Abstract
Chlamydia trachomatis infection is the most common sexually transmitted bacterial infection worldwide with over 91 million cases estimated annually. An effective subunit vaccine against Chlamydia may require a multivalent subunit cocktail of antigens in a single formulation for broad coverage of a heterogeneous MHC population. Herein we describe the identification by CD4+ and CD8+ T cell expression cloning, serological expression cloning, and an in silico analysis of the C. trachomatis genome, of novel C. trachomatis antigens. These antigens elicited human CD4+ T cell responses, and a subset proved to be immunogenic and protective when administered as immunoprophylactic vaccines against C. trachomatis challenge. Candidate vaccines consisting of the prioritized C. trachomatis antigens adjuvanted in GSK proprietary AS01B adjuvant were prioritized based on induction of solid protection against challenge in C57BL/6 and BALB/c mice with C. trachomatis. Some of the vaccines prevented bacterial shedding and colonization of the upper genital tract to varying degrees by mechanisms that may include CD4+ T cells.
Keywords: Vaccine, Chlamydia, Adjuvant
INTRODUCTION
Chlamydia trachomatis infection is one of the most common sexually transmitted diseases worldwide with the majority of cases occurring in Asia, Sub-Saharan Africa, and South America (WHO, 1990, Behets, 2001). Most developing countries that have the highest burden of chlamydial infections, have limited capacity to effectively screen for chlamydial infections and treatment is thus largely based on symptomatic case ascertainment (WHO, 2001). In the United States, C. trachomatis infection is the most commonly reported sexually transmitted bacterial disease, with an estimated 4–5 million cases annually.
Although antibiotic therapy is thought to eliminate chlamydial infection (Ridgway, 1997), it does not treat the established pathology. This, together with the fact that chlamydial infections can often be asymptomatic, points toward preventative measures such as vaccination as the most effective option for control of chlamydial disease.
Numerous C. trachomatis serovars have been described, of which 8 (D, E, F, G, H, I, J, K) cause genital infections (Igietseme & Ward, 2004). Infection can lead to a variety of asymptomatic and symptomatic manifestations including vaginal muco-purulent discharge, endometritis and salpingitis, and pelvic inflammatory disease (PID) (Stephens, 2003). Among infected women, it is estimated that approximately 20% develop PID, 4% chronic pelvic pain, 3% become infertile, and 2% have an adverse pregnancy outcome (Paavonen & Eggert-Kruse, 1999, Mardh, 2004, van Valkengoed, et al., 2004).
The use of various mouse gene-knockout strains (Cotter, et al., 1997, Johansson, et al., 1997, Su, et al., 1997), in vivo depletion of specific lymphocyte populations, and transfer of immune lymphocyte populations to naïve mice led to the notion that C. trachomatis immunity is mediated by mucosal IgA antibodies, IgG molecules that transmigrate the gut epithelium, and Th1 CD4+ T cells secreting IFN-γ (Cain & Rank, 1995, Morrison, et al., 1995, Perry, et al., 1997, Igietseme & Murdin, 2000, Morrison & Morrison, 2001, Igietseme, et al., 2002, Morrison & Caldwell, 2002, Barr, et al., 2005, Brunham & Rey-Ladino, 2005). B cell-deficient mice are comparable to wild-type mice in overcoming primary infection, suggesting B cells have only a minor role in preventing an initial infection. However, B cell-deficient mice are unable to prevent re-infection, suggesting a functional role for B cells in adaptive recall immunity (Morrison & Morrison, 2005). Since the natural response against Chlamydia uses elements of humoral and cell-mediated immunity, it appears that a good chlamydial vaccine would induce high frequencies of Th1 type CD4+ T and B cells.
Early vaccine trials revealed two additional important principles in protection against Chlamydia (Grayston & Wang, 1978). First, protection elicited by C. trachomatis vaccination was specific to the homologous strain, and second, acceleration of inflammatory responses could result when breakthrough infections occur. Thus, it was concluded that a whole cell Chlamydia vaccine would be of limited value since it contains antigens that elicit tissue damaging immune-mediated hypersensitivity reactions. Since then, however, mucosal immunization with elementary bodies (EB) has been successfully used to protect mice against genital challenge (de la Maza & Peterson, 2002). Furthermore, protection against a vaginal Chlamydia challenge in mice has been achieved by immunization with dendritic cells that had been pulsed with EB in vitro.
Herein we describe studies to establish a heterologous challenge model in mice with the human C. trachomatis serovar K strain and to examine if systemic immunizations with AS01B Adjuvant System, a strong Th1 inducing adjuvant (Pichyangkul, et al., 2004, Skeiky, et al., 2004, Mettens, et al., 2008) induced protection against genital chlamydial infection. We postulated that UV-inactivated EB formulated in AS01B Adjuvant System may induce a protective immune response similar to the protection observed in prior studies with EB-pulsed dendritic cells in the C. muridarum model (Su, et al., 1998). We were able to demonstrate that systemic immunization of mice with UV-inactivated EB+AS01B Adjuvant System from a heterologous strain induced protection in chlamydia intravaginal and intrauterine challenge models. We also describe studies on the identification of antigens that elicit a human Th1 CD4+ T cell response (proliferation and IFN-γ production) and/or are outer membrane proteins accessible to neutralizing antibodies. Various cloning and screening techniques were emphasized to detect new Chlamydia antigens including (i) an in silico analysis of the available C. trachomatis genome, (ii) CD4+ T cell expression cloning using CD4+ T cell lines derived from infected women with a library of C. trachomatis randomly sheared genomic DNA, and (iii) CD8+ T cell expression cloning using cells from infected humans and mice together with this same genomic library, and screening of this expression library with human serum from Chlamydia-infected donors. We were able to demonstrate that systemic immunization of mice with one or more of the identified antigens formulated in AS01B Adjuvant System (a liposome monophosphoryl lipid A (MPL)- and QS21-based adjuvant currently in clinical trials with malaria vaccine candidate antigens) from a homologous strain induced protection in a chlamydial intravaginal and intrauterine challenge models which we have developed by a CD4+ T cell-mediated mechanism.
MATERIALS AND METHODS
Chlamydia preparations
C. trachomatis serovar K (strain UW-31/Cx; ATCC VR-887) and E (strain BOUR; ATCC VR-348B) were propagated in McCoy (ATCC CRL-1696) or HeLa 229 cells (ATCC CCL-2.1) as described (Gervassi, et al., 2004). EB were purified by two-step Hypaque-70 discontinuous gradient ultracentrifugation (Nycomed Inc, Princeton NJ) and stored at −80 ºC in sucrose phosphate-glutamate (SPG) buffer, as described previously (Gervassi, et al., 2004). Chlamydial preparations were routinely negative for Mycoplasma as shown by a Mycoplasma-specific PCR. The infectivity of the Chlamydia preparations was defined by determination of inclusion forming units (IFU) on McCoy cells. Titers were expressed in IFU per ml and were measured by growing serial dilutions of EB preparations on McCoy cells and subsequent immunofluorescent (IF) staining of the inclusions after 48 h using a polyclonal anti-EB antibody labeled with FITC (AB1140F, Chemicon, Temecula, CA). EB were inactivated by UV-irradiation with an X lamp for 60 min. Viability was checked by inoculation of UV-irradiated EB onto McCoy cells and IF staining 48 hours post infection. Protein concentrations of UV-irradiated bacterial stocks were calculated using a Bi-Cinchoninic Acid (BCA) assay according to the manufacturer’s instructions (Pierce, Rockford IL). The concentration of UV-inactivated EB was adjusted to 200 μg/ml with SPG, aliquoted and frozen.
