Abstract
Introduction
There have been numerous studies examining the association between depression and bone mineral density (BMD), but the underlying nature of this relationship remains unclear. Independent of this association, there is a growing body of evidence that depression impacts the risk for fracture in older adults. This article reviews the current epidemiological evidence regarding comorbidity of depression, low bone mineral density, and fracture.
Methods
A review of the literature on depression, depressive symptoms, low BMD, osteoporosis, and fracture using electronic databases.
Results
We reviewed 20 studies of the association between depression and BMD and five reports of the relationship between depression and fractures. Potential mediating mechanisms (both physiological and behavioral) are discussed, as well as potential confounding influences (e.g., medication use).
Conclusions
Most studies support the finding that depression is associated with increased risk for both low BMD and fractures, but variation in study design, sample composition, and exposure measurement make comparisons across studies difficult. Researchers should be aware of potential confounders, such as medication use, that may influence results. Future research should focus on identifying mediating pathways and targets for intervention in the relationships between depression, low BMD, and fracture.
Keywords: Antidepressants, Behavior, BMD, Depression, Fracture, Physiology
Introduction
Osteoporosis was first recognized as a disorder of bone metabolism in a 1947 paper by Albright [1]. Since that initial publication, much has been learned about the physiology of bone turnover and how those mechanisms interact with other systems of the body. Numerous studies have demonstrated an association between antidepressant medication use and osteoporotic fracture [2], and it has been suggested that depression may be an unrecognized risk factor for osteoporosis [3]. This article reviews the current evidence regarding comorbidity of depression, low bone mineral density, and fracture, and discusses the unresolved issues regarding these associations, including potential mediating pathways and the potential confounding influence of medications.
Depression, depressive symptoms, and osteoporosis
Major depressive disorder (MDD) is one of the most prevalent psychiatric conditions, affecting approximately 16% of the U.S. adult population [4]. MDD is characterized by feelings of dysphoria and/or anhedonia accompanied by somatic (e.g., appetite or sleep disturbances) and cognitive (e.g., trouble concentrating) symptoms. MDD and depressive symptoms commonly co-occur with physical ailments [5]. Schweiger and colleagues published the first study examining the relationship between depression and bone mineral density (BMD) in 1994. They measured BMD by single-energy quantitative computerized tomography (SE-QCT) in 70 depressed outpatients (53 women) and 88 controls (58 women). They found that the depressed group had BMD values, on average, 15% lower than the control group, after adjusting for age [6]. The majority of analyses have replicated the original 1994 finding of Schweiger et al. lower BMD among persons with depression or depressive symptoms relative to comparison groups [7–18]. However, seven studies have not found a statistically significant association between depression or depressive symptoms and lower BMD [19–25]. These studies are discussed below under their respective study designs.
Cross-sectional studies
Seven studies have reported on the cross-sectional relationship between depression or depressive symptoms and BMD. As illustrated by Table 1, these studies have varied widely in both the selection of participants and measurement of depression. Three of these analyses have included only women [7, 9, 22] and two included only men [15, 23]. Six have used population or community-based samples [7, 9, 12, 15, 18, 23]. With the exception of Mussolino et al. (1999), these cross-sectional studies have used non-diagnostic symptom scales to assess depression status, which are less sensitive than diagnostic measures and presumably included persons only temporarily distressed in the “exposed” group. The confounding influences controlled for (either by statistical adjustment or matching) varied greatly, with few studies controlling for the effects of known confounding influences, such as comorbid medical conditions or medications that affect bone strength. Given the varied methods of measuring exposure, selecting study populations, and controlling for confounding variables, it is not surprising that results have been inconsistent. The majority of cross-sectional studies have found that depressive symptomatology was associated with lower BMD [7, 9, 12, 15, 18]. For example, using the third National Health and Nutrition Examination Survey (NHANES III), a nationally representative sample of U.S. adults, Mussolino et al. (2004) found that MDD (as measured by the Diagnostic Interview Schedule (DIS)) was associated with lower BMD, but this association was only statistically significant among men [18]. Two cross-sectional analyses did not find a statistically significant association between depressive symptomology and BMD [22, 23]; however, both studies had notable limitations. For example, the cross-sectional analysis of Whooley et al. (2004) was limited to 16 cases of elevated depressive symptoms and thus had limited power to detect a statistically significant effect [23]. In a cross-sectional analysis, Sogaard et al. (2005) reported no association between depressive symptoms (as measured by the CONOR Mental Health Index) and BMD, although the authors did not report the data for this analysis, and thus it is not described in the table. As shown by Table 1, the remaining five cross-sectional analyses reported an inverse association between elevated depressive symptomatology and lower BMD.
Table 1.
Cross-sectional studies of depression or depressive symptoms and bone mineral density
| First author |
Year | Location | Sample size |
Source of participantsa |
Measure of exposureb |
Duration of exposure |
Measure of BMDc |
Matching and/or statistical adjustment |
Main findings |
|---|---|---|---|---|---|---|---|---|---|
| Coelho [7] | 1999 | Portugal | 102 | Community sample (only women), age: 40–80 yrs | BDI HSCL-90 | Current | Lumbar spine & femur DEXA | Adjusted for age and BMI | BMD≤−2.5 SD below reference mean (osteoporosis) assoc w/higher DepSx |
| Reginster [22] | 1999 | Belgium | 121 | Outpatient clinic (only women), age: 48–77 yrs | GHQ | Current | Spine, total hip & femoral neck DEXA | Adjusted for age, menopause, weight, height, estrogen use | No assoc btw DepSx & BMD |
| Robbins [12] | 2001 | USA | 1,566 | Population-based Medicare enrollees, age: 65–100 yrs | CESD-10 | Current | Total hip DEXA | Stratified by race and sex. Adjusted for age, BMI, kilocalories expended, estrogen use, smoking, alcohol intake | DepSx assoc w/lower BMD overall DepSx assoc w/lower BMD only among Caucasian women in stratified analyses |
| Mussolino [18] | 2004 | USA | 5,171 | Population-based, age: 30–39 yrs | DIS | Lifetime | Total proximal femur DEXA | Statified by sex. Adjusted for age, race, weight, height, food energy, calcium intake, protein intake, alcohol intake, smoking, physical activity, chronic conditions, weight change | Men: Major depressive episode assoc w/lower BMD Women: No assoc btwn major depressive episode & BMD |
| Whooley [23] | 2004 | USA | 515 | Population-based (only men), age: 50+yrs | GDS | Current | Lumbar spine & total hip DEXA | Adjusted for age, weight change, physical activity, smoking, caffeine intake, calcium use, steroid, diuretic, & benzodiazepine use, perceived health, BMI, chair stand activity | No assoc btwn DepSx & baseline BMD |
| Wong [15] | 2005 | Hong Kong | 2,000 | Population based (only men), age: 65–92 yrs | GDS | Current | Lumbar spine, total hip & total body DEXA | Adjusted for age, weight, calcium intake, physical activity, antipressant use, smoking status, comorbid COPD and CVD | DepSx assoc w/lower total hip BMD DepSx assoc w/increased risk of T-score≤−1 |
| Jacka [9] | 2005 | Australia | 78 | Community sample (only women), age: 45–60 yrs | Self-report DSM-based questionaire | ≤ 1 year | Lumbar spine & total hip | Adjusted for age, weight, estrogen use | DepSx assoc w/lower BMD |
Unless otherwise stated, studies include both men and women. Mean age of study participants is given if age range was unavailable.
