Abstract
Background
Preeclampsia is new onset hypertension during pregnancy with proteinuria. The initiating event in preeclampsia has been postulated to involve reduce placental perfusion which leads to widespread dysfunction of the maternal vascular endothelium.
Objective
The main objective of this brief review is to highlight some of the recent advances in our understanding of mechanisms whereby the endothelin (ET) system, via endothelin type A (ETA) receptor activation, modulates blood pressure in preeclamptic women and in animal models of pregnancy related hypertension.
Methods
This review focuses on the role of ET and tumor necrosis factor (TNF-α) in preeclampsia with emphasis on the pathophysiology of hypertension in response to placental ischemia in animal models of pregnancy. The review covers recently published data from Pubmed in addition to contributions from our laboratory.
Results
Studies in preeclamptic women indicate that the hypertension is associated with increases in ET synthesis. Recent studies also indicate that the ET system is activated in response to reductions in uterine perfusion pressure and in response to chronic elevations in serum levels of TNF-α in pregnant rats. Results also suggest that ETA receptor activation plays a role in mediating the hypertension in these two animal models.
Conclusions
Although recent studies in animal models implicate an important role for the ET system in preeclampsia, the usefulness of selective ETA receptor antagonists for the treatment of hypertension in women with preeclampsia remains unclear. This important question will not be answered until well-controlled clinical studies, using specific ETA receptor antagonists are performed in women with preeclampsia.
Keywords: preeclampsia, pregnancy, hypertension, endothelin, cytokines
Introduction
Hypertensive disorders of pregnancy such as preeclampsia occur in 6 to 8 percent of all pregnancies (1-3). Despite being one of the leading causes of maternal death and a major contributor of maternal and perinatal morbidity, the mechanisms responsible for the pathogenesis of preeclampsia are unknown (1-5). The hypertension associated with preeclampsia develops during pregnancy and remits after delivery implicating the placenta as a central culprit in this disease (1,5). The initiating event in preeclampsia has been postulated to involve reduce placental perfusion which leads to widespread dysfunction of the maternal vascular endothelium. While the mechanisms are not clear, they are likely to involve a delicate balance of vasodilators such as nitric oxide and prostacylin and vasoconstrictors of which the potent vasoactive peptide, endothelin may play an important role (1-6). The focus of this brief review is to provide an up-to-date analysis of recent evidence indicating a potential central role for endothelin in the pathophysiology of hypertension in response to placental ischemia during pregnancy.
In 1988, Yanagsawa and colleagues characterized an endothelial-derived vasoconstrictor, a 21 amino acid peptide subsequently called endothelin (7). Endothelin -1 (ET-1) is derived from a 203 amino acid peptide precursor, preproendothelin, which is cleaved after translation to form proendothelin. In the presence of a converting enzyme located within the endothelial cells, proendothelin or big endothelin is cleaved to produce the 21 amino acid peptide, endothelin. Increased synthesis of ET-1 has been reported in various diseases associated with cardiovascular abnormalities such as hypertension, diabetes, and chronic renal failure (8-11). ET-1 receptor binding sites have been identified throughout the body with the greatest numbers of receptors in the kidneys and lungs (8-11). Although the biochemical and molecular nature of endothelin has been well characterized, the physiological importance of endothelin in the regulation of renal and cardiovascular function in disease processes such as preeclampsia has yet to be fully elucidated.
Methods
The main objective of this brief review is to highlight some of recent advances in our understanding of the mechanisms whereby endothelin system, via endothelin type A (ETA) receptor activation, modulate blood pressure regulation in preeclamptic women and in several animal models of preeclampsia. Here, we highlight relevant data from the literature as well as recently published data from our own laboratory.
The Endothelin System in Preeclamptic Women
Clinical evidence from human studies indicates that endothelin may play an important role in mediating pathophysiological changes that occur during preeclampsia (12-14). Plasma concentration of ET-1 has been measured in normal pregnant and preeclamptic women (12). Some, but not all, investigators have found higher ET-1 plasma concentrations of approximately two- to threefold in women with preeclampsia (12,15) Typically, plasma levels of ET-1 are highest during the latter stage of the disease, suggesting that ET-1 may not be involved in the initiation of preeclampsia, but rather in the progression of disease into a malignant phase. Although the elevation in plasma levels of ET-1 during preeclampsia is only two or threefold above normal, previous studies have reported that this level of plasma ET-1 can have significant long-term effects on systemic hemodynamics and arterial pressure regulation (16) Thus long-term elevations in plasma levels of ET-1 comparable to those measured in women with preeclampsia could play a role in mediating the reductions in renal function and elevations in arterial pressure observed in women with preeclampsia.
