INTRODUCTION
Of significant interest is the development of synthetic methodologies to prepare complex macromolecular structures that contain functionalities, allowing for stimuli-responsive characteristics and further chemical modifications.1–6 The cylindrical molecular brush, which is composed of many side chain polymers distributed densely along a backbone, has attracted much attention because its chemical composition, size and morphology can be controlled precisely by choosing appropriate monomers and tuning the lengths of the backbone and side chains.7–9 To prepare such structures, three synthetic strategies are often employed: “grafting onto”8,9 (grafting side chains onto a pre-established multifunctional backbone by coupling reactions), “grafting from”10–13 (growth of side chains from a multifunctionalized initiating backbone) and “grafting through”14–18 (polymerization of previously synthesized side chains through their terminal groups). Previously, our laboratory has demonstrated the compatibility of the three primary living radical polymerizations (nitroxide mediated radical polymerization (NMRP), atom transfer radical polymerization (ATRP) and reversible addition-fragmentation chain transfer (RAFT) polymerization) together with ring-opening metathesis polymerization (ROMP) in the “grafting from” method.12,19,20 Our interest has focused recently on the “grafting through” method, because it provides exceptional control over the grafting density, the length of the backbone, and the length of the side chains, each independently.
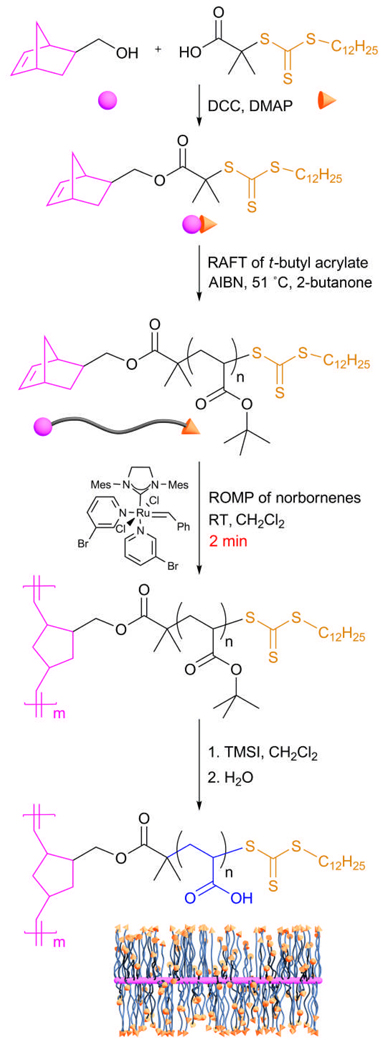
Although the “grafting through” approach offers versatility in the construction of complex macromolecular systems, it is likely to encounter steric hindrance during the polymerization of high molecular weight or sterically-bulky macromonomers.21,22 To overcome this issue, ROMP has often been used, which is driven by the release of enthalpy from cyclic monomer structures and affords a polymer backbone with a relatively loose grafting density. For example, the syntheses of molecular brushes have been reported using ROMP from norbornene-terminated poly(ethylene oxide),16 polystyrene,15 polyphosphazene,14 poly(ε-caprolactone),18 and polylactide,17 macromonomers synthesized mainly from anionic polymerization or ring opening polymerization. NMRP, ATRP and RAFT are controlled radical polymerization techniques that allow for the preparation of well-defined polymers from vinylic monomers, which can be converted into ROMP-based macromonomers. Grubbs recently reported an elegant combination of ATRP, click chemistry and ROMP to produce narrowly-dispersed brush polymers, whereby the termini of ATRP-generated poly(tert-butyl acrylate), poly(methyl acrylate) and polystyrene were converted to norbornenyl groups via click chemistry, and those norbornenyl groups were then utilized for “grafting-through” growth of the final brush polymers.23 In this study, we also take advantage of the reactivity and control of ROMP in “grafting-through” polymerizations, but we obtain the norbornenyl-functionalized macromonomers directly from RAFT24 of t-butyl acrylate using a dual-functionality small molecule that serves as a RAFT agent and carries the norbornene unit. The selectivity of RAFT for the polymerization of t-butyl acrylate in the presence of the norbornenyl group allows this chemistry to be used to prepare α-norbornenyl-functionalized poly(t-butyl acrylate). Subsequent ROMP of the resulting macromonomer then achieves brush synthesis. A kinetic study of this polymerization showed that it was extremely quick and air-insensitive, using the the modified 2nd generation Grubbs’ catalyst. Furthermore, the poly(t-butyl acrylate) side chains were converted to poly(acrylic acid)s, affording water-soluble functional nanoparticles, which are pH-responsive and are readied for future chemical modifications.