Diagnostic measures of depression (MDD or MDE): DIS diagnostic interview schedule. Non-diagnostic measures of depressive symptoms (DepSx): BDI Beck depression inventory, HSCL-90 Hopkins symptom checklist-90, GHQ general health questionnaire, CESD-10 centers for epidemiologic studies depression scale-10 item. GDS geriatric depression scale. Self-report DSM based questionnaire.
DEXA dual-energy x-ray absorptiometry
Case-control studies
Since the initial case-control study of depression and BMD reported by Schweiger and colleagues in 1994, nine additional case-control studies of this association have been published (Table 2). As with the cross-sectional reports, the selection of participants, exposure measurement, and consideration of potential confounders varied greatly. All but one [11] of the case-control studies relied on clinic populations to enroll participants. The use of clinic populations, risks the internal validity of study findings via selection bias, which could lead to false positive findings [26]. Assessment of depression varied across the studies and included depressive symptom scales (e.g., Centers for Epidemiologic Studies Depression Scale (CESD) or Geriatric Depression Scale (GDS)), structured diagnostic interviews (e.g., Diagnostic Interview Schedule (DIS) or Structured Clinical Assessment for Neuropsychiatry (SCAN)), and psychiatric diagnoses. Few studies accounted for the effects of medications known to influence bone strength (i.e., use of thiazide diuretics or hormone replacement therapy). As with the cross-sectional reports, results were inconsistent across these case-control analyses. Three of the ten case-control studies reported no association between depression or depressive symptoms and BMD [20, 21, 25]. Each of these studies had important limitations that should be taken into account when evaluating the evidence for this relationship. The case-control study by Amsterdam and Hooper (1995) had limited power to detect an effect given the small sample size (N=11) [20]. Similarly, the studies by Kavuncu et al. (2002) and Yazici et al. (2005) were limited to young pre-menopausal women (mean age of depressed group: 35.4 years and 44.8 years, respectively). Many of these women had not yet passed through the peak developmental period of risk for depression (age 30–45) [27], and thus the cases of depression in these reports may not have been representative of the depression in the general population. It may be that depression affects BMD by accelerating post-menopausal bone loss in women, a hypothesis that neither of these studies could examine. However, Kavuncu et al. (2002) did find evidence of increased bone turnover, indicated by an elevated urinary deoxypyridinolie-to-creatine ratio, among the depressed group compared to controls [21]. The remaining seven case-control studies reported statistically significant inverse associations between depression and bone mineral density [6, 8, 10, 11, 14, 16, 17]. For example, Michelson et al. (1996) found that BMD as measured by DEXA was 13.6% lower at the femoral neck in women with a history of MDD compared to controls [11]. This study also found that depressed women had higher urinary cortisol levels than controls, a finding that supports the hypothesis that physiologic changes associated with depression, like hypercortisolism, mediate the relationship between depression and low BMD (discussed below).
Table 2.
Case-control studies of depression or depressive symptoms and bone mineral density
| First Author |
Year | Location | Sample size |
Source of participantsa | Measure of exposureb |
Duration of exposure |
Measure of BMDc |
Matching and/or statistical adjustment |
Main findings |
|---|---|---|---|---|---|---|---|---|---|
| Schweiger [6] | 1994 | Germany | 137 | Inpatient clinic, community controls, age: 40–95 yrs | MDC | Current | Lumbar spine SE-QCT | Adjusted for age | MDD assoc w/lower BMD compared to controls |
| Halbreich [8] | 1995 | USA | 68 | Inpatient clinic, age: 20–66 yrs | Psych Dx | Current | Lumbar spine & femoral neck DPA | Matched on age and sex | Psych Dxd assoc w/lower BMD compared to age and sex-matched normative BMD data |
| Michelson [11] | 1996 | USA | 48 | Community volunteers (only women), mean age: 41 yrs | SCID | Past or current | Lumbar spine, hip & radius DEXA | Matched on 5-year age strata, BMI, menopausal status, race | MDD assoc w/lower BMD compared to controls |
| Amsterdam [20] | 1998 | USA | 11 | Outpatient clinic, community controls, age: 27–53 yrs | Psych Dx | <1 yr to 25 yrs | Lumbar spine DEXA | None | No assoc btwn MDD & BMD |
| Vrkljan [14] | 2001 | Croatia | 48 | Inpatient clinic, community controls, age: 29–45 yrs | Psych Dx | Current | Unknown | None | Duration of depression therapy assoc w/lower BMD |
| Kavuncu [21] | 2002 | Turkey | 84 | Inpatient clinic, community controls (only women), mean age: 36 yrs | Psych Dx HDRS | Current | Lumbar spine & hip DEXA | Matched on age and BMI | No assoc btwn MDD & BMD No assoc btwn DepSx severity & BMD |
| Yazici [16] | 2003 | Turkey | 40 | Outpatient clinic, community controls only women), mean age: 31 yrs | SCAN | Current | Lumbar spine & hip DEXA | Matched on age, BMI, calcium intake, physical activity, social class | MDD assoc w/lower BMD |
| Yazici[25] | 2005 | Turkey | 65 | Outpatient clinic, community controls (only women), mean age: 45 yrs | Psych Dx HDRS | Current | Lumbar spine & femoral neck DEXA | Matched on age, BMI, age of menarche, number of pregnancies | No assoc btwn MDD & BMD |
| Kahl [10] | 2005 | Germany | 58 | Borderline PDd clinic, community controls (only women), mean age: 27 yrs | SCID | Current and Lifetime | Lumbar spine, femur & forearm DEXA | Matched on age and sex | MDD+borderline PDd assoc w/lower BMD vs. borderline PD alone MDD + borderline PD assoc w/lower BMD vs. neither |
| Altindag [17] | 2007 | Turkey | 77 | Outpatient clinic, community controls (only women), age: 26–56 yrs | Psych Dx HDRS | Current ≥ 3 months | Lumbar spine & femoral neck DEXA | Matched on age, BMI, age of menarche and parity | MDD assoc w/lower BMD |
Unless otherwise stated, studies include both men and women. Mean age of study participants is given if age range was unavailable
Diagnostic measures of depression (MDD or MDE): MDC Munich diagnostic checklist, Psych Dx psychiatrist diagnosis of major depressive disorder or major depressive episode. SCID structured clinical interview for DSM. SCAN structured clinical assessment for neuropsychiatry. Non-diagnostic measures of depressive symptoms (DepSx): HDRS Hamilton depression rating scale.
SE-QCT Single-energy quantitative computerized tomography, DPA dual-photon absorptiometry, DEXA dual energy x-ray absorptiometry
Psych Dx includes major depressive disorder, schizophrenia, schizoaffective disorder, mania or adjustment disorder
Longitudinal studies
Schweiger and colleagues followed-up the study sample from their 1994 publication and in 2000 published a prospective study of depression and BMD.(13)]. They found evidence of increased bone density loss among the depressed group of men and women relative to the controls after two years of follow-up. The remaining three prospective studies of depressive symptomology and BMD (Table 3) have failed to replicate the Schweiger et al. (2000) finding [19, 23, 24]. It is difficult to compare these reports given the varied study populations and measures of exposure. The 1999 study by Whooley and colleagues was limited to post-menopausal women [24]; Sogaard et al. (2005) included both men and women (pre-and post-menopausal) [19]; and Whooley et al. (2004) included only elderly men [23]. While these three studies have the methodological strength of being population-based rather than clinic samples, all used non-diagnostic measures of depression, and thus it is likely that there is substantial heterogeneity in the “exposed” group in each of these studies, which would tend to bias the analytic results towards the null [28]. For example, Whooley and colleagues in 1999 and 2004 (while both studies are prospective, they are different study populations and the latter is not a follow-up of the former) measured depressive symptoms using the Geriatric Depression Scale [23, 24]. Similarly, Sogaard et al. (2005) used a three-item measure of “long-term mental distress” to measure exposure [19]. Also of note, the prospective analysis of the Whooley (2004) study was limited to four cases of elevated depressive symptoms and therefore had limited power to detect a significant relationship. Interestingly, while these studies did not find an association between depression and BMD, two of the studies that also measured incidence of fracture found that depression was associated with increased risk of osteoporotic fracture (discussed below) [19, 24].
