the institutional committees on human experimentation and with the Helsinki Declaration. The study was overseen by an independent international safety monitoring board. (A list of members of the board appears on the BMJ’s website.)
Design
The 26 week study, conducted at 45 centres in Europe (in Austria, France, Germany, and Switzerland) and in North America, utilised a randomised, double blind, placebo controlled, parallel group design. Patients were randomly allocated either to placebo or 1-4 mg/day rivastigmine (lower dose) or 6-12 mg/day (higher dose) according to a computer generated randomisation code at Novartis Pharma (Basle, Switzerland). To maintain blinding capsules containing rivastigmine and placebo were identical and the number taken was the same at each dose in all groups. Dosages were increased weekly in steps of up to 1.5 mg/day during weeks 1-12 (dose escalation phase) and had to be within the target range by week 7. Decreases in doses were not permitted during this phase. However, if adverse events occurred a dose could be omitted, maintained without increase for two consecutive weeks, or antiemetic drugs could be given. During weeks 13-26 (maintenance) doses could be increased or decreased within the assigned range with the aim of administering the highest dose that was well tolerated.
Efficacy measures fulfilled the US Food and Drug Administration’s dual efficacy requirements for clinical trials for Alzheimer’s disease—that is, improvement on a performance based cognitive instrument and demonstration that the improvement was clinically meaningful.14 Efficacy was assessed on the cognitive subscale of the Alzheimer’s disease assessment scale,15 the clinician interview based impression of change incorporating caregiver information,16 and the progressive deterioration scale.17 Efficacy evaluations were performed at baseline and weeks 12, 18, and 26 or at early withdrawal from the trial. Table 1 summarises the instruments used, symptoms and domains measured, the source of information, the range of scores, and their interpretation.
Table 1.
Instruments used to evaluate the efficacy of rivastigmine in treating Alzheimer’s disease
| Instrument | Symptoms or domains assessed | Source of information | Range of scale and interpretation |
|---|---|---|---|
| Alzheimer’s disease assessment scale (cognitive subscale) | Cognition (memory, language, orientation, praxis) | Patient | 0-70 points 0=no errors (rarely achieved, even in general population) 70=severe impairment |
| Clinician interview based impression of change scale (incorporating caregiver information) | Global assessment of behaviour, general psychopathology, cognition, and activities of daily living | Patient and caregiver during interview with clinician* | 1-7 points 1, 2, 3=marked, moderate, or minimal improvement 4=no change 5, 6, 7=minimal, moderate, or marked deterioration |
| Progressive deterioration scale | Activities of daily living (dressing and eating independently, social interaction, participation in housework and hobbies, awareness of time, handling of financial matters) | Caregiver | 29 items Scores range from 0 to 100 |
Clinicians had no access to data on efficacy or safety.
The mini-mental state examination13 and the global deterioration scale18 were used as staging measures at baseline and week 26.
Safety evaluations included physical examinations, electrocardiography, monitoring vital signs, and laboratory testing. Adverse events were coded using the Sandoz medical technology thesaurus, which is based on a WHO document. Three central laboratories (in Europe, the United States, and Canada) performed all clinical laboratory evaluations, and one cardiologist at a central analysis centre read all electrocardiograms.
Statistical methods
The study sample population of about 200 in each group was planned to enable achievement of 90% power with α=0.05 for detecting at least a 3.0 point improvement on the Alzheimer’s disease assessment scale and an increase from 15-30% among patients scoring <4 on the clinician impression of change scale.
Patients were classed for efficacy analyses as: classical intention to treat, traditional last observation carried forward (randomised patients with at least one evaluation while being treated), and observed cases (randomised patients with an evaluation made while on study drug at designated assessment times). Comparisons with placebo were two tailed with the critical level set at P<0.05. Analyses of efficacy utilised the clinician impression of change scale with analysis of variance and two tailed pairwise Student’s t tests using the pooled error term from the analysis of variance (sas type III analysis); the Alzheimer’s assessment scale and the progressive deterioration scale with analysis of covariance and variance and two tailed pairwise Student’s t tests using the pooled error term from the analysis of covariance and variance (sas type III analysis); the Alzheimer’s assessment scale, the clinician impression of change scale and the progressive deterioration scale (categorical analyses) with Mantel-Haenszel blocking for centre. Safety analyses used an analysis of variance for vital signs, laboratory data, and electrocardiograms, and Fisher’s exact test for the occurrence of abnormalities on physical examinations, electrocardiograms, vital signs, laboratory tests, and adverse events.