While some studies have reported no significant changes in circulating levels of ET-1 during moderate forms of preeclampsia, a possible role for ET-1 as a paracrine or autocrine agent in preeclampsia remains worthy of consideration. Since ET-1 is released towards the vascular smooth muscle in a paracrine fashion, changes in plasma levels of ET during may not reflect the local production of ET. Indeed, this is one of the reasons why it has been difficult to ascertain whether preeclampsia is associated with altered ET production. Local synthesis of ET has been assessed in preeclamptic women and investigators have found preproendothelin mRNA to be elevated in a variety of tissues (17-19). Because of the limitations of clinical studies utilizing selective ET type A receptor antagonists in pregnant women, the importance of locally produced ET in the pathophysiology of preeclampsia remains unclear.
Placental Ischemia-Induced Hypertension During Pregnancy: Role of Endothelin
Experimental induction of chronic uteroplacental ischemia appears to be the most promising animal model to study potential mechanisms of preeclampsia since reductions in uteroplacental blood flow in a variety of animal models causes hypertension similar to what is observed in preeclamptic women (6,20-22). Recently, we have developed a model of placental ischemia in the rat in order to examine potential pathophysiological mechanisms that mediate hypertension during chronic reductions in uteroplacental perfusion pressure (20,21). We found that reducing uteroplacental perfusion pressure (RUPP) results in significant and consistent elevation in arterial pressure of 20-30 mmHg as compared to control pregnant rats at day 19 of gestation (equivalent to the end of the third trimester). We also reported that reducing uteroplacental perfusion pressure in non-pregnant rats had no effect on blood pressure. In addition to hypertension, there are several findings in the RUPP model that are consistent with preeclampsia in women. First, our data indicate that the RUPP-induced hypertension is accompanied by proteinuria, reductions in renal plasma flow and glomerular filtration rate, and a hypertensive shift in the pressure natriuresis relationship (20,21). Second, vascular endothelial function is significantly impaired in the RUPP hypertensive rat (20). This is based on evidence that relaxation responses to acetylcholine in aortic strips isolated from RUPP hypertensive rats were significantly attenuated compared to normal pregnant rats (21). This impairment was likely due to a reduced production of nitric oxide in vascular tissue from RUPP hypertensive rats as well as an increase in the synthesis of thromboxane, ET-1, and 8-isoprostane, a marker of oxidative stress (22-27). Third, the inflammatory cytokines, tumor necrosis factor alpha (TNFα) and Interluken-6 (IL-6) are significantly increased in plasma from RUPP hypertensive rats (28,29). This data is consistent with reports that preeclamptic women have increase circulating levels of inflammatory cytokines (4,21,32-38). Fourth, we have recently reported that circulating sFlt-1 concentration is increased and both plasma free VEGF and PlGF are decreased in RUPP hypertensive rats (30), again consistent with what has been reported in preeclamptic women (31). Finally, we have found that intrauterine growth restriction is prevalent in pups born to RUPP hypertensive rats (20-27). Taken together, these characteristics make the model of RUPP-induced hypertension in the pregnant an ideal experimental tool to investigate the pathophysiological mechanisms that contribute to hypertension in preeclampsia.
Alexander et al. examined the role of ET-1 in mediating the hypertension in this placental ischemic model of preeclampsia (32). Using an RNase protection assay, they found that renal expression of preproendothelin was significantly elevated in both the medulla and the cortex of pregnant rats with chronic RUPP compared with control pregnant rats. Moreover, they reported that chronic administration of the selective ETA receptor antagonist, ABT627 markedly attenuated the increase in mean arterial pressure in pregnant rats with RUPP. In contrast, ETA receptor blockade had no significant effect on blood pressure in the normal pregnant animal. These findings suggest that ET-1 plays a major role in mediating the hypertension produced by chronic reductions in uterine perfusion pressure in pregnant rats.