Table 3.
Comparative longitudinal studies of depression or depressive symptoms and bone mineral density
| First Author |
Year | Location | Sample size |
Follow- up duration |
Source of participantsa |
Measure of exposureb |
Measure of BMDc |
Matching and/or adjustment | Main findings |
|---|---|---|---|---|---|---|---|---|---|
| Whooley [24] | 1999 | USA | 7,414 | 3.7 years | Population-based (only women), age: 65+yrs | GDS | Lumbar spine & hip DEXA | Adjusted for age, marital status, education, arthritis, diabetes, weight change, physical activity, smoking, alcohol use, caffeine intake, calcium use, BMI, estrogen, steroid, diuretic, and benzodiazepine use, perceived health, quadricepts strength | No overall assoc btwn DepSx & BMDDepSx assoc w/lower BMD among highest BMI tertile vs. lower tertiles |
| Schweiger [13] | 2000 | Germany | 39 | 2 years | Inpatient clinic, community controls, age: 40–95 yrs | MDC | Lumbar spine SE-QCT | Adjusted for baseline BMD, age, sex | MDD assoc w/increased BMD loss at followup |
| Whooley [23] | 2004 | USA | 100 | 3.6 years | Population-based (only men), age: 50+yrs | GDS | Lumbar spine & hip DEXA | Adjusted for age, weight change, physical activity, smoking, caffeine intake, calcium use, steroid, diuretic & benzodiazepine use, perceived health, BMI, chair stand activity | No assoc btwn DepSx & mean percent change in BMD |
| Sogaard [19] | 2005 | Norway | 4,690 | 15 years | Population-based, age: 25+yrs | MDQ | Distal & ultradistal radius SXA | Stratified by sex; adjusted for age, marital status, BMI, receiving disability benefits, physical activity, smoking, estrogen use | Women: No assoc btwn DepSx & BMD Men: No assoc btwn DepSx & BMD |
Unless otherwise stated, studies include both men and women. Mean age of study participants is given if age range was unavailable.
Diagnostic measures of depression (MDD or MDE): MDC Munich diagnostic checklist. Non-diagnostic measures of depressive symptoms (DepSx): GDS geriatric depression scale, MDQ self-report mental distress questionnaire.
DEXA dual-energy x-ray absorptiometry, SE-QCT single-energy quantitative computerized tomography, SXA single-energy x-ray absorptiometry.
Depressive symptoms and fracture
Even if depression does not directly affect BMD, it may still be an important risk factor for the clinically significant outcome of low BMD-fractures. Hip fractures are associated with dramatically elevated mortality during the first year after the event [29], and there is evidence that this excess mortality persists for many years after the event [29]. There have been far fewer studies of depression and fractures compared with depression and BMD, and the vast majority have been longitudinal. Because of the likely bi-directional nature of the relationship between osteoporotic fractures and depression, longitudinal studies provide a more transparent characterization this association than do retrospective (cross-sectional or case-control study) designs (Table 4). One retrospective study [30], and all but one of the five prospective studies[31] found significant positive associations between depression or depressive symptoms and risk of fractures [19, 24, 30, 32, 33]. The longitudinal study that did not find an association between depression and fractures nonetheless found an association between urinary cortisol and fracture; as discussed below, hypercortisolism is a well-documented physiologic correlate of depression [34].
Table 4.
Comparative longitudinal studies of depression or depressive symptoms and fractures
| First Author |
Year | Location | Sample size |
Follow- up duration |
Source of participantsa |
Measure of exposureb |
Type of fracture |
Fracture assessment |
Matching and/or statistical adjustment | Main findings |
|---|---|---|---|---|---|---|---|---|---|---|
| Greendale [31] | 1999 | USA | 684 | 7 years | Population-based, age: 70–79 yrs | HSCL-90 | Hip, arm, spine, wrist & non-specified | Self-report of physician diagnosis | Adjusted for age, race, sex, comorbid conditions, physical activity, chair stand activity, BMI, smoking, alcohol use | No assoc btwn DepSx & fracture risk. Higher urinary free cortisol assoc w/increased fracture risk |
| Forsen [33] | 1999 | Norway | 18,612 | 3 years | Population-based (only women), age: 50– 101 yrs | MDI | Hip | Medical record registry | Adjusted for age, medications, BMI, smoking, physical activity, functional impairment | Top 10% of mental distress assoc w/increased fracture risk vs. lowest 10% |
| Whooley [24] | 1999 | USA | 7,414 | 3.7 years | Population-based (only women), age: 65+yrs | GDS | Non-vertebral & vertebral | Self-report confirmed by medical record radiographic films | Adjusted for age, marital status, education, chair stand activity, history of fracture, Hx of falling, arthritis, diabetes, steroid and estrogen use, calcium use, cognitive function, hip BMD | DepSx assoc w/increased fracture risk (both vertebral & non-vertebral) |
| Sogaard [19] | 2005 | Norway | 12,270 | 7 years | Population-based, age: 25+yrs | MDQ | Non-vertebral, hip, pelvis, proximal humerus, forearm | Hospital x-ray register; pathology reports to determine mechanism of trauma | Stratified by sex and nerve medications (women only); adjusted for age, marital status, smoking, alcohol use | Women: DepSx & DepSx + nerve meds assoc w/increased fracture risk. Men: No assoc btwn DepSx & fracture risk |
| Mussolino [32] | 2005 | USA | 6,195 | 18.3 years | Population-based, age: 25–74 yrs | GWBS | Hip | Hospital records & death certificates | Adjusted for age, sex, race, BMI, smoking, alcohol use, physical activity | DepSx assoc w/increased fracture risk |
Unless otherwise stated, studies include both men and women. Mean age of study participants is given if age range was unavailable.
Non-diagnostic measures of depressive symptoms (DepSx): HSCL-90 Hopkins symptom checklist-90, MDI mental distress index, GDS geriatric depression scale, MDQ self-report mental distress questionnaire GWBS general well-being schedule.
The likelihood of fracture is determined by three factors: bone mineral density, the force of the fall, and the angle of impact [35]. It is controversial as to which of these factors mediates the relationship between depression and fractures, but as discussed above there is evidence that depression affects BMD and two studies have found increased risk of falling associated with depression [24, 36]. Thus it is likely that depression impacts the risk of fracture through multiple pathways.