Results
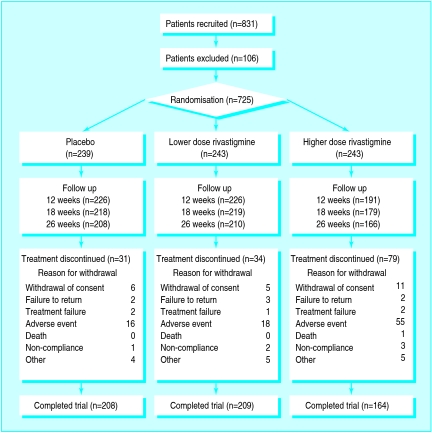
The randomisation of patients and their progress through the study is summarised in figure 1. A total of 831 patients were recruited; 106 of these were excluded. Altogether 243 patients were randomly allocated to higher dose treatment, 243 to lower dose, and 239 to placebo. Demographic variables and disease characteristics at baseline were comparable across groups but there were more females (59% (428/725)) than males (41% (297/725)). Mean age was 72 years (range 45-95 years), and most patients (97% (703/725)) were white. The mean duration of dementia was 39 months; 41% (298/725) of patients had mild disease, 57% (411/725) moderate, and 2% (16/725) had severe disease.18 The mean scores at baseline on the Alzheimer’s disease assessment scale and the progressive deterioration scale for the three groups are shown in table 2. The mean score on the mini-mental state examination was 19.9 (range 10-29).
Figure 1.
Outcome of allocation to treatment and reasons for withdrawal from the study
Table 2.
Mean (range) baseline scores analysed on an intention to treat basis
| Instrument | Rivastigmine groups
|
Placebo (n=243) | |
|---|---|---|---|
| Higher dose (n=243) | Lower dose (n=243) | ||
| Alzheimer’s disease assessment scale (cognitive subscale) | 23.57 (5.7-58.0) | 23.87 (4-60.7) | 23.29 (3.3-57.8) |
| Progressive deterioration scale | 55.22 (9.5-94.6) | 53.8 (9.9-94) | 54.1 (7.1-93.5) |
About 80% of patients (579/725) reported prior or current medical conditions, or both. These were most commonly cardiovascular. About 81% (590/725) were taking concomitant drug treatment at baseline. The mean number of medical conditions per patient was 2.5 and the mean number of concomitant drugs being taken per patient was 4.0. The most common drugs—that is, taken by >10% in each group—included anti-infectives and drugs for cardiovascular, gastrointestinal, respiratory, musculoskeletal, blood, and nervous system disorders.
By the end of the study the mean dose of rivastigmine was 10.4 (SD 2.13) mg/day in the higher dose group and 3.7 (SD 0.59) mg/day in the lower dose group. Of the patients who were taking rivastigmine until the end of the study, 64% (107/166) in the higher dose group and 90% (190/210) in the lower dose group reached the maximum prescribed dose.
Table 3 summarises the effects of rivastigmine on all measures of efficacy.
Table 3.