Generation and expansion of Chlamydia-specific T-cell lines from infected donors and mice. Informed consent was obtained in advance from all the subjects from whom blood products were isolated and this study was approved by Western IRB, Seattle, WA. Peripheral blood mononuclear cells (PBMC) were obtained by density centrifugation over ficoll from the apheresis product of 16 donors including (i) C. trachomatis patients with clinical manifestations, (ii) Chlamydia-infected donors (asymptomatic, but seropositive for C. trachomatis or C. pneumoniae), and (iii) Chlamydia-exposed donors, some of whom were cord-blood donors (asymptomatic, seronegative partners of patients with diagnosed chlamydial infection).. PBMC were frozen until use. HLA typing was performed for HLA class I and class II alleles using polymerase chain amplification with sequence-specific primers to assign alleles for the HLA-A, HLA-B, HLA-C, HLA-DRB1, 3, 4, 5 and HLA-DQB1 loci at the Puget Sound Blood Center.
For stimulation assays, PBMCs were plated in triplicate at 2 – 2.5 × 105 cells/well and cultured with medium, PHA (10 μg/ml), UVEB (10 μg/ml), or each recombinant protein (10 μg/ml) for 72 h. Supernatants were harvested and analyzed for IFN-γ by a double-sandwich ELISA using specific mAb (eBioscience Inc., San Diego, CA), following the manufacturer’s protocol. Values above mean background (medium) + 3 SD were considered positive.
Dendritic cells (DC) were generated by culture of autologous adherent PBMC with 30ng of GM-CSF and 10ng of IL-4 for 7 days, as described (Sanderson, et al., 1995) in RPMI–10% human serum (HS). Four hours after cultures were iniated, the non-adherent cells were collected, washed and re-frozen. Adherent cells were harvested with cell-dissociation medium (Sigma, St. Louis, Mo.) and seeded at 2 × 104 cells per well in 96-well flat-bottom plates in 100 ml of RPMI–10% HS. DC were cultured in the presence of Chlamydia elementary bodies from serovar E were cultured at 104 cells/well in 96-well round bottom plates with varying numbers of monocyte depleted PBMC as responder cells (102–104). Wells that showed obvious growth of T cells characterized by aggregates of dividing cells by visual light microscopy inspection, were then expanded with anti-CD3 antibody (30 ng/ml) for 5 days.
Cells were then washed, resuspended in 10% HuS + 1ng/ml IL-2, IL-4, and cultured for 4 additional days before resting the culture in 10% HuS + 10ng/ml IL-7, 1ng/ml IL-2, IL-4, for 7 days. The cultures were then tested for reactivity with EB from serovar E using autologous PBMC as APC.
Additionally, a murine, H2-Ld restricted CD8+ T-cell line which was shown to lyse cells infected with a variety of CT serovars (B, C, D, F, J, K, L2 and L3) and to protect mice from challenge with CT following adoptive transfer (Starnbach, et al., 1994) was obtained from M. Starnbach, Harvard University. This CTL line was expanded by stimulating the T cells on C. trachomatis infected J774 cells, a transformed mouse macrophage cell line, in the presence of irradiated syngeneic spleen cells as described (Starnbach, et al., 1994). Expanded T cells were used to screen a genomic library of C. trachomatis LGV II.
Construction and Screening of a Chlamydia Expression Library
C. trachomatis serovar E genomic DNA was isolated and sheared by sonication to a size range of 1–4 kb, partially digested with Sau3A and size fractionated. Libraries were constructed in a Lambda ZapII expression cloning system (Stratagene, La Jolla, CA), as described (Berthet, et al., 1998). Transfected E.coli bacteria were then plated to give ~50–80 transfectants per plate and colonies were pooled to establish glycerol stocks. Glycerol stocks were used to establish overnight cultures (2XYT/100 μg/ml ampicillin) that were split 1:5 or 1:10 the next morning. Plates were grown for an additional 1 h and then induced by the addition of isopropyl-β-D-thio-galactopyranoside (IPTG). After induction for 3–4 h, plates were centrifuged and the supernatant discarded. Bacterial pellets were resuspended in 200 μl antibiotic free RPMI/10% FCS and 10 μl was added in duplicate to wells containing DC, also plated in antibiotic free medium. The plates were cultured for 90 min at 37 °C and washed to remove excess EB. The medium was replaced with antibiotic containing complete medium (RPMI/10% pooled human serum/50 μg/ml gentamicin) and 104 T cells were added. The plates were cultured for an additional 3 days, after which 50 μl was removed for assessment of IFN-γ by ELISA and the plates were pulsed with tritiated thymidine (1 μCi/well). After culture for an additional 18 h, cells were harvested and tritium uptake determined using a gas scintillation counter. IFN-γ levels in culture supernatants were determined by ELISA. Pools that demonstrated an increase in either assay were broken down to recover the reactive clone. This was accomplished by picking 96 individual colonies from a positive pool, growing these in a 96-well plate, and repeating the screening of 20 sub-pools derived from the 8 rows and 12 columns. A final repeat of the expression screening of the pure positive Chlamydia clone was performed. The insert was then characterized by DNA sequencing.
A genomic library of C. trachomatis LGV II was also constructed in the retroviral vectors pBIB-KS1,2,3 by limited BamHI, BglII, BstYi, and MboI restriction digests of the genome and ligation into the BamHI site of pBIB-KS1,2,3. This vector set was modified to contain a Kosak translation initiation site and stop codons in order to allow expression of proteins off of short DNA genomic fragments. DNA pools of 80 clones were prepared and transfected into the retroviral packaging line Phoenix-Ampho (Pear, et al., 1993). The chlamydia library in retroviral form was then transduced into P815 (H2d) cells which were used to stimulate the CTL.
Expression of recombinant Chlamydia proteins
The recombinant antigens encoded by CT694, CT695, CT696, pmpG, CT089, CT858, CT875 were expressed and purified. To prepare the various vaccines, stocks of purified proteins were prepared after expressing their genes in E. coli. For this purpose, competent E. coli strains BL21(DE3) plys S and Rosetta 2(DE3)plys S were transformed with expression plasmids and grown on the appropriate antibiotic selection medium. Harvested cells were resuspended in lysis buffer each (20 mM Tris 8.0/100 mM NaCl/2 mM PMSF), and lysed by freeze/thaw followed by sonication. Ni-NTA chromatography was performed on the entire cell lysate, post sonication, under native conditions. Lysates were combined and dH2O-rinsed Ni-NTA (Qiagen) was added and rocked at room temperature. After centrifugation, wash buffer (lysis buffer containing 1% deoxycholate) was added to both Ni-NTA/antigen pellets, and rocked at room temperature. Antigens were eluted using lysis buffer containing 350 mM Imidazole. The elution fractions were combined, placed into a 10MWCO snakeskin dialysis bag (Pierce Biotechnology, Inc.), and placed in beaker containing 4L of 20 mM Tris pH 8.0. The protein concentration was measured by the Pierce (Rockford, IL.) bicinchoninic acid assay, and purity was assessed by SDS-PAGE followed by Coomassie blue staining.