Potential mediators
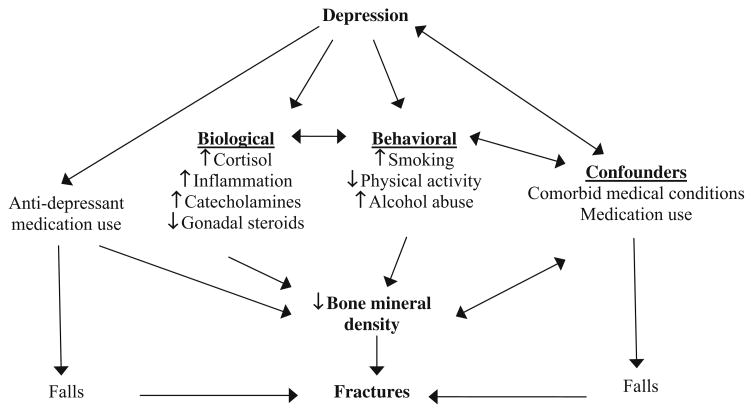
There are numerous mediating processes that may contribute to the relationship between depression and bone mineral density (Fig. 1). Two prominent ways in which depression is hypothesized to directly affect BMD and fracture risk are physiologic changes (e.g., alterations in the hypothalamic-pituitary-adrenal (HPA) axis) and the adoption of poor health behaviors (e.g., smoking and physical inactivity). It is also hypothesized that depression itself is not causally related to bone strength or fracture risk but is associated with these conditions due to confounding influences of comorbid health conditions and use of medications that affect bone metabolism. As shown by the figure, these processes likely interact, and thus it is more appropriately characterized not as an absolutist question of whether a mediating path exists, but rather as a question of the relative importance of a given path at a particular point in development.
Fig. 1.
Pathways linking depression, low bone mineral density, and fracture
Physiology
There are three pathophysiologic pathways leading to low bone mineral density: (1) inadequate acquisition of bone mass early in life, (2) elevated resorption of bone mass later in life, and (3) inefficient bone formation during continuous bone remodeling [35, 37]. These pathways are interdependent and the relative importance of each mechanism changes over development and varies by sex.
Many neuroendocrine hormones affect bone formation and/or bone resorption. Elevated levels hormones that either increase osteoclast (bone resorption) activity (e.g., inflammatory cytokines interleukin-6 (IL-6), IL-1 and tumor necrosis factor-alpha (TNF-α), parathyroid hormone, and C-reactive protein (CRP)) or inhibit osteoblast (bone formation) activity (e.g., calcitonin, leptin and cortisol) are predicted to be associated with low BMD [38]. Levels of many of the hormones that influence bone metabolism are altered in depression. For example, hypercortisolism, a consequence of HPA axis activation, is a correlate of depression [34], and cortisol has potent effects on bone metabolism [39]. Levels of IL-6 [40] and other inflammatory markers, such as CRP [41], are elevated in depression, and elevated levels of these pro-inflammatory markers are associated with low BMD [41, 42]. Sympathetic nervous system (SNS) activity as measured by catecholamine synthesis and hormone levels (e.g., tyrosine hydroxylase, norepinephrine) [43, 44] is also elevated in depression, and levels of these hormones are associated with reduced BMD [38]. Hyperinsulinemia is also associated with depressed mood [45], and insulin is thought to preserve bone mass [46]. Depression is associated with decreased levels of gonadal hormones estrogen [47] and testosterone [48], which are key regulators of bone formation [49].
Depression stimulates the action of hormones that increase bone resorption (e.g., cortisol) as well as those that potentiate bone formation (e.g., insulin). However, there have been only three studies that have directly examined the effect of depression on markers of bone metabolism. Herran and colleagues (2000) compared markers of bone turnover between first-episode cases of MDD and controls and found that markers of bone turnover (e.g., osteocalcin, telopeptide, and cross-laps) were elevated in the cases versus the controls. They also found that hormones that affect osteoclast function (e.g., parathyroid hormone) and osteoblast function (e.g., cortisol) were altered in the cases compared to the controls [50]. Kahl et al. (2005) reported elevated levels of cross-laps, osteocalcin, cortisol, TNF-á and IL-6 among cases of comorbid MDD and borderline personality disorder compared to controls [10]. Finally, Altindag et al. (2007) found elevated levels of plasma cortisol and cross-laps and lower levels of osteocalcin among depressed outpatients relative to controls [17].
Behaviors
Depression is associated with many poor health behaviors that have physiologic consequences which affect BMD [51]. Depression is associated with smoking [52], which is associated with lower BMD by inhibiting estrogen activity and inhibiting calcium absorption by the intestines [53]. Depression is also associated with increased alcohol use [54], and chronic alcohol use is also associated with low BMD by inhibition of bone cell proliferation and function [55, 56]. Depression is associated with fatigue and physical inactivity [57, 58], and physical activity is associated with increased BMD [36].
The association between depression and overall obesity as indicated by body mass index (BMI) is unclear and appears to be moderated by age, race and sex [59]. There is more consistent evidence regarding the positive association between depressive symptoms and abdominal or centralized obesity as measured by waist-hip-ratio [60, 61]. Body weight is positively associated with BMD [62] and is thought to preserve BMD by two mechanisms: first, synthesis of estrogen in adipose cells [63], which in turn promotes bone formation [35, 49], and second, through providing physical resistance to skeletal movement, which stimulates osteoblast activity [36]. The centralization of body weight is associated with alterations in markers of HPA axis and SNS dysregulation [64, 65]. Thus, while higher BMI may be protective of BMD loss with age, centralized obesity may be detrimental to bone mass because it is symptomatic of HPA hyperactivation, which inhibits bone formation and promotes bone resorption (discussed above).
Potential confounders
Comorbid medical conditions
Several medical conditions have been shown to affect risk of depression, bone mineral density, and fracture. Diabetes is associated with increased risk of depression [66], and diabetes (both type 1 and type 2) is associated with altered (both increased and decreased) BMD [67, 68]. Type 2 diabetes is more consistently associated with normal or increased BMD [69], whereas, type 1 diabetes is generally associated with decreased BMD [70]. Both types of diabetes are associated with increased risk of fractures [71, 72]. Other medical conditions associated with both depression and BMD include epilepsy [73], Crohn’s disease [74], rheumatoid arthritis [75], and systemic lupus erythematosus [76]. Many of these associations are thought to be iatrogenic in nature due to the medications used to treat these conditions.
Medication use
Another potential confounder of the relation between depression and BMD is the use of medications that have the potential to affect either bone strength or risk of fracture. Several classes of medications are known to decrease bone mass: glucocorticoids (e.g., for treatment of autoimmune disorders) [75, 77], lithium [78], and anti-convulsants [79]. Other medications are known to increase bone mass: estrogen (e.g., postmenopausal hormone replacement therapy) [80], statins [81], and thiazide diuretics [81].
Bone mineral density and antidepressant medications
Seven studies that examined the relationship between depression and BMD controlled for the influence of antidepressant medication use, either by statistical adjustment or exclusion criteria (Tables 1, 2, 3). Both studies that used statistical modeling methods found that the association between depression and low BMD persisted after adjusting for antidepressant use [11, 15]. Three studies only included cases that had a history of antidepressant use [8, 11, 12], and all found that depression was associated with decreased BMD. Two studies only included cases that had never used antidepressant medications [7, 16], and both found that depression was associated with risk of osteoporosis. One study found an inverse association between years of unspecified depression therapy and BMD [14].