EXPERIMENTAL
Materials
Unless otherwise noted, all solvents and reagents were purchased from Aldrich Chemical Co. (St. Louis, MO) and used as received. tert-Butyl acrylate (tBA, 98%) and dichloromethane (CH2Cl2, 99+%) were distilled over CaH2 before use. The norbornenyl-functionalized alcohol,20 S-1-dodecyl-S’-(R,R’-dimethyl-R”-acetic acid)trithiocarbonate25 and the modified 2nd generation Grubbs’ catalyst26 were prepared following literature methods.
Synthesis of ROMP-active RAFT chain transfer agent, with norbornenyl (NB) and trithiocarbonate (TTC) units (NB-TTC)
Into a 500 mL RB flask with a stir bar were placed exo-5-norbornene-2-methanol (2.52 g, 20.7 mmol), S-1-dodecyl-S’-(R,R’-dimethyl-R”-acetic acid)trithiocarbonate (8.93 g, 24.5 mmol), N,N’-dicyclohexylcarbodiimide (5.31 g, 25.7 mmol), and 4-(dimethylamino)pyridine (0.98 g, 8.02 mmol). Dry CH2Cl2 (220 mL) was then injected into the flask to dissolve all the reagents. The reaction was allowed to stir at room temperature for 60 h, at which time, TLC showed complete conversion of the alcohol. After filtration through fluted filter paper, concentration in vacuo and flash chromatography eluting with 10% ethyl acetate-hexane the product NB-TTC was afforded as an orange-colored oil. Yield: 89%. IR: 3059, 2925, 2853, 1735, 1569, 1465, 1378, 1361, 1324, 1253, 1154, 1126, 1066, 998, 971, 904, 816, 750, 706 cm−1; 1H NMR (300 MHz, CDCl3, ppm) δ 6.05 (br s, 2H, norbornenyl alkenyl protons), 4.17 (dd, J = 7 and 11 Hz, 1H, CH2OC(O)), 3.92 (dd, J = 10 and 10 Hz, 1H, CH2OC(O)), 3.25 (t, J = 7 Hz, 2H, CH2SC(S)S), 2.80 (s, 1H, allylic proton of norbornenyl group), 2.65 (s, 1H, allylic proton of norbornenyl group), 1.50–1.80 (br m, 10H, C(O)C(CH3)2S, one bridge proton of norbornenyl group, CHCH2OC(O) and SCH2CH2C10H23) 1.00–1.50 (br m, 21H, aliphatic protons), 0.85 (ill-defined t, 3H, C(S)SCH2C10H20CH3); 13C NMR (125 MHz, CDCl3, ppm) 14.37, 22.93, 25.63, 25.68, 28.16, 29.15, 29.34, 29.58, 29.66, 29.68, 29.80, 29.86, 32.15, 37.07, 37.93, 41.85, 43.91, 45.17, 56.22, 70.30, 136.55, 137.08, 173.24, 221.65.