Prophylactic immunization and chlamydial challenge
Mice were immunized twice at the base of the tail with 10 μg UVEB from serovar K or E formulated in 100 μl AS01B Adjuvant System at 3 weeks interval. The AS01B Ajuvant System consists of MPL, QS21 and liposomes (Pichyangkul, et al., 2004, Skeiky, et al., 2004, Mettens, et al., 2008). Vaccine doses of candidate antigens were formulated by mixing 50 μl of recombinant antigen(s) with 50 μl of AS01B Adjuvant System (2x) for 1 dose of vaccine (candidate antigen/AS01B), according to instructions provided by GlaxoSmithKline Biologicals (Rixensart, Belgium). Female BALB/c, and C57BL/6 mice were obtained from Charles River (Wilmington, MA). All mice were used between 6 and 8 weeks of age. All operations were performed under anesthesia using a mixture of Xylazine and Ketamine except for the progesterone injection and the vaginal swabbing described below. All mice were injected subcutaneously with 1.25 mg of progesterone (Depo-Provera) on days 10 and 3 prior to genital challenge. Mice were infected 4 weeks after the last immunization using 5 × 105 IFU of C. trachomatis serovar K. Chlamydia were deposited onto the ectocervix of anesthetized mice using a positive displacement pipette in a final volume of 20 μl SPG. For intrauterine challenge experiments mice were immunized intramuscularly twice, at a three week interval with 10 μg of UVEB formulated in AS01B Adjuvant System. Mice were treated with progesterone as described. Four weeks after the last immunization the mice were anesthetized, a small incision was made in the abdomen, and 2 μl of serovar K (2 × 106 IFU) was injected using a Hamilton syringe (Hamilton Company, Reno, NV) into the lumen of each horn of the uterus, just above the level of the cervix.
Immunology Endpoints
Serum IgG antibody specificities to chlamydial antigens were tested by ELISA using sera from C. trachomatis patients and study subjects with no acute C. trachomatis infection. Plates were coated with 0.1 mg/wel1 of the respective antigen. Serum IgG titers at an OD of 1.0 were calculated by using a curve fit program by plotting serial serum dilution from 1:100 to 1:16400 with the respective optical density values.
Microbiological and histopathological end-points
Chlamydial shedding in the lower genital tract was determined using a Dacroswab (Dacron) inserted in the vagina and rotated 25 times while keeping in contact with the ectocervix. Dacroswab tips were frozen in 1ml SPG at −80 ºC until cultured on McCoy cells to determine chlamydial IFU. For culture of vaginal swabs, samples were thawed and a 100 μl volume of acid washed glass beads (Sigma-Aldrich) was added to all the tubes. Vials were vortexed for 2 minutes and 100 μl of each sample was inoculated onto a McCoy monolayer grown to determine chlamydial IFU confluence in a 24-well plate. Plates were centrifuged at 1000 × g at 37 °C and then cultured for 48 h to determine IFU. Bacterial load in the upper genital tracts was determined by removing the entire genital tracts above the level of the cervix and homogenizing the tissue on ice in SPG. For histological analysis, genital tracts were removed, fixed in Bouin’s for 24 h, rinsed with PBS and dehydrated with methanol before embedding in paraffin. 5–6 μm sections were de-paraffined and stained with eosin and hematoxylin. Inflammatory infiltrate in the uterus and oviducts was scored in a blinded manner on a scale from 1 to 5 arbitrarily representing minimal, slight mild, moderate and severe inflammation, respectively.
Statistical analysis
The differences between mean and median bacterial numbers of control mice and vaccinated mice were compared by Student’s t test and/or one-way Anova to analyze differences in IFU/swab and inflammatory scores of experimental groups. P values of < 0.05 were considered significant.
RESULTS
Screening and Prioritization of Identified Candidate Antigens Using Human Sera
The first screening approach made use of prior observations that strong systemic antibody responses generally result from genital infections with C. trachomatis in those patients with clinical manifestations. Using this approach and using pooled sera from 5 C. trachomatis patients to screen 40,000 plaque forming units (PFU) of a λ CT L-2 randomly sheared genomic library, 26 primary picks or unique sequences were identified. Of these, four putative membrane proteins were identified (data not shown).
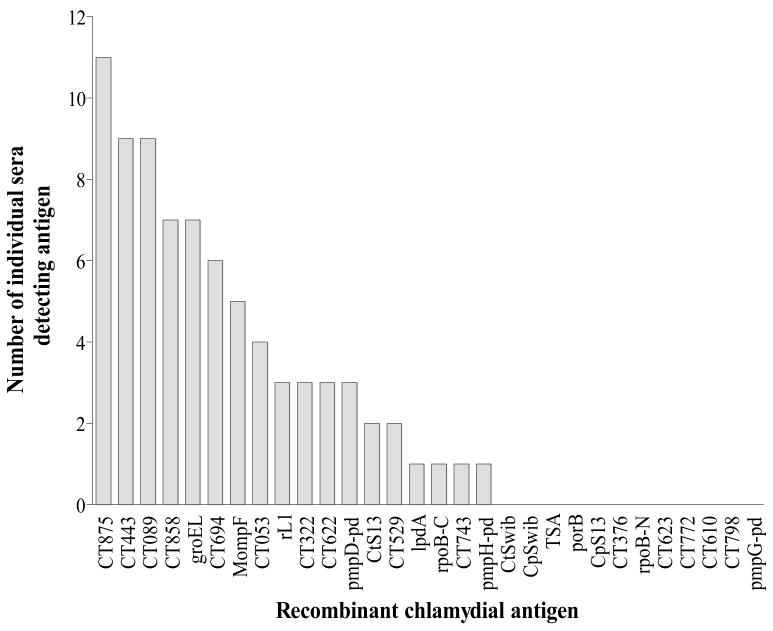
After identifying the first 26 genes using a pool of C. trachomatis patient sera, we subsequently characterized the IgG responses of sera from 27 more donors (11 C. trachomatis patients and 16 Chlamydia-exposed donors with no history of disease (6 C. trachomatis exposed donors, 2 C. pneumoniae and 8 cord-blood donors)), to a panel of these chlamydial antigens. Figure 1 shows the number of serum samples that bound a particular recombinant antigen in an ELISA (OD ≥ 1.0). Interestingly, two of the 8 cord blood sera responded to the C. trachomatis–specific antigen CT694 suggesting that Chlamydia–specific maternal antibodies crossed the placenta in these subjects. Eight antigens were bound by antibodies in four or more donors, and the following five antigens were recognized by serum IgG from at least seven donors: CT875 (n=11); CT858 (n=7); CT443 (n=9); groEL (n=7); CT089 (n=9) (Figure 1).
Figure 1. Recognition of recombinant chlamydial proteins by human serum IgG.
Serum IgG antibody specificities to chlamydial antigens were tested by ELISA using sera from 11 C. trachomatis patients and 16 study subjects with no acute C. trachomatis infection (6 C. trachomatis exposed donors, 2 C. pneumoniae and 8 cord-blood donors). Plates were coated with 0.1 mg/wel1 of the respective antigen. Serum IgG titers at an OD of 1.0 were calculated by using a curve fit program by plotting serial serum dilution from 1:100 to 1:16400 with the respective optical density values.