There have been few studies of the direct effects of antidepressant medications on bone turnover. Studies have found that phosphodiesterase inhibitors, which are approved as antidepressant treatments in the UK but not in the US, increase bone formation in animal models [82, 83]. Animal studies have also indicated that serotonin may influence bone mass, particularly during stages of bone growth [84, 85]. A recent study showed that daily injections of the selective serotonin reuptake inhibitor (SSRI) fluoxetine in mice increased bone formation relative to controls, but that this effect was not observed in estrogen-deficient animals [86]. However, these initial results have not been replicated [87]. These results suggest that the effect of antidepressants on bone metabolism may depend on developmental stage and sex steroids (e.g., menopausal status in humans).
Five studies have directly examined the relationship between antidepressants and BMD in humans, with the majority reporting that use of these medications is associated with BMD. An analysis of the NHANES III data by Kinjo and colleagues found no association between antidepressant use and BMD [79]. However, Cauley and colleagues found that current use of SSRIs, but not tricyclic antidepressants (TCAs), was associated with low lumbar and hip BMD [88]. This finding that the association between antidepressant use and BMD is restricted to the SSRI class of antidepressants has been supported by the findings of two recent studies by Diem et al. (2007) [89] and Hanley et al. (2007) [90]. Notably, the study by Diem and colleagues found that the association between SSRI use and BMD persisted even after adjustment for depressive symptoms as measured by the geriatric depression scale. Another recent study of SSRI use in a prospective population-based cohort found that daily SSRI use at baseline was associated with 4% lower total hip BMD at followup five years later [91]. While this latter study controlled for depressive symptoms (as measured by the Short Form-36) they did not have a diagnostic measure of depression [91]. In sum, none of the currently published studies of antidepressant use and BMD have adjusted for depression as measured by a diagnostic or clinical instrument, and subsequently it is unclear whether this association is an example of confounding by indication. In light of the apparent association between antidepressant use and fracture (discussed below), it remains unresolved as to whether antidepressant medications increase the risk of fracture by directly reducing BMD as opposed to simply impairing balance and concentration, thus increasing the likelihood of falls.
Fractures and antidepressant medications
Numerous studies have examined the association between antidepressant use (both SSRIs and TCAs) and fracture risk. The majority have found that use of these medications, regardless of class (i.e., SSRI, TCA) is associated with increased risk of fracture [2]. However, most of these studies did not control for depression or depressive symptoms, and thus these analyses beg the question of whether the association between antidepressant use and fractures is due to confounding by indication. Those studies that did adjust for depressive symptomology found that the association between antidepressants and fracture was attenuated when depression was included in the model [92]. Studies that have examined duration of antidepressant medication use have shown that current use of these medications is a stronger predictor of fractures than former use [93–95], which indicates that these medications may be a more important confounder regarding the risk of falling or fracture than any effect they may have on BMD, since antidepressants often take weeks to significantly alter cell metabolism [96].
Three prospective studies of depression and fracture (Table 4) have adjusted for use of sedative and/or antidepressant medications, and all found that depression is associated with increased risk of fracture after taking the effects of such medications into account [19, 24, 33]. Thus it is possible that studies examining the relationship between antidepressant medications and fracture may overestimate the risk associated with these agents if they fail to adjust for indexes of depression [33].
Conclusion
Low bone mineral density (BMD) is a common condition among older adults, and the prevalence of osteopenia and osteoporosis is expected to increase dramatically in the next 50 years as the population pyramid shifts toward old age. Low BMD is associated with pronounced increased risk for debilitating fractures, particularly of the hip, vertebrae and distal forearm. Many of the prominent risk factors for low BMD, such as sex, age, race/ethnicity, and body type, are unalterable. It is therefore crucial to identify modifiable risk factors in order to reduce the public health burden of osteoporosis and osteopenia and the fractures associated with them.
Major depressive disorder is a common psychiatric disorder that is treatable with pharmacological and/or cognitive-behavioral therapy. Depression has been shown to be associated with low BMD in several studies, but even if depression influences BMD, it is unclear whether those changes are clinically meaningful. Depression has also been associated with increased risk of fractures. It is possible that depression affects the risk of low BMD and associated fractures in multiple ways, through both physiologic and behavioral mechanisms, and it is crucial to account for potential confounding influences, including antidepressant medications, when examining this relationship. Future studies should focus on establishing the mediating pathways that connect depression to fracture risk, in hopes of identifying targets for intervention and prevention.
Acknowledgments
This work was supported by National Institute of Mental Health grants T32-MH14592, R01-MH47447 and F31-MH78443.
References
- 1.Albright F. Osteoporosis. Ann Intern Med. 1947;27:861–882. doi: 10.7326/0003-4819-27-6-861. [DOI] [PubMed] [Google Scholar]
- 2.Takkouche B, Montes-Martinez A, Gill S, et al. Psychotropic medications and the risk of fracture: a meta-analysis. Drug Safety. 2007;30:171–184. doi: 10.2165/00002018-200730020-00006. [DOI] [PubMed] [Google Scholar]
- 3.Cizza G, Ravn P, Chrousos G. Depression: a major, unrecognized risk factor for osteoporosis? Trends Endocrinol Metab. 2001;12:198–203. doi: 10.1016/s1043-2760(01)00407-6. [DOI] [PubMed] [Google Scholar]
- 4.Kessler R, Berglund P, Delmer O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 5.Evans D, Charney D, Lewis L, et al. Mood disorders in the medically ill: scientific review and recommendations. Biol Psychiatry. 2005;58:175–189. doi: 10.1016/j.biopsych.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Schweiger U, Deuschle M, Korner A, et al. Low lumbar bone mineral density in patients with major depression. Am J Psychiatry. 1994;151:1691–1693. doi: 10.1176/ajp.151.11.1691. [DOI] [PubMed] [Google Scholar]