Mean (95% confidence interval) change from baseline on measures of efficacy of rivastigmine at week 26
| Instrument | Treatment group and analysis
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Higher dose rivastigmine
|
Lower dose rivastigmine
|
Placebo
|
|||||||||
| Intention to treat analysis | Last observation carried forward analysis | Observed cases analysis | Intention to treat analysis | Last observation carried forward analysis | Observed cases analysis | Intention to treat analysis | Last observation carried forward analysis | Observed cases analysis | |||
| Alzheimer’s disease assessment scale (cognitive subscale) | 0.26 | 0.83 | 1.17 | −1.37 | −1.24 | −1.24 | −1.34 | −1.45 | −1.41 | ||
| (−0.66 to 1.06) | (−0.19 to 1.79) | (−0.07 to 2.27) | (−2.27 to −0.53) | (−2.23 to −0.37) | (−2.31 to −0.29) | (−2.19 to −0.41) | (−2.33 to −0.47) | (−2.43 to −0.37) | |||
| P value v placebo | <0.1 | <0.001 | <0.001 | >0.05 | >0.05 | >0.05 | |||||
| No (%) patients with ⩾4 point improvement | 57/242 (24) | 53/199 (27) | 45/157 (29) | 36/242 (15) | 36/226 (16) | 34/202 (17) | 39/238 (16) | 40/225 (18) | 38/205 (19) | ||
| P value v placebo | <0.1 | <0.05 | <0.05 | >0.05 | >0.05 | >0.05 | |||||
| Clinician interview based impression of change scale | 3.91 | 3.88 | 3.93 | 4.24 | 4.17 | 4.20 | 4.38 | 4.32 | 4.34 | ||
| (3.71 to 4.09) | (3.69 to 4.11) | (3.67 to 4.13) | (4.02 to 4.38) | (4.0 to 4.4) | (3.99 to 4.41) | (4.22 to 4.58) | (4.1 to 4.5) | (4.09 to 4.51) | |||
| P value v placebo | <0.001 | <0.001 | <0.05 | >0.05 | >0.05 | >0.05 | |||||
| No (%) patients with improvement (scores 1, 2 or 3) | 80/219 (37) | 78/193 (40) | 63/155 (41) | 69/233 (30) | 71/224 (32) | 62/198 (31) | 46/230 (20) | 44/220 (22) | 44/197 (22) | ||
| P value v placebo | <0.001 | <0.001 | <0.001 | <0.05 | <0.01 | <0.05 | |||||
| Progressive deterioration scale | 0.05 | 0.5 | 1.3 | −3.37 | −3.31 | −2.9 | −2.18 | −2.23 | −1.9 | ||
| (−1.57 to 1.77) | (−1.32 to 2.52) | (−0.95 to 3.55) | (−4.99 to −1.61) | (−5.1 to −1.5) | (−4.83 to −0.97) | (−3.91 to −0.49) | (−4.02 to −0.38) | (−3.84 to 0.04) | |||
| P value v placebo | <0.1 | <0.05 | <0.04 | >0.05 | >0.05 | ||||||
| No (%) patients with ⩾10% improvement | 70/241 (29) | 66/198 (33) | NP | 45/241 (19) | 45/225 (20) | NP | 45/237 (19) | 45/223 (20) | NP | ||
| P value v placebo | <0.01 | <0.01 | >0.05 | >0.05 | |||||||
| Global deterioration scale | −0.06 | −0.03 | NP | −0.22 | −0.2 | NP | −0.26 | −0.24 | NP | ||
| (−0.2 to 0.0) | (−0.13 to 0.13) | (−0.3 to −0.1) | (−0.31 to −0.09) | (−0.4 to −0.2) | (−0.31 to −0.09) | ||||||
| P value v placebo | <0.05 | <0.05 | |||||||||
| Mini-mental state examination | 0.21 | 0.34 | NP | −0.62 | −0.60 | NP | −0.47 | −0.54 | NP | ||
| (−0.24 to 0.64) | (−0.25 to 0.85) | (−1.05 to −0.15) | (−1.08 to −0.12) | (−0.96 to −0.04) | (−0.99 to −0.01) | ||||||
| P value v placebo | <0.05 | <0.05 | |||||||||
NP=analysis not planned.
Cognitive subscale of the Alzheimer’s disease assessment scale
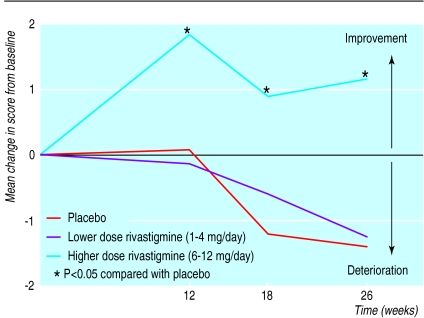
Cognitive function worsened progressively in patients taking placebo. The mean deterioration in the cognitive subscale was 1.41 points over 26 weeks among observed cases (fig 2). The mean score on the subscale improved among patients in the higher dose group (mean improvement 1.17 points). Differences between the two groups in the mean change from baseline scores were statistically significant at weeks 12, 18, and 26 for all three analyses. Of the patients completing the study 55% (86/157) of those in the higher dose group improved from baseline measurements compared with 45% (93/205) of those treated with placebo (analysis of observed cases). The proportion of patients with a clinically meaningful improvement in their scores (defined as a change of four points or more from baseline) at the end of the study was significantly greater among patients receiving higher dose rivastigmine than among those taking placebo (24% (57/242) higher dose group v 16% (39/238) placebo in intention to treat analysis; 27% (53/199) higher dose group v 18% (40/225) last observation carried forward; and 29% (45/157) higher dose group v 19% (38/205) observed cases analysis (P<0.05)).