Prioritization of Identified Candidate Antigens Using Human PBMC
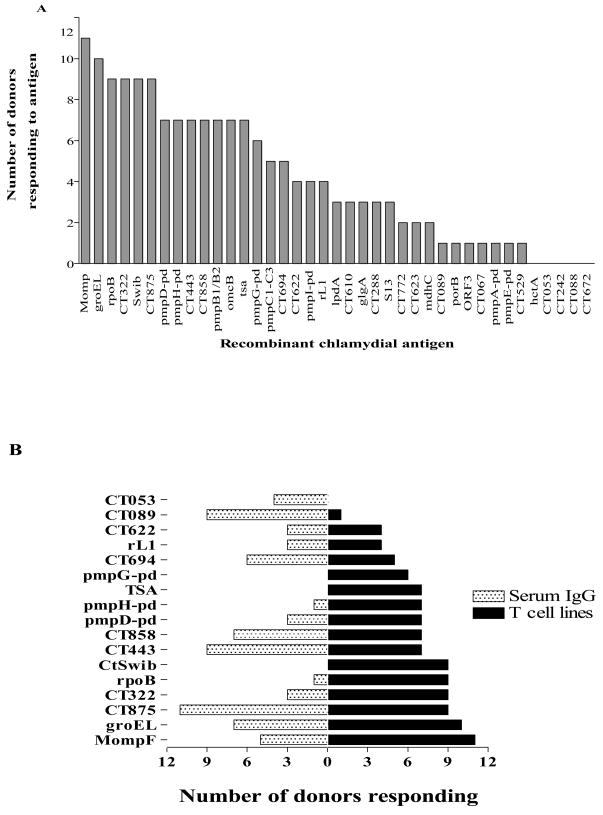
To select antigens on the basis of T cell responses, T cell lines were generated by stimulating in vitro the PBMC of Chlamydia-exposed, asymptomatic, seronegative partners of patients with diagnosed chlamydial infection with inactivated chlamydial EB or human monocyte-derived dendritic cells infected with C. trachomatis serovar E or LGV II. Besides the cohort of seronegative donors, T cell lines were also generated from C. trachomatis patients with clinical manifestations. The established T cell lines were shown to have an HLA-restricted response to C. trachomatis as measured by proliferation (3H-Thymidine incorporation) and IFN-γ production (ELISA), and were used to screen a genomic DNA library generated from serovar E by CD4+ T cell expression cloning according to a protocol described for Leishmania and M. tuberculosis (Alderson, et al., 2000, Skeiky, et al., 2000, Probst, et al., 2001). By screening the library with T cell lines from 3 C. trachomatis patients with clinical manifestations, 13 healthy Chlamydia-infected or Chlamydia-exposed, asymptomatic donors who were presumed to be immune or protected from C. trachomatis, we identified a total of 51 unique C. trachomatis sequences including five hypothetical proteins, pmpG, two enzymes and two ribosomal proteins (Figure 2; Table 1). Six of these genes were independently identified by both the serological and T cell expression cloning approaches.
Figure 2. Recognition of recombinant chlamydial proteins by T cell lines and sera from multiple Chlamydia–infected donors.
(A)The graph summarizes the data generated with Chlamydia-specific T cell lines from 16 donors. (B) This graph summarizes the data generated with sera from 27 study subjects and Chlamydia-specific T cell lines generated from 16 individuals.
Table 1.
Selection of short-listed antigens. The criteria for short-listed antigens included the frequency of human T cell responses to recombinant antigens expressing chlamydial antigens, biological relevance, surface exposure/antibody targets, primary sequence identity with human proteins, C. trachomatis specificity, and protection in the intra-vaginal murine challenge model
| Antigen | Hits T cells Human n=16 |
Hits T cells Murine UVEB immunized |
Hits T cells Murine AS01/UVEB challenged |
Hit Serum IgG Human n=11 |
a Reduction in Shedding day 7 Median log reduction b(# of experiment) |
a Mean Inflammatory score of UGT (# of mice) |
Expression/ Purification |
Chlamydia Specificity |
AA identity Human |
8 AA Stretch with human 100% id |
|---|---|---|---|---|---|---|---|---|---|---|
| Momp | 11 | + | + | 3 | 0.16–3 (10) | 1.1 (35) 5 (3) | Good | Chlamydia | 0 | No |
| CT460 Swib | 9 | − | − | 0 | 0.42 (1) | at 107 infection 3.1 (4) | Good | Chlamydia | Insig. | No |
| CT875 | 9 | − | − | 6 | 0 (4) | at 107 infection | Difficult Good | C.t. | 0 | No |
| pmpD-pd | 7 | + | − | 5 | 0–1.6 (4) | 2.0 (15) | (some breakdown) | C.t. | 0 | No |
| CT858 | 7 | − | − | 4 | 0.75–1 (3) | 1.3 (8) | Difficult | Chlamydia | Insig. | No |
| pmpG-pd | 6 | + | w | 0 | 0–0.25 (3) | 2.1 (4) | Good Good | C.t. | 0 | No |
| CT622 | 4 | + | + | 4 | 0–0.5 (3) | 2.0 (16) | (some breakdown Good) | Chlamydia | Insig | No |
| CT089 | 1 | − | − | n.t. | 0.33–0.6 (2) | 2.6 (4) | (some breakdown ) | Chlamydia | Insig. | No |
| CT610 | 3 | − | − | n.t. | 1 (1) | n.d. | Very good | Chlamydia | Insig. | No |
| pmpH-pd | 7 | + | − | 0 | 0–1.42 (3) | 1.5 (12) | Good N (good), C | C.t. | 0 | No |
| CT315 rpoB | 9 | − | + | 2 | 1–1.33 (3) | 1.2 (16) | (fair) | Common | 37.3 | No |
| CT322 | 9 | − | + | 1 | 0.4–1.75 (3) | 2.3 (16) | Very good | Common | 55% | Yes |
w: Weak response, only positive by proliferation.
Antigen screening in vaginal challenge model.
Number of repeated experiments. Table shows the lowest and highest reduction in bacterial shedding as compared to the AS01B sham-immunized control. UGT: Upper genital tract. Chlamydia denotes both C. trachomatis and Chlamydia pneumoniae
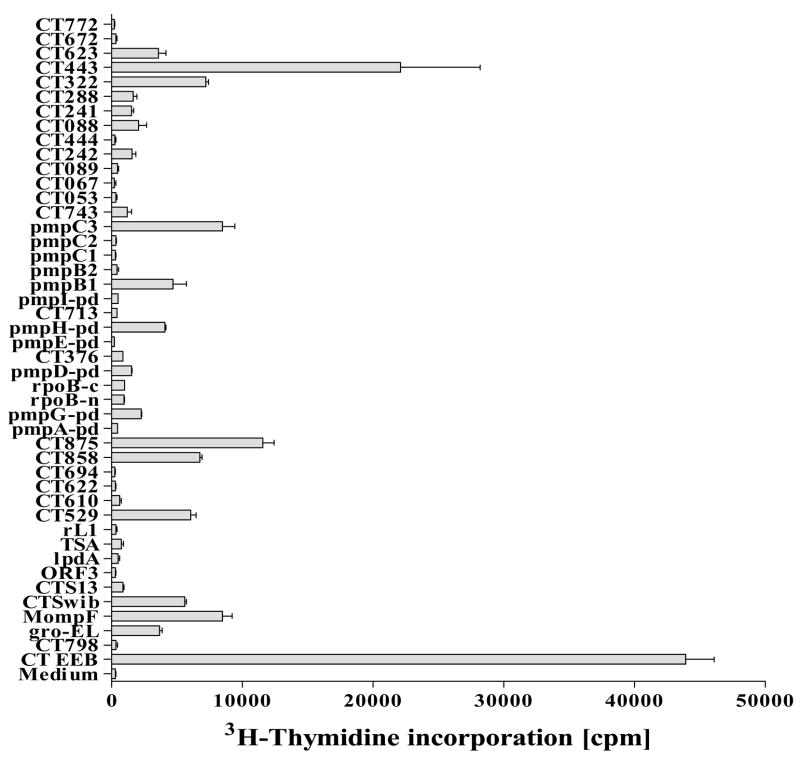
Candidate antigens from the T cell expression cloning approaches were ranked based on their abilities to induce human PBMC (from Chlamydia-infected healthy donors) to proliferate in vitro (Figure 3), by C. trachomatis specificity, biological relevance, surface exposure/antibody target, absence of identity between the Chlamydia antigen primary sequence and any known human proteins.