RAFT-based synthesis of α-norbornenyl poly(t-butyl acrylate) macromonomer
To a 50 mL Schlenk flask with a stir bar, NB-TTC (0.1031 g, 0.2203 mmol) and t-butyl acrylate (10.0 mL, 68.27 mmol) were added, followed by the addition of AIBN (as a freshly-prepared stock solution in 2-butanone, 0.20 mg/mL, 10 mL, 5 mol%). After 4 cycles of freeze-pump-thaw, the polymerization was conducted in a 51 °C oil bath. At every hour, an aliquot was withdrawn and analyzed by 1H-NMR spectroscopy to determine the extent of monomer conversion. At 3 h, the reaction was quenched by freezing the reaction mixture in a liquid nitrogen bath. The reaction mixture was then precipitated in 20% H2O-methanol 3 times and the polymer (NB-PtBA macromonomer) was collected. Yield: 1.23 g, 45%. MnNMR = 17.3 kDa, MnGPC = 16.9 kDa, Mw/Mn = 1.16; Tg = 43 °C; IR: 2977, 2932, 1727, 1479, 1448, 1392, 1367, 1338, 1257, 1148, 1035, 908, 845, 751, 705, 637, 471, 428 cm−1; 1H NMR (300 MHz, CDCl3, ppm) δ 6.05–6.10 (norbornenyl alkene), 4.60–4.72 (CHSC(S)S), 3.80–4.20 (CH2OC(O)), 3.30–3.40 (CH2SC(S)S), 2.60–2.90 (allylic protons of norbornenyl groups), 2.05–2.40 (polymer backbone protons) 1.00–1.95 (t-butyl protons and C(S)SCH2C10H20CH3), 0.80–0.90 (C(S)SCH2C10H20CH3); 13C NMR (75 MHz, CDCl3, ppm) 28.0–28.2, 35.7–37.3, 41.5–43.5, 80.2, 174.3.
ROMP-based synthesis of molecular brushes bearing poly(t-butyl acrylate) side chains
To a solution of Grubbs’ catalyst in CH2Cl2 (8.4 × 10−3 mg/mL, 100 µL, 1 equiv.) under argon in a scintillation vial capped with a septum was added the NB-PtBA macromonomer (102.3 mg, 320 equiv.) in CH2Cl2 (1.0 mL) via syringe. The reaction was allowed to stir at room temperature for 4 h, and was then quenched by addition of ethyl vinyl ether (a few drops) via syringe. The final PtBA brush product was obtained after precipitating the reaction mixture in 20% H2O-methanol. It was later found that the reaction could be performed under air. Tg=43 °C; IR: 2977, 2931, 1728, 1479, 1448, 1392, 1367, 1256, 1148, 1035, 845, 751, 632, 471 cm−1; 1H NMR (300 MHz, CD2Cl2, ppm) δ 4.60–4.72 (CHSC(S)S), 3.80–4.20 (CH2OC(O)), 3.30–3.40 (CH2SC(S)S), 2.05–2.40 (grafted chain backbone protons) 0.70–1.95 (t-butyl protons and C(S)SCH2C11H23); 13C NMR (75 MHz, CD2Cl2, ppm) 28.0–28.2, 35.7–38.3, 41.0–43.7, 80.2, 175.9.
Hydrolysis of t-butyl groups to acrylic acid groups
PtBA brush (23.6 mg) was loaded into a vial and dissolved in CH2Cl2 (5.0 mL). A solution of trimethylsilyl iodide (400 µL, diluted by 1.0 mL CH2Cl2) was added. After 90 min, the excess solvent and reagent were removed in vacuo. The residue was then redissolved in THF and decolorized by addition of Na2S2O3 to afford a colorless solution. Dialysis of the solution against nanopure water for 5 days cleaved the silyl ester bonds and gave the final PAA brush product, which was collected after removal of water by lyophilization. Tg= 101 °C; at pH 4, HavAFM = 1.5 nm; LavAFM = 32 nm; at pH 9, HavAFM = 1.5 nm; LavAFM = 58 nm; DavTEM = 68 nm; IR: 2917, 2849, 1718, 1560, 1542, 1458, 1259, 1195, 1131, 1076, 800 cm−1; 1H NMR (300 MHz, DMSO-d6, ppm) δ 14.10–15.70 (COOH), 4.60–4.72 (CHSC(S)S), 3.80–4.20 (CH2OC(O)), 3.30–3.40 (CH2SC(S)S), 2.05–2.40 (polymer backbone protons), 0.70–1.95 (polymer backbone protons and C(S)SCH2C11H23); 13C NMR (75 MHz, DMSO-d6, ppm) 35.0–39.0, 41.3–43.5, 176.2.