Role of Endothelin in Tumor Necrosis Factor-Induced Hypertension During Pregnancy
Several lines of evidence support the hypothesis that the ischemic placenta contributes to endothelial cell activation/dysfunction of the maternal circulation by enhancing the synthesis of cytokines such as tumor necrosis factor alpha (TNFα) (33-38). Inflammatory cytokines such as TNFα have been shown to induced structural as well as functional alterations in endothelial cells(33-35). Also supporting a potential role of TNFα in preeclampsia are findings that plasma levels of TNFα are significantly elevated in women with preeclampsia by approximately two-fold (36-38).
Sera from pregnant rats exposed to chronic RUPP increases ET-1 production by cultured endothelial cells (39). The exact mechanism linking enhanced renal production of ET-1 to placental ischemia in pregnant rats or in preeclamptic women is unknown. One potential mechanism for enhanced ET-1 production is via transcriptional regulation of the ET-1 gene by TNF-α. TNF-α is elevated in preeclamptic women and has been implicated in the disease processes (40). LaMarca and colleagues recently reported that chronic infusion of TNF-α in pregnant rats, at a rate to mimic plasma levels (2-3 fold increase) observed in women with preeclampsia significantly increases in blood pressure (41). The increase in arterial pressure produced by a 2-3 fold elevation in plasma levels of TNF-α in pregnant rats is associated with significant increases in local production of ET-1 in the kidney, placenta, and vasculature (41). Moreover, the increase in mean arterial pressure in response to TNF-α is completely abolished in pregnant rats treated with an ETA receptor antagonist (41). Collectively, these findings suggest that endothlelin, via ETA receptor activation, plays an important role in mediating TNF-α - induced hypertension in pregnant rats.
Summary
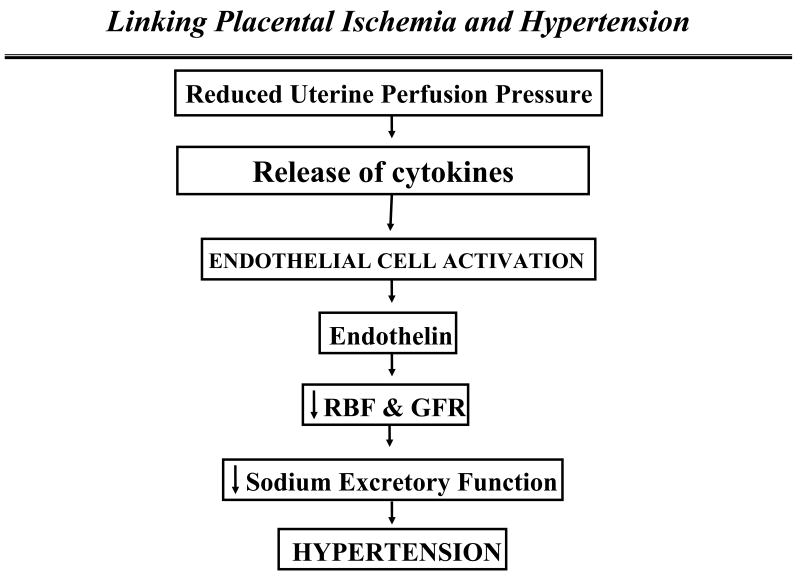
Preeclampsia is defined as new onset hypertension with proteinuria during pregnancy. The initiating event in preeclampsia is postulated to be reduced uteroplacental perfusion which leads to widespread dysfunction of the maternal vascular endothelium. Inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF alpha) are thought to be important links between placental ischemia and cardiovascular and renal dysfunction (See Figure 1). Recent studies have indicated that chronic reductions in placental perfusion in pregnant animals are associated with enhanced production of TNF alpha and endothelin. In addition, chronic infusion of TNF alpha into normal pregnant rats results in significant increases in arterial pressure and a decrease in renal hemodynamics. TNF alpha activates the endothelin system in placenta, renal and vascular tissues. Moreover, the increase in mean arterial pressure in response to placental ischemia or TNF-α is completely abolished in pregnant rats pretreated with an ETA receptor antagonist.
Figure 1.