Figure 3. Proliferation by PBMC of Chlamydia-exposed donors to candidate Chlamydia antigens.
Proliferation of donor PBMC (n= 16) from C. trachomatis patients, healthy Chlamydia-infected donors or Chlamydia-exposed, asymptomatic, seronegative partners of patients with diagnosed chlamydial infection to Chlamydia.. PBMC (2 × 105 cells/well) were cultured in the presence of antigen (10 μg/ml) for 5 days. After 5 days, the plates were pulsed with tritiated thymidine. After culture for a further 18 h, cells were harvested, and tritium uptake was determined by using a gas scintillation counter. Proliferation results are reported as mean counts per minute of cultures from 16 donors stimulated with antigen or medium alone.
The following genes (listed in functional categories) are a subset of those that were identified by CD4+ T cell expression cloning: Enzymes (mdhC, had/fab1, lpdA, nrdA, glgA), Hypothetical proteins (CT610, CT622, CT875, CT288, CT694); Outer membrane proteins (pmpB, pmpC, pmpD, pmpG, pmpH, omcB, Momp); DNA/RNA-binding proteins (Swib, rpoB, elongation factors); Ribosomal proteins (L1, S1, S2, S13); Proteases (CT858, 2 CLP-protease subunits); Heat-shock proteins (groEL, dnaK); Detoxification (TSA); Type III secretion (CT674); Plasmid encoded protein (ORF3) (Table 1).
The 41 recombinant antigens that met all four criteria through this prioritization process were encoded by 38 C. trachomatis genes out of a total of ~75 genes initially identified, were expressed and purified.
Immunization with inactivated chlamydial elementary bodies (EB) protects against genital chlamydial infection in C57BL/6 mice
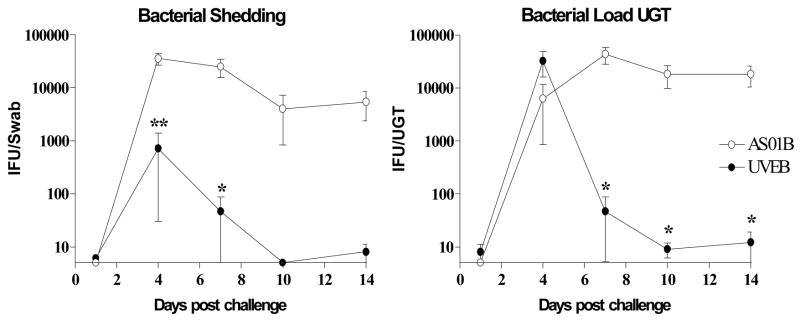
It has been shown that mice immunized intravenously with DC pulsed with inactivated chlamydial EB are protected against chlamydial infection of the female genital tract (Su, et al., 1998). In the present study, we determined whether the DC immunization can be substituted by formulating inactivated EB in AS01B Adjuvant System, a potent Th1 inducing adjuvant formulation (Pichyangkul, et al., 2004, Skeiky, et al., 2004, Mettens, et al., 2008). Female mice were immunized twice three weeks apart with either serovar E UV-irradiated elementary bodies [UVEB] formulated in AS01B Adjuvant System or with AS01B Adjuvant System alone. Four weeks after the last immunization mice were challenged with 5×105 IFU of C. trachomatis serovar K deposited onto the ectocervix of mice. Groups of 5 mice were swabbed to determine chlamydia shedding in the lower genital tract [LGT] and then sacrificed for upper genital tact [UGT] resection to quantify the bacterial load in the uterine horns and ovaries on days 1, 4, 7, 10 and 14, respectively. Chlamydia was quantified by determining IFU in swabs and UGT lysates. Data are shown in Figure 4 as mean vaginal shedding and bacterial load in the UGT of C57BL/6 mice.
Figure 4. Kinetics of genital C. trachomatis serovar K infection in UVEB-immunized or AS01B Adjuvant System-sham-immunized C57BL/6 mice.
Groups of 5 C57BL/6 mice were immunized twice with either UVEB from C. trachomatis serovar E formulated in AS01B Adjuvant System [closed circles] or were AS01B sham-immunized [open circles]. Four weeks after the final immunization, progesterone-treated mice were challenged with an intravaginal dose of 5×105 IFU of C. trachomatis serovar K. Bacterial shedding was quantified by taking swabs on the indicated days and determining the IFU using McCoy cells. Each group was only swabbed once on one of the indicated days post challenge. Bacterial load in the upper genital tract [UGT] was quantified by preparing UGT lysates from sacrificed mice on the indicated days and determining the IFU count using McCoy cells. Data represent means ± SE of IFU from 5 mice. **: P < 0.01, *: P < 0.05 compared with results from AS01B Adjuvant System control mice by unpaired t test. The figure is representative of three separate experiments.
No live Chlamydia were detected on day 1 post infection in either the vaginal swabs or the UGT lysates of serovar E UVEB-immunized or sham-immunized mice. On day 4 post infection, chlamydial shedding increased to 35000 IFU per swab in the AS01B-sham-immunized control group indicating that serovar K had productively infected the lower genital tract of C57BL/6 mice. Bacterial shedding remained at this level on day 7 before declining over the next two time points. Similar studies were performed in C3H mice and the less resistant BALB/c strain of mice, and demonstrated that while the kinetics of infection were similar in all 3 strains, BALB/c mice tended to have the highest overall bacterial loads at days 4 and 7 after infection (data not shown) but shedding decreased by day 14 in this strain of mice as well.
As shown in Figure 4, immunization with serovar E UVEB formulated in AS01B Adjuvant System induced a significant reduction in bacterial shedding on days 4 and 7 post infection by 1.5 and 2.5 logs, respectively, when compared to the AS01B sham-immunized control group. At the later time points, bacterial shedding was only detected in 1/10 vaccinated mice compared with 7/10 mice in the AS01B Adjuvant System control group. Similar to the LGT, chlamydia infection of the UGT was measurable on day 4 but not day 1 post infection indicating that the C. trachomatis serovar K challenge caused a productive infection ascending to the upper genital tract. In contrast to the chlamydial infection of the LGT, UGTs of both UVEB-immunized mice as well as the AS01B–sham immunized controls had comparable peak chlamydial loads at day 4. UVEB immunization, however, significantly reduced the mean chlamydial infection of the UGT by at least 3 logs [P < 0.05] at the later time points. Thus, the UVEB immunization induced protection against genital chlamydia infection by inhibiting chlamydia shedding in the LGT and hastening the clearance of chlamydial infection of the UGT. Importantly, these studies also provide evidence that immunization with inactivated EB from C. trachomatis serovar E induced cross protection against vaginal challenge with serovar K in C57BL/6 mice.
Protection Studies in an intravaginal mouse model for antigen evaluation
Various animal models of Chlamydia genital infections have been established, including mouse, rat, rabbit, guinea pig and monkey models. Considering the need for large numbers of animals to evaluate the newly identified antigens and the practicality of using mice from the standpoints of reagent availability for analyzing the host immune responses, we chose to develop further our mouse vagina C. trachomatis challenge model. This was done by comparing the infectivity of various human serovars in different strains of mice, adjusting progesterone treatment, and adapting inoculation, swabbing, culture and histopathological techniques. In the murine system intravaginal infections with human C. trachomatis strains does not induce salpingitis, so we developed based on the work of Tuffrey et al. (Tuffrey, et al., 1986, Tuffrey, et al., 1990) the low volume intrauterine challenge model to follow an ascending chlamydial infection from the lower uterine horns to the salpinges and ovaries. Under these conditions, C. trachomatis serovar K induced salpingitis and oophoritis on day 5 post inoculation with a peak in inflammation on day 14, confirming an ascending infection from the lower uterine horns to the ovaries. These experiments led to the establishment of a vaginal challenge model using C. trachomatis serovar K in progesterone treated BALB/c and C57BL/6 mice.