RESULTS AND DISCUSSION
The dual ROMP-active and RAFT chain transfer agent containing both a norbornene and a trithiocarbonate functionality, NB-TTC, was prepared in 89% yield by esterification of exo-5-norbornene-2-methanol20 (1.0 equiv.) and S-1-dodecyl-S’-(R,R’-dimethyl-R”-acetic acid)trithiocarbonate. 1H NMR spectroscopy showed a series of characteristic resonances, including those of the norbornene alkenyl protons at 6.05 ppm, CH2OC(O) at 4.17 and 3.92 ppm, CH2SC(S)S at 3.25 ppm and the terminal CH3 group at 0.85 ppm, whose integrations agreed with the theoretical ratios.
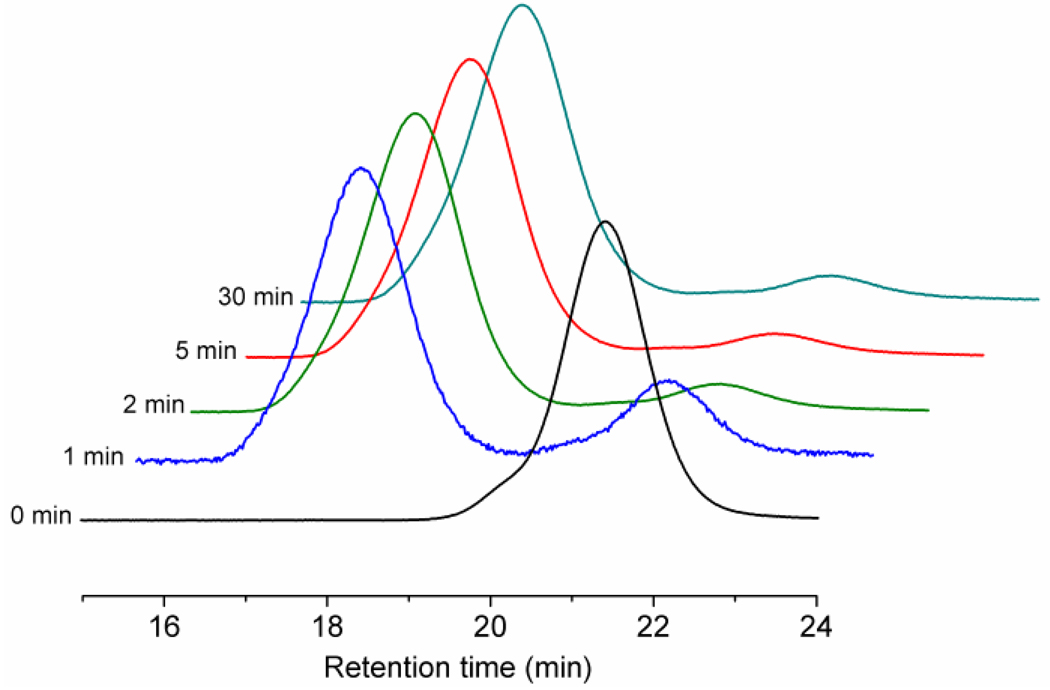
The chain transfer agent was then used in the polymerization of t-butyl acrylate to afford the NB-PtBA macromonomers (Scheme 1). An undesirable amount of the norbornenyl groups participated in the RAFT polymerization in our first trials, as was confirmed by both gel permeation chromatography (GPC), which gave unsymmetrical peaks, and 1H NMR spectroscopy, which revealed partial reduction of the norbornene alkenyl proton resonance intensities. To suppress the norbornenyl group radical polymerization, a series of experiments was conducted to optimize the polymerization conditions. Ultimately, the polymerization was performed in 2-butanone (50% v/v) with 0.05 equiv. AIBN at 51 °C and quenched at 40% conversion by freezing the reaction mixture in a liquid nitrogen bath. By this method, the RAFT polymerization was controlled, as indicated by retention of both the norbornenyl terminus and the trithiocarbonate terminus in the resulting macromonomer. The 1H NMR spectrum showed good agreement between the resonance intensities for the protons from the norbornenyl double bond at 6.05–6.10 ppm and the protons from the CH2SC(S)S group resonating at 3.30–3.40 ppm. GPC analysis of the macromonomer showed a symmetric peak with a polydispersity index (PDI) of 1.16 (Figure 1, 0 min), also demonstrating that the polymerization was well controlled. There was a slight high molecular weight shoulder observable in the GPC trace, which can be attributed to a small amount of norbornenyl participation during the radical polymerization and/or biradical coupling reactions. However, the Mn obtained from 1H NMR spectroscopy (17.3 kDa) agreed with the Mn value from GPC (16.9 kDa, relative to polystyrene standards) and both Mn values were close to the theoretical Mn value (16.4 kDa, based on the monomer conversion). Overall, the involvement of norbornenyl groups in RAFT was suppressed and a linear NB-terminated poly(t-butyl acrylate) was synthesized, which could then serve as a macromonomer and/or a macroRAFT chain transfer agent.