Although recent studies indicate that the endothelin system plays an important role in mediating the hypertension in response to reductions in uterine perfusion pressure and in response to chronic elevations in serum levels of TNF-α in pregnant rats, the usefulness of selective endothelin type A receptor antagonists for the treatment of hypertension in women with preeclampsia remains unclear. This important question will not be answered until well-controlled clinical studies, using selective ET type A receptor antagonists, are performed in women with preeclampsia
Acknowledgments
This work was supported by NIH grants HL38499 and HL51971. The NIH National Research Service Award HL78147 and HL10137-01 supported Drs. LaMarca and Alexander.
References
- 1.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005 February 26;365(9461):785–99. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 2.Roberts JM, Pearson G, Cutler J, Lindheimer M. Summary of the NHLBI Working Group on Research on Hypertension During Pregnancy. Hypertension. 2003 March 1;41(3):437–45. doi: 10.1161/01.HYP.0000054981.03589.E9. [DOI] [PubMed] [Google Scholar]
- 3.Sibai BM, Caritis S, Hauth J National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. What we have learned about preeclampsia? Semin Perinatol. 2003 Jun;27(3):239–46. doi: 10.1016/s0146-0005(03)00022-3. [DOI] [PubMed] [Google Scholar]
- 4.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1989 November;161(5):1200–4. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- 5.Redman CW. Current topic: pre-eclampsia and the placenta. Placenta. 1991 July;12(4):301–308. doi: 10.1016/0143-4004(91)90339-h. [DOI] [PubMed] [Google Scholar]
- 6.Khalil RA, Granger JP. Vascular mechanisms of increased arterial pressure in preeclampsia: lessons from animal models. Am J Physiol Regul Integr Comp Physiol. 2002 Jul;283(1):R29–45. doi: 10.1152/ajpregu.00762.2001. [DOI] [PubMed] [Google Scholar]
- 7.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 8.Kohan D. Endothelins in the normal and diseased kidney. Am J Kidney Dis. 1997;29:2–26. doi: 10.1016/s0272-6386(97)90004-4. [DOI] [PubMed] [Google Scholar]
- 9.Schiffrin EL. Endothelin: potential role in hypertension and vascular hypertrophy. Hypertension. 1995;25:1135–1143. doi: 10.1161/01.hyp.25.6.1135. [DOI] [PubMed] [Google Scholar]
- 10.Simonson MS, Dunn MJ. Endothelin peptides and the kidney. Annu Rev Physiol. 1993;55:249–265. doi: 10.1146/annurev.ph.55.030193.001341. [DOI] [PubMed] [Google Scholar]
- 11.Schiffrin EL. Vascular endothelin in hypertension. Vascul Pharmacol. 2005;43(1):19–29. doi: 10.1016/j.vph.2005.03.004. Review. [DOI] [PubMed] [Google Scholar]
- 12.Taylor RN, Varma M, Teng NN, Roberts JM. Women with preeclampsia have higher plasma endothelin levels than women with normal pregnancies. J Clin Endocrinol Metab. 1990;71:1675–1677. doi: 10.1210/jcem-71-6-1675. [DOI] [PubMed] [Google Scholar]
- 13.Mastrogiannis DS, O'Brien WF, Krammer J, Benoit R. Potential role of endothelin-1 in normal and hypertensive pregnancies. Am J Obstet Gynecol. 1991;165:1711–1716. doi: 10.1016/0002-9378(91)90020-r. [DOI] [PubMed] [Google Scholar]
- 14.McMahon LP, Redman CW, Firth JD. Expression of the three endothelin genes and plasma levels of endothelin in pre-eclamptic and normal gestations. Clin Sci (Colch) 1993;85:417–424. doi: 10.1042/cs0850417. [DOI] [PubMed] [Google Scholar]