Figure 2.
Mean change in baseline scores on cognitive subscale of Alzheimer’s disease assessment scale, observed cases analysis. P<0.05 compared with placebo (two tailed pairwise Student’s t tests using pooled error term from analysis of covariance and analysis of variance
Clinician interview based impression of change
At week 26 patients treated with placebo had deteriorated (mean rating 4.34) (table 4). Patients in the higher dose rivastigmine group had improved (mean rating 3.93). The difference between the two groups at week 26 was statistically significant for all three efficacy analyses. At week 26 significantly more patients in both rivastigmine groups had ratings of marked, moderate, or minimal improvement on this scale when compared with those taking placebo (20% (46/230) placebo group v 30% (69/233) lower dose group (P<0.05) and 37% (80/219) higher dose group (P<0.001) in the intention to treat analysis; 22% (49/226) placebo group v 32% (71/224) lower dose group (P<0.01) and 40% (78/193) higher dose group (P<0.001) in the last observation carried forward; 22% (44/197) placebo group v 31% (62/198) lower dose group (P<0.05) and 41% (63/155) higher dose group (P<0.001) in the observed cases analysis).
Table 4.
Mean scores (95% confidence intervals) on the clinician interview based impression of change scale (incorporating caregiver information) at weeks 12, 18, and 26 among the observed cases population
| Week | Placebo | Rivastigmine groups
|
|
|---|---|---|---|
| Lower dose | Higher dose | ||
| 12 | 3.96 (3.83 to 4.17) | 4.01 (3.83 to 4.17) | 3.88 (3.72 to 4.08) |
| 18 | 4.09 (3.92 to 4.28) | 4.06 (3.92 to 4.28) | 3.85 (3.7 to 4.1) |
| 26 | 4.34 (4.09 to 4.51) | 4.20 (3.99 to 4.41) | 3.93* (3.67 to 4.13) |
P<0.05 compared with placebo (pairwise Student’s t tests using pooled error terms from analysis of variance).
Progressive deterioration scale
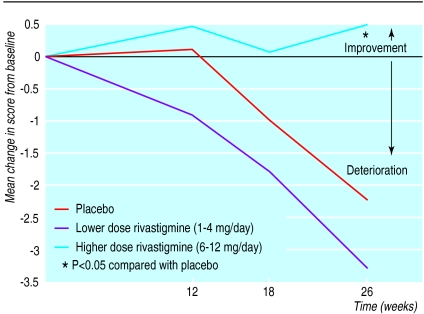
At week 26 the difference in mean change from baseline in scores on the progressive deterioration scale between patients receiving placebo and those receiving higher dose rivastigmine was statistically significant in the analysis of the last observation carried forward (P<0.05) (fig 3). Of the 581 patients completing the study, a significant difference in those showing any improvement in these scores was observed for those taking higher dose rivastigmine compared with those taking placebo (49% (98/198) v 39% (88/223) respectively; P=0.04 in analysis of the last observation carried forward). Significantly more patients in the higher dose group improved by at least 10% than did in the placebo group both in the intention to treat analysis (29% (70/241) v 19% (45/237), P<0.01) and the analysis of the last observation carried forward (33% (66/198) v 20% (45/223), P<0.01).
Figure 3.
Mean change in baseline scores on the progressive deterioration scale, analysis of last observation carried forward. P<0.05 compared with placebo (two tailed pairwise Student’s t tests using pooled error term from analysis of covariance and analysis of variance
Global deterioration scale and mini-mental state examination
At week 26 patients who had received rivastigmine 6-12 mg/day had a significantly better response than those in the placebo group in the mean change from baseline scores on the mini-mental state examination and the global deterioration scale. Patients receiving placebo deteriorated by 0.47 points from baseline on the mini-mental state and those receiving rivastigmine 6-12 mg/day improved by 0.21 points over baseline using the intention to treat analysis. Significantly less deterioration occurred on the global deterioration scale among patients taking 6-12 mg/day rivastigmine compared with those taking placebo.