Scheme 1.
The RAFT-ROMP “grafting through” synthetic route to molecular brushes bearing poly(acrylic acid) side chains.
Figure 1.
GPC curves from the ROMP kinetic study of α-norbornenyl-functionalized PtBA.
The brush synthesis was achieved by ROMP of the terminal norbornenyl group of the NB-PtBA macromonomer (Scheme 1), using the modified 2nd generation Grubbs’ catalyst.17 ROMP of bulky macromonomers using the 1st and 2nd generations Grubbs’ catalysts generally require longer reaction times than those of small monomers.17,20,26 The modified 2nd generation Grubbs’ catalyst exhibits fast initiation and propagation,26 suggesting that it could be an ideal catalyst for the ROMP of bulky macromonomers. Therefore, a kinetic study was performed for the ROMP of the norbornenyl-terminated poly(t-butyl acrylate) using 320 equiv. of PtBA macromonomer relative to catalyst at room temperature in CH2Cl2. The polymerization was conducted in air without Ar protection. At increasing time intervals, an aliquot of the reaction mixture was collected, quenched by ethyl vinyl ether and analyzed by GPC (Fig. 1). At 1 min, ca. 75% conversion was achieved and the reaction had reached completion at 2 min. GPC analysis showed that the macromonomer peak at 21.4 min had nearly disappeared and a peak for the molecular brush appeared at 17.4 min. By 1H NMR spectroscopy, the characteristic protons resonating at 6.05–6.10 ppm and 2.60–2.00 ppm disappeared, supporting complete consumption of the norbornenyl groups. Conducting the reaction in open air or under argon protection showed no discernible difference by GPC and NMR. Even when left for 12 h, no deleterious side reactions were observed. After quenching with ethyl vinyl ether and isolation by precipitation into a H2O: methanol mixture (1:5, v:v), the molecular brushes were obtained in >90% yield with well-defined molecular weight and composition.
As observed by GPC, the PtBA molecular brushes had a Mn value of ca. 1,000,000 Da and a uniform size distribution, with a PDI, similar to that of the macromonomer, of 1.20 (Figure 1). Molecular weight determination was complicated by an inability to observe the chain ends by 1H NMR spectroscopy and because of GPC observation performed by RI using linear polystyrene standards for calibration. Although extensive, multiple detection techniques will be necessary to obtain an accurate molecular weight value, the data here confirm molecular brush growth to establish a high molecular weight product. By comparison of the relative areas of integration of the macromonomer and the molecular brush peaks in the gel permeation chromatogram, ca. 93% of the macromonomer had been incorporated into the final brush structure. Based upon this degree of conversion, together with the ratio of initiator to macromonomer, the theoretical degree of polymerization was 300, giving a theoretical Mn of 5.1 MDa. Within all the experiments, however, there remained a small peak at 21.4 min elution time in the GPC chromatograms of the products, which is believed to be due to the presence of residual macromonomer. One possible explanation is that these macromonomers lacked a norbornenyl end group, due to the mechanism of RAFT and the potential for a portion of the tBA chains to be initiated by AIBN during the macromonomer synthesis.