- 7.Coelho R, Silva C, Maia A, et al. Bone mineral density and depression: a community study of women. J Psychosom Res. 1999;46:29–35. doi: 10.1016/s0022-3999(98)00064-6. [DOI] [PubMed] [Google Scholar]
- 8.Halbreich U, Rojansky N, Palter S, et al. Decreased bone mineral density in medicated psychiatric patients. Psychosom Med. 1995;57:485–491. doi: 10.1097/00006842-199509000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Jacka F, Pasco J, Henry M, et al. Depression and bone mineral density in a community setting of perimenopausal women: Geelong Osteoporosis Study. Menopause. 2005;12:88–91. doi: 10.1097/00042192-200512010-00015. [DOI] [PubMed] [Google Scholar]
- 10.Kahl K, Rudolf S, Stoeckelhuber B, et al. Bone mineral density, markers of bone turnover, and cytokines in young women with borderline personality disorder with and without comorbid major depressive disorder. Am J Psychiatry. 2005;162:168–174. doi: 10.1176/appi.ajp.162.1.168. [DOI] [PubMed] [Google Scholar]
- 11.Michelson D, Stratakis C, Hill L, et al. Bone mineral density in women with depression. NEJM. 1996;335:1176–1181. doi: 10.1056/NEJM199610173351602. [DOI] [PubMed] [Google Scholar]
- 12.Robbins J, Hirsch C, Whitmer R, et al. The association of bone mineral density and depression in an older population. J Am Geriatr Soc. 2001;49:732–736. doi: 10.1046/j.1532-5415.2001.49149.x. [DOI] [PubMed] [Google Scholar]
- 13.Schweiger U, Weber B, Meuschle M, et al. Lumbar bone mineral density in patients with major depression: evidence of increased bone loss at followup. Am J Psychiatry. 2000;157:118–120. doi: 10.1176/ajp.157.1.118. [DOI] [PubMed] [Google Scholar]
- 14.Vrkljan M, Thaller V, Lovricevic I, et al. Depressive disorder as a possible risk factor for osteoporosis. Coll Antropol. 2001;25:485–492. [PubMed] [Google Scholar]
- 15.Wong S, Lau E, Lynn H, et al. Depression and bone mineral density: is there a relationship in elderly Asian men? Results from Mr. Os (Hong Kong) Osteoporos Int. 2005;16:610–615. doi: 10.1007/s00198-004-1730-2. [DOI] [PubMed] [Google Scholar]
- 16.Yazici K, Akinci A, Sutcu A, et al. Bone mineral density in premenopausal women with major depressive disorder. Psychiatry Res. 2003;117:271–275. doi: 10.1016/s0165-1781(03)00017-9. [DOI] [PubMed] [Google Scholar]
- 17.Altindag O, Altindag A, Asoglu M, et al. Relation of cortisol levels and bone mineral density among premenopausal women with major depression. Int J Clin Pract. 2007;61:416–420. doi: 10.1111/j.1742-1241.2006.01276.x. [DOI] [PubMed] [Google Scholar]
- 18.Mussolino M, Jonas B, Looker A. Depression and bone mineral density in young adults: results from NHANES III. Psychosom Med. 2004;66:533–537. doi: 10.1097/01.psy.0000132873.50734.7d. [DOI] [PubMed] [Google Scholar]
- 19.Sogaard A, Joakimsen R, Tverdal A, et al. Long-term mental distress, bone mineral density and non-vertebral factures: the Tromso Study. Osteoporos Int. 2005;16:887–897. doi: 10.1007/s00198-004-1784-1. [DOI] [PubMed] [Google Scholar]
- 20.Amsterdam J, Hooper M. Bone mineral density in major depression. Prog Neuro-Psychopharmacol Biol Psychiatry. 1998;22:267–277. doi: 10.1016/s0278-5846(98)00003-7. [DOI] [PubMed] [Google Scholar]
- 21.Kavuncu V, Kuloglu M, Kaya A, et al. Bone metabolism and bone mineral density in premenopausal women with mild depression. Yonsei Med J. 2002;43:101–108. doi: 10.3349/ymj.2002.43.1.101. [DOI] [PubMed] [Google Scholar]
- 22.Reginster J, Deroisy R, Paul I, et al. Depressive vulnerability is not an independent risk factor for osteoporosis in post-menopausal women. Maturitas. 1999;33:133–137. doi: 10.1016/s0378-5122(99)00057-2. [DOI] [PubMed] [Google Scholar]
- 23.Whooley M, Cauley J, Zmuda J, et al. Depressive symptoms and bone mineral density in men. J Geriatr Psychiatry Neurol. 2004;17:88–92. doi: 10.1177/0891988704264537. [DOI] [PubMed] [Google Scholar]
- 24.Whooley M, Kip K, Cauley J, et al. Depression, falls and risk of fracture in older women. Arch Intern Med. 1999;159:484–490. doi: 10.1001/archinte.159.5.484. [DOI] [PubMed] [Google Scholar]
- 25.Yazici A, Bagis S, Tot S, et al. Bone mineral density in premenopausal women with major depression. Joint Bone Spine. 2005;72:540–543. doi: 10.1016/j.jbspin.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 26.Berkson J. Limitations of the application of fourfold table analysis to hospital data. Biometrics. 1946;2:47–53. [PubMed] [Google Scholar]
- 27.Eaton WW, Anthony J, Gallo J, et al. Natural history of Diagnostic Interview Schedule/DSM-IV major depression: the Baltimore Epidemiologic Catchment Area followup. Arch Gen Psych. 1997;54:993–999. doi: 10.1001/archpsyc.1997.01830230023003. [DOI] [PubMed] [Google Scholar]
- 28.Copeland K, Checkoway H, McMichael A, et al. Bias due to misclassification in the estimation of relative risk. Am J Epidemiol. 1977;105:488–495. doi: 10.1093/oxfordjournals.aje.a112408. [DOI] [PubMed] [Google Scholar]
- 29.Forsen L, Sogaard A, Meyer H, et al. Survival after hip fracture: short and long-term excess mortality according to age and gender. Osteoporos Int. 1999;10:73–78. doi: 10.1007/s001980050197. [DOI] [PubMed] [Google Scholar]
- 30.Kelsey J, Prill M, Keegan T, et al. Reducing the risk of distal forearm fracture: preserve bone mass, slow down and don’t fall! Osteoporos Int. 2005;16:681–690. doi: 10.1007/s00198-004-1745-8. [DOI] [PubMed] [Google Scholar]
- 31.Greendale G, Unger J, Rowe J, et al. The relation between cortisol excretion and fractures in healthy older people: results from the MacArthur Studies. J Am Geriatr Soc. 1999;47:799–803. doi: 10.1111/j.1532-5415.1999.tb03835.x. [DOI] [PubMed] [Google Scholar]
- 32.Mussolino M. Depression and hip fracture: the NHANES I Epidemiologic followup study. Public Health Rep. 2005;120:71–75. doi: 10.1177/003335490512000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forsen L, Meyer H, Sogaard A, et al. Mental distress and risk of hip fracture: do broken hearts lead to broken bones? J Epidemiol Community Health. 1999;53:343–347. doi: 10.1136/jech.53.6.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carroll B, Curtis G, Davies B, et al. Urinary free cortisol excretion in depression. Psychol Med. 1976;6:43–50. doi: 10.1017/s0033291700007480. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization. Assessment of fracture risk and its application to screening for post-menopausal osteoporosis: report of the WHO study group. World Health Organ Tech Rep Ser. 1994;843:1–126. [PubMed] [Google Scholar]