Safety
Of the 725 patients initially randomly allocated 581 (80%) completed treatment. The proportion who discontinued treatment for any reason was significantly higher in the higher dose group than in the lower dose or placebo groups (33% (79/243) v 14% (34/243) and 13% (31/239), respectively) as was the proportion who discontinued because of adverse events (23% (55/242) v 7% (18/242) and 7% (16/239), respectively). Most of the discontinuations related to adverse events occurred during dose escalation (69% (38/55) in the higher dose group).
The safety of the drug could be evaluated in 242 patients in each of the rivastigmine treatment groups and in 239 patients in the placebo group. A summary of the adverse events that occurred at least 5% more often with rivastigmine than with placebo or that occurred with an incidence significantly different from placebo is given in table 5. Overall, significantly more patients reported at least one treatment related adverse event in the higher dose group (91% (220/242)) than in the lower dose (71% (172/242)) or placebo (72% (172/239)) groups.
Table 5.
Number (percentage) of adverse effects occurring at least 5% more often in patients taking rivastigmine than in patients taking placebo or occurring with an incidence significantly different from placebo
| Adverse effect | Rivastigmine groups
|
Placebo (n=239) | ||
|---|---|---|---|---|
| Higher dose* (n=242) | Lower dose (n=242) | |||
| Nausea | 121 (50) | 41 (17)* | 23 (10) | |
| Vomiting | 82 (34) | 19 (8) | 14 (6) | |
| Dizziness | 48 (20) | 25 (10) | 17 (7) | |
| Headache | 45 (19) | 16 (7) | 18 (8) | |
| Diarrhoea | 40 (17) | 23 (10) | 21 (9) | |
| Anorexia | 34 (14) | 8 (3) | 4 (2) | |
| Abdominal pain | 29 (12) | 11 (5) | 7 (3) | |
| Fatigue | 23 (10) | 5 (2) | 6 (3) | |
| Malaise | 23 (10) | 3 (1) | 5 (2) | |
P<0.05 compared with placebo (pairwise comparison based on Fisher’s exact test).
Adverse events related to treatment were generally not severe and occurred most frequently during the dose escalation phase. The adverse events most commonly reported with rivastigmine were cholinergic: nausea, vomiting, diarrhoea, abdominal pain, and anorexia. Dizziness, headache, fatigue, and malaise also occurred more frequently with higher doses of rivastigmine than with placebo. Apart from the incidence of nausea, there was no significant difference in the incidence of adverse events between the lower dose group and the group treated with placebo. The frequency of serious adverse events was similar in all groups (about 18%).
There were no obvious overall trends or clinically relevant differences between treatment groups in vital signs (mean systolic and diastolic blood pressure, abnormalities of blood pressure, heart rate, and body temperature), physical examination, haematological or biochemical analyses (including hepatic enzyme levels), electrocardiographic measurement, or urine analysis. Mean body weight increased in the placebo group (mean change +0.72 kg at week 26) but decreased in the rivastigmine groups (mean change −1.39 kg in the higher dose group and −0.13 kg in the lower dose). The difference in the mean change in body weight between the placebo group and the higher dose rivastigmine group was statistically significant (Fisher’s exact test P<0.05). In the higher dose group 24% of patients (55/234) lost >7% of body weight compared with 9% of patients (21/236) in the lower dose group and 7% (16/236) in the placebo group.
Discussion
This study provides evidence of the efficacy of rivastigmine in alleviating the core cognitive and functional symptoms of patients with mild to moderately severe Alzheimer’s disease over 6 months. Rivastigmine was effective on each of the measures of efficacy applied, reflecting improvements in cognition as rated by psychometricians, global functioning as rated by an independent clinician, and activities of daily living as rated by a caregiver. The effects of rivastigmine were dose dependent.
Cognitive and global assessments
Compared with the 55% of patients taking placebo who experienced a decline in cognitive function during the study, patients treated with 6-12 mg/day of rivastigmine improved. Cognitive function in patients with Alzheimer’s disease who are not treated can be expected to deteriorate. Estimates of the rate of decline vary from as little as 1.28 points on the cognitive subscale of the Alzheimer’s disease assessment scale over 24 weeks19 to as much as 9 points over 1 year.20 Other estimates of the average rate of decline are 5.2 points on the cognitive subscale over 1 year,15 7 points over 1 year,21 and about 5 points over 5 to 9 months.22 The stabilisation of cognitive decline seen over 6 months in this study in 55% of patients taking 6-12 mg/day of rivastigmine is therefore relevant to clinical practice.