- 15.Benigni A, Orisio S, Gaspari F, Frusca T, Amuso G, Remuzzi G. Evidence against a pathogenetic role for endothelin in pre-eclampsia. Br Obstet Gynecol. 1992;99:798. doi: 10.1111/j.1471-0528.1992.tb14409.x. [DOI] [PubMed] [Google Scholar]
- 16.Wilkins FC, Alberola A, Mizelle HL, Opgenorth TJ, Granger JP. Systemic hemodynamics and renal function during long-term pathophysiological increases in circulating endothelin. Am J Physiol. 1995;268(2 Pt2):R375–R381. doi: 10.1152/ajpregu.1995.268.2.R375. [DOI] [PubMed] [Google Scholar]
- 17.Napolitano M, Miceli F, Calce A, Vacca A, Gulino A, Apa R, Lanzone A. Expression and relationship between endothelin-1 messenger ribonucleic acid (mRNA) and inducible/endothelial nitric oxide synthase mRNA isoforms from normal and preeclamptic placentas. J Clin Endocrinol Metab. 2000 Jun;85(6):2318–23. doi: 10.1210/jcem.85.6.6623. [DOI] [PubMed] [Google Scholar]
- 18.Rogers RG, Thorpe JM., Jr Pregnancy-induced hypertension: Genesis of and response to endothelial injury and the role of endothelin1. Obstet Gynecol Surv. 1997;52:723–727. doi: 10.1097/00006254-199712000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Faxen M, Nasiell J, Lunell NO, Blanck A. Differences in mRNA expression of endothelin-1, c-fos and c-jun in placentas from normal pregnancies and pregnancies complicated with preeclampsia and/or intrauterine growth retardation. Gynecol Obstet Invest. 1997;44(2):93–6. doi: 10.1159/000291494. [DOI] [PubMed] [Google Scholar]
- 20.Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of hypertension during preeclampsia linking placental ischemia with endothelial dysfunction. Hypertension. 2001 Sep;38(3 Pt 2):718–22. doi: 10.1161/01.hyp.38.3.718. [DOI] [PubMed] [Google Scholar]
- 21.Granger JP, LaMarca BB, Cockrell K, Sedeek M, Balzi C, Chandler D, Bennett W. Reduced uterine perfusion pressure (RUPP) model for studying cardiovascular-renal dysfunction in response to placental ischemia. Methods Mol Med. 2006;122:383–392. doi: 10.1385/1-59259-989-3:381. [DOI] [PubMed] [Google Scholar]
- 22.Conrad KP. Animal models of pre-eclampsia: do they exist. Fetal Medicine Rev. 1990;2:67–88. [Google Scholar]
- 23.Crews JK, Herrington JN, Granger JP, Khalil RA. Decreased endothelium-dependent vascular relaxation during reduction of uterine perfusion pressure in pregnant rats. Hypertension. 2000;35:367–372. doi: 10.1161/01.hyp.35.1.367. [DOI] [PubMed] [Google Scholar]
- 24.Alexander BT, Kassab SE, Miller MT, Abram SR, Reckelhoff JF, Bennett WA, Granger JP. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension. 2001;37:1191–1195. doi: 10.1161/01.hyp.37.4.1191. [DOI] [PubMed] [Google Scholar]
- 25.Llinas MT, Alexander BT, Abram SR, Sedeek M, Granger JP. Enhanced production of thromboxane A2 in response to chronic reductions in uterine perfusion pressure in pregnant rats. Am J Hypertens. 2002;15:793–797. doi: 10.1016/s0895-7061(02)02975-8. [DOI] [PubMed] [Google Scholar]
- 26.Llinas MT, Alexander BT, Capparelli M, Carroll MA, Granger JP. Cytochrome P-450 inhibition attenuates hypertension induced by reductions in uterine perfusion pressure in pregnant rats. Hypertension. 2004;43:623–628. doi: 10.1161/01.HYP.0000117721.83371.9f. [DOI] [PubMed] [Google Scholar]
- 27.Alexander BT, Llinas MT, Kruckeberg WC, Granger JP. L-arginine attenuates hypertension in pregnant rats with reduced uterine perfusion pressure. Hypertension. 2004;43:832–836. doi: 10.1161/01.HYP.0000119192.32360.a9. [DOI] [PubMed] [Google Scholar]