- 36.Korpelainen R, Korpelainen J, Heikkinen J, et al. Lifelong risk factors for osteoporosis and fractures in elderly women with low body mass index-a population-based study. Bone. 2006;39:385–391. doi: 10.1016/j.bone.2006.01.143. [DOI] [PubMed] [Google Scholar]
- 37.Shoback D, Marcus R, Bikle D. Metabolic Bone Disease. In: Greenspan F, Gardner D, editors. Basic and clinical endocrinology. Lange Medical Books/McGraw-Hill; New York: 2004. pp. 295–361. [Google Scholar]
- 38.Raisz L. Physiology and pathophysiology of bone remodeling. Clin Chem. 1999;48:1353–1358. [PubMed] [Google Scholar]
- 39.Kann P, Laudes M, Piepkorn B, et al. Suppressed levels of serum cortisol following high-dose oral dexamethasone administration differ between healthy postmenopausal females and patients with established primary vertebral osteoporosis. Clin Rheumatol. 2001;20:25–29. doi: 10.1007/s100670170099. [DOI] [PubMed] [Google Scholar]
- 40.Licinio J, Wong M. The role of inflammatory mediators in the biology of major depression: central nervous system cytokines modulate the biological substrate of depressive symptoms, regulate stress-response systems, and contribute to neurotoxicity and neuroprotection. Mol Psychiatry. 1999;4:317–327. doi: 10.1038/sj.mp.4000586. [DOI] [PubMed] [Google Scholar]
- 41.Ganesan K, Teklehaimanot S, Tran T, et al. Relationship of c-reactive protein and bone mineral density in community-dwelling elderly females. J Natl Med Assoc. 2005;97:329–333. [PMC free article] [PubMed] [Google Scholar]
- 42.Papanicolaou D, Wilder R, Manolagas S, et al. The pathophysiologic roles of interleukin-6 in human disease. Ann Intern Med. 1998;128:127–137. doi: 10.7326/0003-4819-128-2-199801150-00009. [DOI] [PubMed] [Google Scholar]
- 43.Lake C, Pickar D, Ziegler M, et al. High plasma norepinephrine levels in patients with major affective disorder. Am J Psychiatry. 1982;139:1315–1318. doi: 10.1176/ajp.139.10.1315. [DOI] [PubMed] [Google Scholar]
- 44.Maes M, Vandewoude M, Schotte C, et al. Positive relationship between the catecholaminergic turnover and the DST results in depression. Psychol Med. 1990;20:493–499. doi: 10.1017/s0033291700017001. [DOI] [PubMed] [Google Scholar]
- 45.Winokur A, Maislin G, Phillips J, et al. Insulin resistance after oral glucose tolerance testing in patients with major depression. Am J Psychiatry. 1988;145:325–330. doi: 10.1176/ajp.145.3.325. [DOI] [PubMed] [Google Scholar]
- 46.Thrailkill K, Lumpkin C, Bunn R-C, et al. Is insulin an anabolic agent in bone? Dissecting the diabetic bone for clues. Am J Physiol Endocrinol Metab. 2005;289:735–745. doi: 10.1152/ajpendo.00159.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rehman H, Masson E. Neuroendocrinology of female aging. Gend Med. 2005;2:41–56. doi: 10.1016/s1550-8579(05)80008-7. [DOI] [PubMed] [Google Scholar]
- 48.Carnahan R, Perry P. Depression in aging men: the role of testosterone. Drugs Aging. 2004;21:361–376. doi: 10.2165/00002512-200421060-00002. [DOI] [PubMed] [Google Scholar]
- 49.Khosla S, Melton LJ, 3rd, O’Fallon W, et al. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab. 1998;83:2266–2274. doi: 10.1210/jcem.83.7.4924. [DOI] [PubMed] [Google Scholar]
- 50.Herran A, Amado J, Garcia-Unzueta M, et al. Increased bone remodeling in first-episode major depressive disorder. Psychosom Med. 2000;62:779–782. doi: 10.1097/00006842-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 51.Kanis J. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. Osteoporos Int. 1994;4:368–381. doi: 10.1007/BF01622200. [DOI] [PubMed] [Google Scholar]
- 52.Anda R, Williamson D, Escobedo L, et al. Depression and the dynamics of smoking: a national perspective. JAMA. 1990;264:1541–1545. [PubMed] [Google Scholar]
- 53.Kapoor D, Jones T. Smoking and hormones in health and endocrine disorders. Eur J Endocrinol. 2005;152:491–499. doi: 10.1530/eje.1.01867. [DOI] [PubMed] [Google Scholar]
- 54.Grant B, Harford T. Comorbidity between DSM-IV alcohol use disorders and major depression: results of a national survey. Drug Alcohol Depend. 1995;39:197–206. doi: 10.1016/0376-8716(95)01160-4. [DOI] [PubMed] [Google Scholar]
- 55.Chakkalakal D. Alcohol-induced bone loss and deficient bone repair. Alcohol Clin Exp Res. 2005;29:2077–2090. doi: 10.1097/01.alc.0000192039.21305.55. [DOI] [PubMed] [Google Scholar]
- 56.Friday K, Howard G. Ethanol inhibits human bone cell proliferation and function in vitro. Metabolism. 1991;40:562–565. doi: 10.1016/0026-0495(91)90044-w. [DOI] [PubMed] [Google Scholar]
- 57.Camancho T, Roberts R, Lazarus N, et al. Physical activity and depression: evidence from the Alameda County Study. Am J Epidemiol. 1991;134:220–231. doi: 10.1093/oxfordjournals.aje.a116074. [DOI] [PubMed] [Google Scholar]
- 58.Kritz-Silverstein D, Barrett-Connor E, Corbeau C. Cross-sectional and prospective study of exercise and depressed mood in the elderly: the Rancho Bernardo Study. Am J Epidemiol. 2001;153:596–603. doi: 10.1093/aje/153.6.596. [DOI] [PubMed] [Google Scholar]
- 59.Heo M, Pietrobelli A, Fontaine K, et al. Depressive mood and obesity in US adults: comparison and moderation by sex, age and race. Int J Obes. 2006;30:513–519. doi: 10.1038/sj.ijo.0803122. [DOI] [PubMed] [Google Scholar]
- 60.Weber-Hamann B, Hentschel F, Kniest A, et al. Hypercortisolemic depression is associated with increased intra-abdominal fat. Psychosom Med. 2002;64:274–277. doi: 10.1097/00006842-200203000-00010. [DOI] [PubMed] [Google Scholar]
- 61.Bjorntorp P. Abdominal fat distribution and disease: an overview of epidemiological data. Ann Med. 1992;24:15–18. doi: 10.3109/07853899209164140. [DOI] [PubMed] [Google Scholar]
- 62.Dargent-Molina P, Poitiers F, Breart G. In elderly women weight is the best predictor of a very low bone mineral density: evidence from the EPIDOS study. Osteoporos Int. 2000;11:881–888. doi: 10.1007/s001980070048. [DOI] [PubMed] [Google Scholar]
- 63.Schiff I. Menopause. In: Becker K, editor. Principles and practice of endocrinology and metabolism. J.B. Lippincott Company; Philadelphia, PA: 1995. pp. 915–924. [Google Scholar]
- 64.Bjorntorp P, Rosmond R. Hypothalamic origin of the metabolic syndrome X. Ann NY Acad Sci. 1999;18:297–307. doi: 10.1111/j.1749-6632.1999.tb07803.x. [DOI] [PubMed] [Google Scholar]
- 65.Ljung T, Holm G, Friberg P, et al. The activity of the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system in relation to waist/hip circumference ratio in men. Obes Res. 2000;8:487–495. doi: 10.1038/oby.2000.61. [DOI] [PubMed] [Google Scholar]
- 66.Anderson R, Freedland K, Clouse R, et al. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24:1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 67.Sert M, Tetiker T, Kirim S, et al. Type 2 diabetes mellitus and osteopenia: is there an association? Acta Diabetol. 2003;40:105–108. doi: 10.1007/s005920300014. [DOI] [PubMed] [Google Scholar]