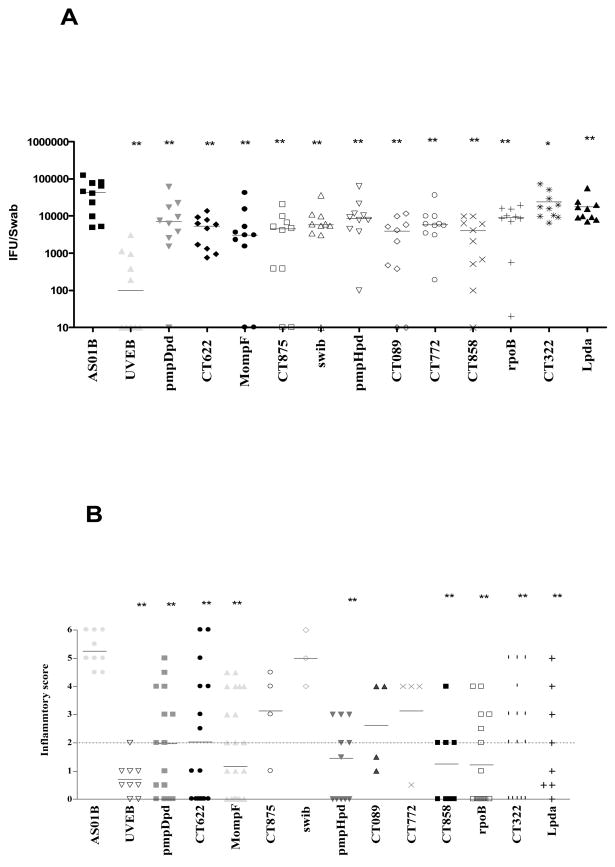
To date, 38 individual serovar E antigens have been examined in the intravaginal challenge model developed testing each antigen twice in replicate experiments. Upon in vivo testing, several of the antigens identified in the discovery program have conferred partial protection in this intravaginal challenge model (Figure 5) in which the data represent median ± SE of IFU from 5 mice. **: P < 0.01, *: P < 0.05 compared with results from AS01B Adjuvant System control mice.
Figure 5. Antigen screening in the C. trachomatis vaginal challenge model.
Groups of BALB/c mice were immunized with the various antigens (10 μg/per antigen) in AS01B Adjuvant System. UVEB from serovar E formulated with AS01B Adjuvant System served as a positive control of protection. Progesterone-treated mice were challenged with an intra-vaginal dose of 1×106 IFU of serovar K. Bacterial shedding was quantified by taking Swabs on day 7 post infection and determining the IFU using McCoy cells (A), and by histopathological examination of the upper genital tract for uterine inflammation at day 14 post vaginal challenge (B). **: P < 0.01, *: P < 0.05 compared with results from AS01B Adjuvant System control mice by unpaired t test. The figure is representative of three separate experiments.
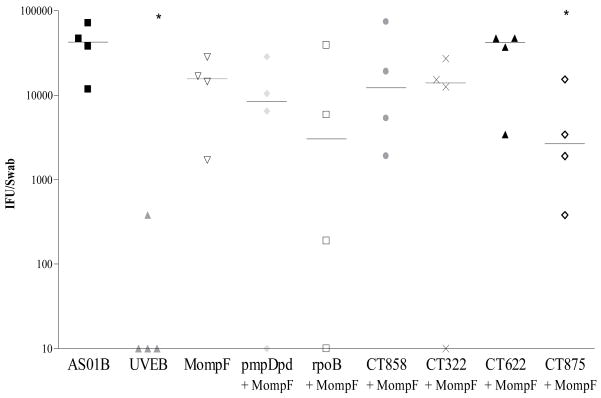
When antigens were mixed together and delivered with AS01B Adjuvant System some of the combinations, such as Momp (an antibody target) and CT875 (an excellent human T-cell antigen), provided better protection against challenge than single antigen administration as measured 7 days after challenge (Figure 6).
Figure 6. Bacterial shedding after vaginal challenge with serovar K in mice immunized with Momp-based antigen combinations.
Groups of BALB/c mice were immunized with the various antigens (10 μg/per antigen) in AS01B Adjuvant System. UVEB from serovar E formulated with AS01B Adjuvant System served as a positive control of protection. Progesterone-treated mice were challenged with an intra-vaginal dose of 1×106 IFU of serovar K. Bacterial shedding was quantified by taking Swabs on day 7 post-infection and determining the IFU using McCoy cells. Horizontal bars represent median IFU from the respective groups of mice. **: P < 0.01, *: P < 0.05 compared with results from AS01B Adjuvant System control mice by unpaired t test. The figure is representative of two separate experiments.
Discussion
In the course of vaccine development for C.trachomatis, one criterion that should be satisfied by candidate chlamydial vaccine antigens is recognition by the immune system during the course of infection in the majority of individuals of the target population evaluated. We describe studies to identify and prioritize newly discovered human C. trachomatis T cell and serological antigens as vaccine candidates for further evaluation, and to characterize priority antigens in animal models of immunogenicity and protection using innovative adjuvant/delivery systems appropriate for human use. To evaluate candidate chlamydial antigens, we have used a panel of donors, with no history of genital chlamydial disease. We have included an ethnically diverse group, and have used T-cell proliferation from in vitro-stimulated PBMC to measure recognition of the relevant antigen during previous exposure to C. trachomatis. This preliminary screening of candidate antigens has identified 51 unique antigens, of which 8 were short-listed as being recognized by the majority of C. trachomatis-infected individuals without evident disease, with strong T-cell proliferation and serological responses.
Our preliminary studies demonstrate that prophylactic vaccines can be developed and tested in animal models of Chlamydia infection. We demonstrated that immunization with heterologous UVEB from serovar E formulated in AS01B Adjuvant System, a MPL and QS21 containing Th1 inducing adjuvant, induced protection against genital C. trachomatis serovar K infection in murine intravaginal and intrauterine challenge models. UVEB immunization not only significantly inhibited chlamydial shedding in the lower genital tract but also controlled the bacterial load in the upper genital tract after intra-vaginal challenge with serovar K. Importantly, the protection induced by the UVEB/AS01B Adjuvant System vaccine is heterologous, since immunization with EB from C. trachomatis serovar E protected against serovar K challenge. Amplified fragment length polymorphism analysis showed considerable heterogeneity in the human C. trachomatis strains with the highest phylogenitical diversity between serovar K and serovar E and a similarity coefficient of 88% (Meijer, et al., 1999) suggesting that immunization with serovar E UVEB may also protect against genital infections with other human serovars. To our knowledge, this is first time that a non-replicating EB has been shown to elicit a high level of protection against genital tract infection by human C. trachomatis serovars.
Nevertheless, it is not feasible to develop a human vaccine based on chlamydial EB due to the perceived high cost of manufacturing as well as to the safety concerns raised in the initial human trials using a chlamydia EB vaccine (Grayston & Wang, 1978). Our data, however, indicate that antigens contained in the EB, when combined with AS01B, elicited a protective immune response in naïve animals suggesting that it may be possible to develop a recombinant subunit vaccine for genital C. trachomatis infections.
Our priority candidate vaccine antigens when formulated appropriately with the AS01B Adjuvant Systems, have also demonstrated the ability to elicit a protective response to reduce bacterial shedding and prevent ascension of the infection to the UGT.