To afford water-soluble poly(acrylic acid) (PAA) brushes, the t-butyl groups were cleaved by reaction with trimethylsilyl iodide (TMSI), followed by hydrolysis of the intermediate silyl esters. Treatment of the PtBA molecular brush with TMSI in CH2Cl2 at room temperature for 90 min, concentration under reduced pressure, dissolution into THF, decoloration with sodium thiosulfate, dialysis against water, and lyophilization gave the PAA molecular brush structure. The deprotection was confirmed by expected characteristic absorption bands in the IR spectra, and by the loss of t-butyl protons (1.50 ppm) in the 1H NMR spectrum and the loss of t-butyl carbons (29.0 and 81.1 ppm) in the 13C NMR spectrum, each obtained in DMSO-d6. For each molecular brush, the resonances corresponding to the protons of the double bonds along the backbone were not discerned, in any solvent, suggesting that there is limited solvent access to the most internal regions of the molecular brush frameworks and/or limited mobility for the central norbornenyl backbone.
Differential scanning calorimetry (DSC) analysis indicated that there was no observable difference between the thermal transition temperatures for the linear vs. brush structure, suggesting that the apparent mobility differences were either not present in the bulk state or were too subtle to be observed by the relatively insensitive DSC technique. Both the PtBA macromonomer and the resulting molecular brush showed a Tg at 43 °C, which is typical for PtBA. Upon removal of the tBA protecting groups, the Tg of the PAA molecular brush had increased to 101 °C. Collecting accurate Tg values for PAA is complicated by many factors,27 including the extent of hydration; the thermal history has a significant impact, therefore, the second heating run was used for determination of the Tg value.
The solution-state sizes of the PtBA and PAA molecular brushes were determined by dynamic light scattering (DLS) in THF and water, respectively. The average diameters of the PtBA brush were 17 ± 2 nm by number averaging, 19 ± 2 nm by volume averaging and 24 ± 2 nm by intensity averaging. Solvation of the PAA brushes into DMSO and dialysis against nanopure water allowed for measurement of the hydrodynamic diameter, Dh, of the PAA brushes. The aqueous solution after dialysis had a pH of 4. At this pH, the particles showed an apparent diameter of ca. 340 nm, which was much larger than that of the PtBA brush, indicating intermolecular aggregation in the aqueous solution. Given the fact that the pKa of acrylic acid units is around 4.0–4.5,28 the pH of the solution was then increased. Initially, titration with NaOH to pH 9 gave a decrease in the Dh to 36 ± 10 nm (number averaged), falling into a reasonable range of the size of a single PAA brush if hydration is considered, in comparison with the PtBA brushes in THF solution. Further increase of pH to 14 showed similar results as those at pH 9. When the solution was titrated back to pH 0 using HCl, aggregates reformed, having an initial number-average diameter of ca. 1028 nm, and macroscopic precipitation occurred overnight.