- 68.Kao W, Krammerer C, Schneider J, et al. Type 2 diabetes is associated with increased bone mineral density in Mexican-American women. Arch Med Res. 2003;34:399–406. doi: 10.1016/j.arcmed.2002.07.001. [DOI] [PubMed] [Google Scholar]
- 69.de Liefde I, van der Klift M, de Laet C, et al. Bone mineral density and fracture risk in type 2 diabetes mellitus: the Rotterdam Study. Osteoporos Int. 2005;16:1713–1720. doi: 10.1007/s00198-005-1909-1. [DOI] [PubMed] [Google Scholar]
- 70.Lopez-Ibarra P, Pastor M, Escobar-Jimenez F, et al. Bone mineral density and time of clinical diagnosis of adult-onset type 1 diabetes mellitus. Endocr Pract. 2001;7:346–351. doi: 10.4158/EP.7.5.346. [DOI] [PubMed] [Google Scholar]
- 71.Forsen L, Meyer H, Midthjell K, et al. Diabetes mellitus and the incidence of hip fracture: results from the Nord-Trondelag Health Survey. Diabetologia. 1999;42:920–925. doi: 10.1007/s001250051248. [DOI] [PubMed] [Google Scholar]
- 72.Vestergaard P, Rejnmark L, Mosekilde L. Relative fracture risk in patients with diabetes mellitus, and the impact of insulin and oral antidiabetic medication on relative fracture risk. Diabetologia. 2005;48:1292–1299. doi: 10.1007/s00125-005-1786-3. [DOI] [PubMed] [Google Scholar]
- 73.Vestergaard P. Epilepsy, osteoporosis and fracture risk-a meta-analysis. Acta Neurol Scand. 2005;112:277–286. doi: 10.1111/j.1600-0404.2005.00474.x. [DOI] [PubMed] [Google Scholar]
- 74.Hela S, Nihel M, Faten L, et al. Osteoporosis and Crohn’s disease. Joint Bone Spine. 2005;72:403–407. doi: 10.1016/j.jbspin.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 75.Romas E. Bone loss in inflammatory arthritis: mechanisms and therapeutic approaches with biophosphonates. Best Pract Res Clin Rheumatol. 2005;19:1065–1079. doi: 10.1016/j.berh.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 76.Bertoli A, Alarcon G, Calvo-Alen M, et al. Systemic lupus erythematosus in a multiethnic US cohort: clinical features, course and outcome in patients with late-onset disease. Arthritis Rheum. 2006;54:1580–1587. doi: 10.1002/art.21765. [DOI] [PubMed] [Google Scholar]
- 77.Harpavat M, Keljo D, Reguerio M. Metabolic bone disease in inflammatory bowel disease. J Clin Gastroenterol. 2004;38:218–224. doi: 10.1097/00004836-200403000-00005. [DOI] [PubMed] [Google Scholar]
- 78.Misra M, Papakostas G, Klibanski A. Effects of psychiatric disorders and psychotropic medications on prolactin and bone metabolism. J Clin Psychiatry. 2004;65:1607–1618. doi: 10.4088/jcp.v65n1205. [DOI] [PubMed] [Google Scholar]
- 79.Kinjo M, Setoguchi S, Schneeweiss S, et al. Bone mineral density in subjects using central nervous system-active medications. Am J Med. 2005;118:1414.e7–1414.e12. doi: 10.1016/j.amjmed.2005.07.033. [DOI] [PubMed] [Google Scholar]
- 80.Vestergaard P, Rejnmark L, Mosekilde L. Anxiolytics, sedatives, antidepressants, neuroleptics and the risk of fracture. Osteoporos Int. 2006;17:807–816. doi: 10.1007/s00198-005-0065-y. [DOI] [PubMed] [Google Scholar]
- 81.Rejnmark L, Olsen M, Johnsen S, et al. Hip fracture risk in statin users-a population-based Danish case-control study. Osteoporos Int. 2004;15:452–458. doi: 10.1007/s00198-003-1568-z. [DOI] [PubMed] [Google Scholar]
- 82.Kinoshita H, Kobayashi S, Ebara S, et al. Phosphodiesterase inhibitors, pentoxifylline and rolipram, increase bone mass mainly by promoting bone formation in normal mice. Bone. 2000;27:811–817. doi: 10.1016/s8756-3282(00)00395-1. [DOI] [PubMed] [Google Scholar]
- 83.Waki Y, Horita T, Miyamoto K-I, et al. Effects of XT-44, a phosphodiesterase 4 inhibitor, in osteoblastgenesis and osteoclastgenesis in culture and its therapeutic effects in rat osteopenia models. Jpn J Pharmacol. 1999;79:477–483. doi: 10.1254/jjp.79.477. [DOI] [PubMed] [Google Scholar]
- 84.Bliziotes M, Gunness M, Eshleman A, et al. The role of dopamine and serotonin in regulating bone mass and strength: studies on dopamine and serotonin transporter null mice. J Musculoskele Neuronal Interact. 2002;2:291–295. [PubMed] [Google Scholar]
- 85.Warden S, Robling A, Sanders M, et al. Inhibition of the serotonin (5-hydroxytryptamine) transporter reduces bone accrual during growth. Endocrinology. 2005;146:685–693. doi: 10.1210/en.2004-1259. [DOI] [PubMed] [Google Scholar]
- 86.Battaglino R, Vokes M, Schulze-Spate U, et al. Fluoxetine treatment increases trabecular bone formation in mice. J Cell Biochem. 2007;100:1387–1394. doi: 10.1002/jcb.21131. [DOI] [PubMed] [Google Scholar]
- 87.Bonnet N, Bernard P, Beaupied H, et al. Various effects of antidepressant drugs on bone microarchitecture, mechanical properties and bone remodeling. Toxicol Applied Pharmacol. 2007;221:111–118. doi: 10.1016/j.taap.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 88.Cauley J, Fullman R, Stone K, et al. Factors associated with the lumbar spine and proximal femur bone mineral density in older men. Osteoporos Int. 2005;16:1525–1537. doi: 10.1007/s00198-005-1866-8. [DOI] [PubMed] [Google Scholar]
- 89.Diem S, Blackwell T, Stone K, et al. Use of antidepressants and rates of hip bone loss in older women. Arch Intern Med. 2007;167:1240–1245. doi: 10.1001/archinte.167.12.1240. [DOI] [PubMed] [Google Scholar]
- 90.Haney E, Chan B, Diem S, et al. Association of low bone mineral density with selective serotonin reuptake inhibitor use by older men. Arch Intern Med. 2007;167:1246–1251. doi: 10.1001/archinte.167.12.1246. [DOI] [PubMed] [Google Scholar]
- 91.Richards J, Papaioannou A, Adachi J, et al. Effect of selective serotonin reuptake inhibitors on the risk of fracture. Arch Intern Med. 2007;167:188–194. doi: 10.1001/archinte.167.2.188. [DOI] [PubMed] [Google Scholar]
- 92.Ensrud K, Blackwell T, Mangione C, et al. Central nervous system active medications and risk for fractures in older women. Arch Intern Med. 2003;163:949–957. doi: 10.1001/archinte.163.8.949. [DOI] [PubMed] [Google Scholar]
- 93.Liu B, Anderson G, Mittmann N, et al. Use of selective serotonin reuptake inhibitors or tricyclic antidepressants and risk of hip fractures in elderly people. Lancet. 1998;351:1303–1307. doi: 10.1016/s0140-6736(97)09528-7. [DOI] [PubMed] [Google Scholar]
- 94.Hubbard R, Farrington P, Smith C, et al. Exposure to tricyclic and selective serotonin reuptake inhibitor antidepressants and the risk of hip fracture. Am J Epidemiol. 2003;158:77–84. doi: 10.1093/aje/kwg114. [DOI] [PubMed] [Google Scholar]
- 95.Thapa P, Gideon P, Cost T, et al. Antidepressants and the risk of falls among nursing home residents. NEJM. 1998;339:875–882. doi: 10.1056/NEJM199809243391303. [DOI] [PubMed] [Google Scholar]
- 96.Svensson T. Brain noradrenaline and the mechanisms of action of antidepressant drugs. Acta Psychiatr Scand Suppl. 2000;402:18–27. doi: 10.1034/j.1600-0447.2000.02604.x. [DOI] [PubMed] [Google Scholar]