Attempts to produce a vaccine against genital C. trachomatis infections began some decades ago. Efforts to vaccinate monkeys, mice, sheep and guinea pigs with C. trachomatis MOMP, which has been thought of as the best candidate for an acellular vaccine, have resulted in limited success (Taylor, et al., 1988, Tan, et al., 1990, Tuffrey, et al., 1992, Batteiger, et al., 1993, Su, et al., 1995, Pal, et al., 2002).
The specific immunological mechanisms involved in protection against a chlamydial genital infection are not well understood. It is thought that humoral and cellular immune responses may be critical at different stages of the infection (Ward, 1992). It is hypothesized that antibodies may inhibit the entry of EB by blocking cellular receptors at the site of entry, may opsonize EB or could kill EB by an antibody-dependent cellular cytotoxic attack. For these reasons, we applied serological expression cloning using a pool of C. trachomatis patient sera to identify new chlamydial antigens. This effort resulted in the identification of 26 genes, 6 of which were independently identified by our T cell expression cloning approaches.
Various reports also suggest that CD4+ T cells and various cytokines, including IL-2, TNF-α and IFN-γ, also appear to play important roles in protective immunity. For this reason, we utilized various T cell cloning methodologies and the host immune response to identify relevant antigens from gene expression libraries. Using these approaches, followed by evaluation of purified recombinant proteins based on human T-cell stimulation, we identified a total of 51 unique C. trachomatis sequences including five hypothetical proteins, pmpG, two enzymes and two ribosomal proteins.
It may be expected that a candidate vaccine for C. trachomatis will need to contain multiple T-cell epitopes to afford protection in an outbred human population. To be effective, a combination vaccine against Chlamydia will most probably also need to elicit effective Th1 cell-mediated immune responses as well as accessory or neutralizing antibodies (Rank, et al., 1990, Cotter, et al., 1997, Perry, et al., 1997, Su, et al., 1997). The finding that 8 of the 51 identified unique antigens had the ability to induce human PBMC (from Chlamydia-infected healthy donors) to proliferate in vitro, were C. trachomatis specific, had biological relevance, were surface exposed, had an absence of identity between the Chlamydia antigen primary sequence and any known human proteins. The best candidate vaccine antigens would be those that when combined are able to provide protection in at least one animal model of genital Chlamydia, will elicit protection against more than one serovar, and are known to be recognized by the immune systems of humans infected with C. trachomatis.
References
- 1.Alderson MR, Bement T, Day CH, et al. Expression Cloning of an Immunodominant Family of Mycobacterium tuberculosis Antigens Using Human CD4(+) T Cells. Journal of Experimental Medicine. 2000;191:551–560. doi: 10.1084/jem.191.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barr EL, Ouburg S, Igietseme JU, et al. Host inflammatory response and development of complications of Chlamydia trachomatis genital infection in CCR5-deficient mice and subfertile women with the CCR5delta32 gene deletion. J Microbiol Immunol Infect. 2005;38:244–254. [PubMed] [Google Scholar]
- 3.Batteiger BE, Rank RG, Bavoil PM, Soderberg LS. Partial protection against genital reinfection by immunization of guinea-pigs with isolated outer-membrane proteins of the chlamydial agent of guinea-pig inclusion conjunctivitis. J Gen Microbiol. 1993;139:2965–2972. doi: 10.1099/00221287-139-12-2965. [DOI] [PubMed] [Google Scholar]
- 4.Behets FM, Miller WC, Cohen MS. Syndromic treatment of gonococcal and chlamydial infections in women seeking primary care for the genital discharge syndrome: decision-making. Bulletin World Health Organization. 2001;79:6. [PMC free article] [PubMed] [Google Scholar]
- 5.Berthet FX, Rasmussen PB, Rosenkrands I, Andersen P, Gicquel B. A Mycobacterium tuberculosis operon encoding ESAT-6 and a novel low-molecular-mass culture filtrate protein (CFP-10) Microbiology. 1998;144 ( Pt 11):3195–3203. doi: 10.1099/00221287-144-11-3195. [DOI] [PubMed] [Google Scholar]
- 6.Brunham RC, Rey-Ladino J. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat Rev Immunol. 2005;5:149–161. doi: 10.1038/nri1551. [DOI] [PubMed] [Google Scholar]
- 7.Cain TK, Rank RG. Local Th1-like responses are induced by intravaginal infection of mice with the mouse pneumonitis biovar of Chlamydia trachomatis. Infect Immun. 1995;63:1784–1789. doi: 10.1128/iai.63.5.1784-1789.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cotter TW, Ramsey KH, Miranpuri GS, Poulsen CE, Byrne GI. Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon gene knockout mice. Infect Immun. 1997;65:2145–2152. doi: 10.1128/iai.65.6.2145-2152.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de la Maza LM, Peterson EM. Vaccines for Chlamydia trachomatis infections. Curr Opin Investig Drugs. 2002;3:980–986. [PubMed] [Google Scholar]
- 10.Gervassi A, Alderson MR, Suchland R, Maisonneuve JF, Grabstein KH, Probst P. Differential regulation of inflammatory cytokine secretion by human dendritic cells upon Chlamydia trachomatis infection. Infect Immun. 2004;72:7231–7239. doi: 10.1128/IAI.72.12.7231-7239.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grayston JT, Wang SP. The potential for vaccine against infection of the genital tract with Chlamydia trachomatis. Sex Transm Dis. 1978;5:73–77. doi: 10.1097/00007435-197804000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Igietseme JU, Murdin A. Induction of protective immunity against Chlamydia trachomatis genital infection by a vaccine based on major outer membrane protein-lipophilic immune response-stimulating complexes. Infect Immun. 2000;68:6798–6806. doi: 10.1128/iai.68.12.6798-6806.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Igietseme JU, Ward ME. Chlamydia update. Expert Rev Vaccines. 2004;3:639–642. doi: 10.1586/14760584.3.6.639. [DOI] [PubMed] [Google Scholar]
- 14.Igietseme JU, Black CM, Caldwell HD. Chlamydia vaccines: strategies and status. BioDrugs. 2002;16:19–35. doi: 10.2165/00063030-200216010-00003. [DOI] [PubMed] [Google Scholar]
- 15.Johansson M, Schon K, Ward M, Lycke N. Studies in knockout mice reveal that anti-chlamydial protection requires TH1 cells producing IFN-gamma: is this true for humans? . Scand J Immunol. 1997;46:546–552. doi: 10.1046/j.1365-3083.1997.d01-167.x. [DOI] [PubMed] [Google Scholar]