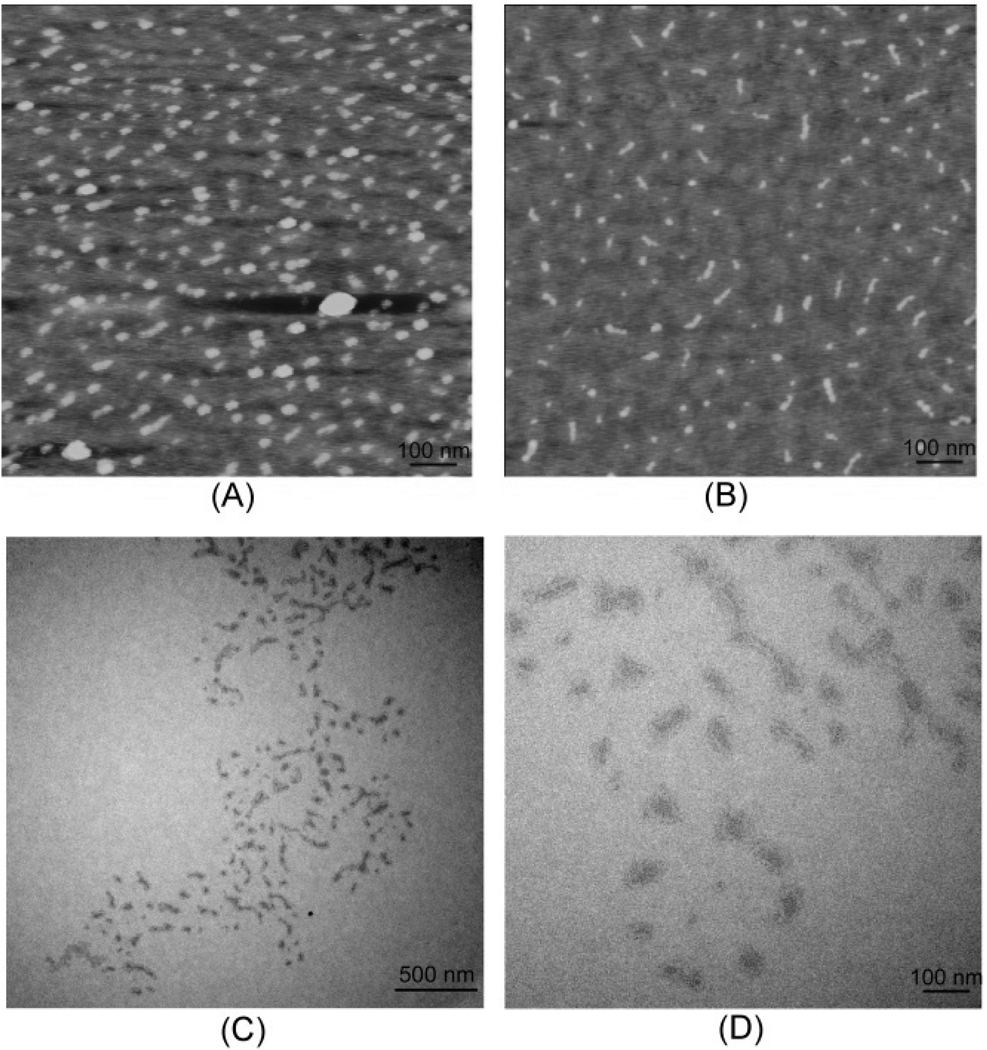
The PAA brushes were also studied by atomic force microscopy (AFM) (Figure 2(A) and 2(B)). The images from the PAA solution at pH 4 showed collapsed globular structures, whose height was ca. 1.5 nm and diameter was ca. 32 nm. The size was much smaller than the hydrodynamic size of the aggregates measured by DLS at pH 4, but similar to that of individual PAA brushes at pH 9. Disassembly of aggregates and conformational reorganization within the macromolecular structures may have occurred when the particles were adsorbed onto the mica surface, suggesting that the intermolecular attractive forces were relatively weak and the structures were quite flexible. The images from PAA brushes deposited from a solution at pH 9 showed particles with rod-like morphology, whose height was again ca. 1.5 nm but length was about 58 nm, suggesting a more extended conformation at elevated pH. A similar phenomenon was also reported previously.29 Based on the theoretical DP of the backbone (from the extent of macromonomer conversion) of 300, the average norbornenyl unit length calculated from the AFM image collected from the pH 9 sample preparation was 0.18 nm, suggesting that the backbone was not fully extended (0.5 nm per norbornenyl repeat unit). Similarly, in the work by Fréchet and Grubbs,30 nanorings from ROMP of sterically-bulky dendritic macromonomers also did not adopt fully extended conformations, rather they had an average backbone unit length of ca. 0.25 nm.
Figure 2.
Tapping-mode AFM images of PAA brushes on mica after being spin-coated from aqueous solutions (2 µL) at pH 4 (A) and pH 9 (B) onto freshly-cleaved mica and allowed to dry under ambient conditions. TEM images of PAA brushes on a carbon-coated copper grid after being deposited from aqueous solution at pH 4, staining with uranyl acetate solution (1% wt.) (pH = 4), removal of the excess solution and allowing to dry under ambient conditions, collected at different magnifications (C, D).
Transmission electron microscopy (TEM) was also used to study the size and morphology of the PAA brushes (Figure 2(C) and (D)). The TEM images were quite difficult to obtain and are challenging to interpret. Some particle-particle interactions are captured, but individual particles could be selected out and counted to calculate the average particle size, which was ca. 68 nm. This value fell into a reasonable range, compared with the sizes measured by DLS and AFM. As was observed by AFM, most of the molecular brushes deposited at pH 4 were seen as globular structures, although a few elongated images are discernible.