Mean ratings on the clinician interview based impression of change were consistently and significantly superior for the group taking 6-12 mg/day of rivastigmine when compared with placebo, and significantly more patients treated with rivastigmine (in both dosage groups) experienced global improvement than did those taking the placebo.
Effects on activities of daily living
Perhaps the most relevant effects of rivastigmine observed in this study are those on activities of daily living. Poor performance of these activities is correlated with admission to long term care facilities.23,24 Poor performance is also recognised as an important determining factor of the use of support services by caregivers.25 The improvements in these activities shown here are the first to be reported in a prospective analysis of a global clinical trial. More than one third of patients treated with 6-12 mg/day of rivastigmine showed more than a 10% improvement.
Tolerability and safety
Adverse events leading to the discontinuation of treatment were seen in 27% of patients taking 6-12 mg/day of rivastigmine. The majority of these occurred during the dose titration phase, which used a forced dose escalation procedure and introduced an artificial element into the trial design. Outside a clinical trial it is likely that the dose escalation phase would be more individualised and tolerance would improve.
The most common adverse events were related to effects on acetylcholinesterase and were gastrointestinal. Most were mild and short lived and were observed after increases in doses. There was no evidence that rivastigmine compromised cardiovascular function in these elderly patients, many of whom had concomitant cardiovascular disease. The overall incidence of serious adverse events was similar in all three groups. Despite the age of the patients, the high incidence of coexisting illnesses, and the use of concomitant drug treatment, rivastigmine produced no clinically relevant changes in laboratory tests, electrocardiograms, on physical examination, or in vital signs except for a small, statistically significant decrease in mean body weight at higher doses.
This study provides clear evidence that rivastigmine is effective in the treatment of patients with probable Alzheimer’s disease and produces clinical benefits in a significant percentage of patients on the three domains measured. Improvements were still evident at the end of the 6 month study, although further data are required to determine the persistence of the results. Adverse events were generally mild or moderate and occurred early in treatment. The positive outcome of this study occurred despite the variability in clinical practice between countries and the difficulties presented by differences in language and culture. The results are qualitatively similar to those of a study of similar design carried out in the United States9 and add further evidence that rivastigmine offers clinically meaningful benefits to patients with Alzheimer’s disease.
Acknowledgments
Professor Agid wishes to acknowledge the special contribution made to the study by his colleague Professor Bruno Dubois.
Funding: This study was supported by funding from Novartis Pharma AG, Basle, Switzerland.
Footnotes
Competing interests: RA, AC-S, RH, and MG are employees of Novartis. The study was commissioned by Novartis Pharma in Switzerland. None of the other authors has any conflict of interest.
References
- 1.Knapp MJ, Knopman DS, Solomon PR, Pendlebury WW, Davis CS, Gracon SI, et al. A 30-week randomized controlled trial of high-dose tacrine in patients with Alzheimer’s disease. JAMA. 1994;271:985–991. [PubMed] [Google Scholar]
- 2.Davis KL, Thal LJ, Gamzu ER, Davis CS, Woolson RF, Gracon SI, et al. A double-blind, placebo-controlled multicenter study of tacrine for Alzheimer’s disease. N Engl J Med. 1992;327:1253–1259. doi: 10.1056/NEJM199210293271801. [DOI] [PubMed] [Google Scholar]
- 3.Farlow M, Gracon SI, Hershey LA, Lewis KW, Sadowsky CH, Dolan-Ureno J. A controlled trial of tacrine in Alzheimer’s disease. JAMA. 1992;268:2523–2529. [PubMed] [Google Scholar]
- 4.Roger SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT. A 24-week, double-blind, placebo controlled trial of donepezil in patients with Alzheimer’s disease. Neurology. 1998;50:136–145. doi: 10.1212/wnl.50.1.136. [DOI] [PubMed] [Google Scholar]