- 16.Mardh PA. Tubal factor infertility, with special regard to chlamydial salpingitis. Curr Opin Infect Dis. 2004;17:49–52. doi: 10.1097/00001432-200402000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Meijer A, Morre SA, van den Brule AJ, Savelkoul PH, Ossewaarde JM. Genomic relatedness of Chlamydia isolates determined by amplified fragment length polymorphism analysis. J Bacteriol. 1999;181:4469–4475. doi: 10.1128/jb.181.15.4469-4475.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mettens P, Dubois PM, Demoitie MA, et al. Improved T cell responses to Plasmodium falciparum circumsporozoite protein in mice and monkeys induced by a novel formulation of RTS, S vaccine antigen. Vaccine. 2008;26:1072–1082. doi: 10.1016/j.vaccine.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 19.Morrison RP, Caldwell HD. Immunity to murine chlamydial genital infection. Infect Immun. 2002;70:2741–2751. doi: 10.1128/IAI.70.6.2741-2751.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morrison RP, Feilzer K, Tumas DB. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect Immun. 1995;63:4661–4668. doi: 10.1128/iai.63.12.4661-4668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrison SG, Morrison RP. Resolution of secondary Chlamydia trachomatis genital tract infection in immune mice with depletion of both CD4+ and CD8+ T cells. Infect Immun. 2001;69:2643–2649. doi: 10.1128/IAI.69.4.2643-2649.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrison SG, Morrison RP. The protective effect of antibody in immunity to murine chlamydial genital tract reinfection is independent of immunoglobulin A. Infect Immun. 2005;73:6183–6186. doi: 10.1128/IAI.73.9.6183-6186.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paavonen J, Eggert-Kruse W. Chlamydia trachomatis: impact on human reproduction. Hum Reprod Update. 1999;5:433–447. doi: 10.1093/humupd/5.5.433. [DOI] [PubMed] [Google Scholar]
- 24.Pal S, Davis HL, Peterson EM, de la Maza LM. Immunization with the Chlamydia trachomatis mouse pneumonitis major outer membrane protein by use of CpG oligodeoxynucleotides as an adjuvant induces a protective immune response against an intranasal chlamydial challenge. Infect Immun. 2002;70:4812–4817. doi: 10.1128/IAI.70.9.4812-4817.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pear WS, Nolan GP, Scott ML, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci U S A. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perry LL, Feilzer K, Caldwell HD. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and -independent pathways. Journal of Immunology. 1997;158:3344–3352. [PubMed] [Google Scholar]
- 27.Pichyangkul S, Gettayacamin M, Miller RS, et al. Pre-clinical evaluation of the malaria vaccine candidate P. falciparum MSP1(42) formulated with novel adjuvants or with alum. Vaccine. 2004;22:3831–3840. doi: 10.1016/j.vaccine.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 28.Probst P, Stromberg E, Ghalib HW, Mozel M, Badaro R, Reed SG, Webb JR. Identification and characterization of T cell-stimulating antigens from Leishmania by CD4 T cell expression cloning. J Immunol. 2001;166:498–505. doi: 10.4049/jimmunol.166.1.498. [DOI] [PubMed] [Google Scholar]
- 29.Rank RG, Batteiger BE, Soderberg LS. Immunization against chlamydial genital infection in guinea pigs with UV-inactivated and viable chlamydiae administered by different routes. Infect Immun. 1990;58:2599–2605. doi: 10.1128/iai.58.8.2599-2605.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ridgway GL. Treatment of chlamydial genital infection. J Antimicrob Chemother. 1997;40:311–314. doi: 10.1093/jac/40.3.311. [DOI] [PubMed] [Google Scholar]
- 31.Sanderson S, Campbell DJ, Shastri N. Identification of a CD4+ T cell-stimulating antigen of pathogenic bacteria by expression cloning. J Exp Med. 1995;182:1751–1757. doi: 10.1084/jem.182.6.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skeiky YA, Ovendale PJ, Jen S, et al. T cell expression cloning of a mycobacterium tuberculosis gene encoding a protective antigen associated with the early control of infection [In Process Citation] Journal of Immunology. 2000;165:7140–7149. doi: 10.4049/jimmunol.165.12.7140. [DOI] [PubMed] [Google Scholar]
- 33.Skeiky YA, Alderson MR, Ovendale PJ, et al. Differential immune responses and protective efficacy induced by components of a tuberculosis polyprotein vaccine, Mtb72F, delivered as naked DNA or recombinant protein. Journal of Immunology. 2004;172:7618–7628. doi: 10.4049/jimmunol.172.12.7618. [DOI] [PubMed] [Google Scholar]
- 34.Starnbach MN, Bevan MJ, Lampe MF. Protective cytotoxic T lymphocytes are induced during murine infection with Chlamydia trachomatis. J Immunol. 1994;153:5183–5189. [PubMed] [Google Scholar]
- 35.Stephens RS. The cellular paradigm of chlamydial pathogenesis. Trends Microbiol. 2003;11:44–51. doi: 10.1016/s0966-842x(02)00011-2. [DOI] [PubMed] [Google Scholar]
- 36.Su H, Parnell M, Caldwell HD. Protective efficacy of a parenterally administered MOMP-derived synthetic oligopeptide vaccine in a murine model of Chlamydia trachomatis genital tract infection: serum neutralizing IgG antibodies do not protect against chlamydial genital tract infection. Vaccine. 1995;13:1023–1032. doi: 10.1016/0264-410x(95)00017-u. [DOI] [PubMed] [Google Scholar]
- 37.Su H, Feilzer K, Caldwell HD, Morrison RP. Chlamydia trachomatis genital tract infection of antibody-deficient gene knockout mice. Infect Immun. 1997;65:1993–1999. doi: 10.1128/iai.65.6.1993-1999.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Su H, Messer R, Whitmire W, Fischer E, Portis JC, Caldwell HD. Vaccination against chlamydial genital tract infection after immunization with dendritic cells pulsed ex vivo with nonviable Chlamydiae. J Exp Med. 1998;188:809–818. doi: 10.1084/jem.188.5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan TW, Herring AJ, Anderson IE, Jones GE. Protection of sheep against Chlamydia psittaci infection with a subcellular vaccine containing the major outer membrane protein. Infect Immun. 1990;58:3101–3108. doi: 10.1128/iai.58.9.3101-3108.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor HR, Whittum-Hudson J, Schachter J, Caldwell HD, Prendergast RA. Oral immunization with chlamydial major outer membrane protein (MOMP) Invest Ophthalmol Vis Sci. 1988;29:1847–1853. [PubMed] [Google Scholar]
- 41.Tuffrey M, Alexander F, Taylor-Robinson D. Severity of salpingitis in mice after primary and repeated inoculation with a human strain of Chlamydia trachomatis. J Exp Pathol (Oxford) 1990;71:403–410. [PMC free article] [PubMed] [Google Scholar]
- 42.Tuffrey M, Falder P, Gale J, Taylor-Robinson D. Salpingitis in mice induced by human strains of Chlamydia trachomatis. Br J Exp Pathol. 1986;67:605–616. [PMC free article] [PubMed] [Google Scholar]
- 43.Tuffrey M, Alexander F, Conlan W, Woods C, Ward M. Heterotypic protection of mice against chlamydial salpingitis and colonization of the lower genital tract with a human serovar F isolate of Chlamydia trachomatis by prior immunization with recombinant serovar L1 major outer-membrane protein. J Gen Microbiol. 1992;138(Pt 8):1707–1715. doi: 10.1099/00221287-138-8-1707. [DOI] [PubMed] [Google Scholar]
- 44.van Valkengoed IG, Morre SA, van den Brule AJ, Meijer CJ, Bouter LM, Boeke AJ. Overestimation of complication rates in evaluations of Chlamydia trachomatis screening programmes--implications for cost-effectiveness analyses. Int J Epidemiol. 2004;33:416–425. doi: 10.1093/ije/dyh029. [DOI] [PubMed] [Google Scholar]
- 45.Ward ME. Chlamydial vaccines--future trends. J Infect. 1992;25(Suppl 1 11-26):11–26. doi: 10.1016/0163-4453(92)91882-c. [DOI] [PubMed] [Google Scholar]
- 46.WHO . Sexually transmitted infections increasing -- 250 million new infections annually. WHO Feature. 1990;152:6. [PubMed] [Google Scholar]
- 47.WHO. Global prevalence and incidence of selected curable sexually transmitted diseases: overview and estimates. Geneva, Switzerland: 2001. pp. 1–34. World Health Organization DHA. [Google Scholar]