- 28.LaMarca BB, Bennett WA, Alexander BT, Cockrell K, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of tumor necrosis factor-alpha. Hypertension. 2005;46:1022–1025. doi: 10.1161/01.HYP.0000175476.26719.36. [DOI] [PubMed] [Google Scholar]
- 29.Gadonski G, LaMarca BB, Sullivan E, Bennett W, Chandler D, Granger JP. Hypertension Produced by Reductions in Uterine Perfusion in the Pregnant Rat. Role of Interleukin 6. Hypertension. 2006 Oct;48(4):711–6. doi: 10.1161/01.HYP.0000238442.33463.94. [DOI] [PubMed] [Google Scholar]
- 30.Gilbert JS, Babcock SA, Granger JP. Hypertension Produced by Reduced Uterine Perfusionin Pregnant Rats Is Associated With Increased Soluble Fms-Like Tyrosine Kinase-1 Expression. Hypertension. 2007 Oct 8; doi: 10.1161/HYPERTENSIONAHA.107.096594. [DOI] [PubMed] [Google Scholar]
- 31.Karumanchi SA, Bdolah Y. Hypoxia and sFlt-1 in preeclampsia: the “chicken-and-egg” question. Endocrinology. 2004;145:4835–4837. doi: 10.1210/en.2004-1028. [DOI] [PubMed] [Google Scholar]
- 32.Alexander BT, Rinewalt AN, Cockrell KL, Bennett WA, Granger JP. Endothelin-A receptor blockade attenuates the hypertension in response to chronic reductions in uterine perfusion pressure. Hypertension. 2001;37:485–489. doi: 10.1161/01.hyp.37.2.485. [DOI] [PubMed] [Google Scholar]
- 33.Lefer AM, Ma XL. Cytokines and growth factors in endothelial dysfunction. Crit Care Med. 1993;21:S9–S14. doi: 10.1097/00003246-199302001-00003. [DOI] [PubMed] [Google Scholar]
- 34.Meekins JW, McLaughlin PJ, West DC, McFadyen IR, Johnson PM. Endothelial cell activation by tumour necrosis factor-alpha (TNF-α) and the development of pre-eclampsia. Clin Exp Immunol. 1994;98:110–114. doi: 10.1111/j.1365-2249.1994.tb06615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benyo DF, Smarason A, Redman CW, Sims C, Conrad KP. Expression of inflamatory cytokines in placentas from women with preeclampsia. J Clin Invest. 2001;86:2505–2512. doi: 10.1210/jcem.86.6.7585. [DOI] [PubMed] [Google Scholar]
- 36.Kupferminc MJ, Peaceman AM, Wigton TR, Rehnberg KA, Socol ML. Tumor necrosis factor-α is elevated in plasma and amniotic fluid of patients with severe preeclampsia. Am J Obstet Gynecol. 1994;170:1752–1757. [PubMed] [Google Scholar]
- 37.Visser W, Beckmann I, Bremer HA, Lim HL, Wallenburg HC. Bioactive tumour necrosis factor α in pre-eclamptic patients with and without the HELLP syndrome. Br J Obstet Gynaecol. 1994;101:1081–1082. doi: 10.1111/j.1471-0528.1994.tb13587.x. [DOI] [PubMed] [Google Scholar]
- 38.Vince GS, Starkey PM, Austgulen R, Kwiatkowski D, Redman CW. Interleukin-6, tumour necrosis factor and soluble tumour necrosis factor receptors in women with pre-eclampsia. Br J Obstet Gynaecol. 1995;102:20–25. doi: 10.1111/j.1471-0528.1995.tb09020.x. [DOI] [PubMed] [Google Scholar]
- 39.Roberts L, LaMarca BB, Fournier L, Bain J, Cockrell K, Granger JP. Enhanced endothelin synthesis by endothelial cells exposed to sera from pregnant rats with decreased uterine perfusion. Hypertension. 2006;47:615–618. doi: 10.1161/01.HYP.0000197950.42301.dd. [DOI] [PubMed] [Google Scholar]
- 40.Marsden PA, Brenner BM. Transcriptional regulation of the endothelin-1 gene by TNF-α. Am J Physiol. 1992;262:C854–C861. doi: 10.1152/ajpcell.1992.262.4.C854. [DOI] [PubMed] [Google Scholar]
- 41.LaMarca BB, Cockrell K, Sullivan E, Bennett W, Granger JP. Role of endothelin in mediating tumor necrosis factor-induced hypertension in pregnant rats. Hypertension. 2005;46:82–86. doi: 10.1161/01.HYP.0000169152.59854.36. [DOI] [PubMed] [Google Scholar]