- 5.Rogers SL, Friedhoff LT. The efficacy and safety of donepezil in patients with Alzheimer’s disease: results of a US multicentre, randomized, double-blind, placebo-controlled trial. Dementia. 1996;7:293–303. doi: 10.1159/000106895. [DOI] [PubMed] [Google Scholar]
- 6.Enz A, Floersheim P. Cholinesterase inhibitors: an overview of their mechanisms of action in Alzheimer’s disease. In: Becker R, Giacobini E, editors. Alzheimer’s disease: from molecular biology to therapy. Boston: Birkhäuser; 1996. pp. 211–215. [Google Scholar]
- 7.Sramek JJ, Anand R, Wardle TS, Irwin P, Hartman RD, Cutler NR. Safety/tolerability trial of SDZ ENA 713 in patients with probable Alzheimer’s disease. Life Sci. 1996;58:1201–1207. doi: 10.1016/0024-3205(96)00081-1. [DOI] [PubMed] [Google Scholar]
- 8.Anand R, Gharabawi G, Enz A. Efficacy and safety results of the early phase studies with Exelon (ENA-713) in Alzheimer’s disease: an overview. J Drug Dev Clin Pract. 1996;8:109–116. [Google Scholar]
- 9.Corey-Bloom J, Anand R, Veach J. A randomized trial evaluating the efficacy and safety of ENA 713 (rivastigmine tartrate), a new acetylcholinesterase inhibitor, in patients with mild to moderately severe Alzheimer’s disease. Int J Geriatr Psychopharmacol. 1998;1:55–65. [Google Scholar]
- 10.Anand R, Gharabawi G. Clinical development of Exelon (ENA-713): the ADENA programme. J Drug Dev Clin Pract. 1996;8:117–122. [Google Scholar]
- 11.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- 12.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS/ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 13.Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 14.FDA guidance on Alzheimer’s drug clinical utility assessments. Washington, DC: FDC Reports; 1992. Food, Drug, and Cosmetic Reports; pp. 13–15. [Google Scholar]
- 15.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 16.Reisberg B, Schneider L, Doody R, Anand R, Feldman H, Haraguchi H, et al. Clinical global measurse of dementia: position paper from the International Working Group on Harmonization of Dementia Drug Guidelines. Alzheimer Dis Assoc Disord. 1997;11(suppl 3):8–18. [PubMed] [Google Scholar]
- 17.DeJong R, Osterlund OW, Roy GW. Measurement of quality-of-life changes in patients with Alzheimer’s disease. Clin Ther. 1989;11:545–554. [PubMed] [Google Scholar]
- 18.Reisberg B, Ferris SH, deLeon MJ, Crook T. The global deterioration scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139:1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 19.Antuono PG.for the Mentane Study Group. Effectiveness and safety of velnacrine for the treatment of Alzheimer’s disease Arch Intern Med 19951551766–1772. [PubMed] [Google Scholar]
- 20.Stern RG, Mohs RC, Davidson M, Schmeidler J, Silverman J, Kramer-Ginsberg E. A longitudinal study of Alzheimer’s disease: measurement, rate and predictors of cognitive deterioration. Am J Psychiatry. 1994;151:390–396. doi: 10.1176/ajp.151.3.390. [DOI] [PubMed] [Google Scholar]
- 21.Thal LJ, Carta A, Clarke WR, Ferris SH, Friedland RP, Petersen RC, et al. A 1-year multicenter placebo-controlled study of acetyl-L-carnitine in patients with Alzheimer’s disease. Neurology. 1996;47:705–711. doi: 10.1212/wnl.47.3.705. [DOI] [PubMed] [Google Scholar]
- 22.Solomon PR, Knapp MJ, Gracon SI, Groccia M, Pendlebury WW. Long-term tacrine treatment in patients with Alzheimer’s disease [letter] Lancet. 1996;348:275–276. doi: 10.1016/s0140-6736(05)65594-8. [DOI] [PubMed] [Google Scholar]
- 23.Riter RN, Fries BE. Predictors of the placement of cognitively impaired residents on special care units. Gerontologist. 1992;32:184–190. doi: 10.1093/geront/32.2.184. [DOI] [PubMed] [Google Scholar]
- 24.Mittelman MS, Ferris SH, Steinberg G, Shulman E, Mackell JA, Ambinder A, et al. An intervention that delays institutionalization of Alzheimer’s disease patients: treatment of spouse-carers. Gerontologist. 1993;33:730–740. doi: 10.1093/geront/33.6.730. [DOI] [PubMed] [Google Scholar]
- 25.The Canadian study of health and aging. Patterns of caring for people with dementia in Canada. Canadian J Ageing. 1994;13:470–487. [Google Scholar]