CONCLUSIONS
The present study has introduced a methodology for the synthesis of cylindrical molecular brushes with low polydispersity, well defined side-chain and chain-end structures, and well-controlled grafting density. This synthetic strategy is accomplished by combining RAFT and ROMP in a “grafting through” methodology. The practical advantages of this strategy include time efficiency and air insensitivity. In addition, because the ROMP-active norbornenyl group is introduced into the initial RAFT chain transfer agents, the final brush polymer structures retain the trithiocarbonate end groups, so that it would be possible to follow with further “grafting from” chain growth. The brushes bearing carboxylic acid groups also may be used for further chemical modifications, in addition to their observed pH-responsive transformations.
Acknowledgements
The authors gratefully acknowledge financial support from the National Heart Lung and Blood Institute of the National Institutes of Health as a Program of Excellence in Nanotechnology (HL080729) and the National Science Foundation under grant number DMR-0451490. Fellowship support from the McDonnell International Scholars Academy (Z. L.) is also acknowledged with appreciation.
References
- 1.Lutz J-F. J Polym Sci Part A Polym Chem. 2008;46:3459–3470. [Google Scholar]
- 2.Nikopoulou A, Iatrou H, Lohse DJ, Hadjichristidis N. J Polym Sci Part A Polym Chem. 2007;45:3513–3523. [Google Scholar]
- 3.Zhang Y, Xu Z, Li X, Chen Y. J Polym Sci Part A Polym Chem. 2007;45:3303–3310. [Google Scholar]
- 4.Gu L, Feng C, Yang D, Li Y, Hu J, Lu G, Huang X. J Polym Sci Part A Polym Chem. 2009;47:3142–3153. [Google Scholar]
- 5.Oh JK, Perineau F, Charleux B, Matyjaszewski K. J Polym Sci Part A Polym Chem. 2009;47:1771–1781. [Google Scholar]
- 6.Wiltshire JT, Qiao GG. J Polym Sci Part A Polym Chem. 2009;47:1485–1498. [Google Scholar]
- 7.Driva P, Lohse DJ, Hadjichristidis N. J Polym Sci Part A Polym Chem. 2008;46:1826–1842. [Google Scholar]
- 8.Gao HF, Matyjaszewski K. J Am Chem Soc. 2007;129:6633–6639. doi: 10.1021/ja0711617. [DOI] [PubMed] [Google Scholar]
- 9.Schappacher M, Deffieux A. Science. 2008;319:1512–1515. doi: 10.1126/science.1153848. [DOI] [PubMed] [Google Scholar]
- 10.Vivek AV, Dhamodharan R. J Polym Sci Part A Polym Chem. 2007;45:3818–3832. [Google Scholar]
- 11.Börner HG, Beers K, Matyjaszewski K, Sheiko SS, Möller M. Macromolecules. 2001;34:4375–4383. [Google Scholar]
- 12.Cheng C, Khoshdel E, Wooley KL. Macromolecules. 2007;40:2289–2292. [Google Scholar]
- 13.Zhang MF, Breiner T, Mori H, Müller AHE. Polymer. 2003;44:1449–1458. [Google Scholar]
- 14.Allcock HR, de Denus CR, Prange R, Laredo WR. Macromolecules. 2001;34:2757–2765. [Google Scholar]
- 15.Breunig S, Heroguez V, Gnanou Y, Fontanille M. Macro Symp. 1995:151–166. [Google Scholar]
- 16.Heroguez V, Breunig S, Gnanou Y, Fontanille M. Macromolecules. 1996;29:4459–4464. [Google Scholar]
- 17.Jha S, Dutta S, Bowden NB. Macromolecules. 2004;37:4365–4374. [Google Scholar]
- 18.Mecerreyes D, Dahan D, Lecomte P, Dubois P, Demonceau A, Noels AF, Jérôme R. J Polym Sci Part A Polym Chem. 1999;37:2447–2455. [Google Scholar]
- 19.Cheng C, Khoshdel E, Wooley KL. Nano Lett. 2006;6:1741–1746. doi: 10.1021/nl0611900. [DOI] [PubMed] [Google Scholar]
- 20.Cheng C, Qi K, Khoshdel E, Wooley KL. J Am Chem Soc. 2006;128:6808–6809. doi: 10.1021/ja061892r. [DOI] [PubMed] [Google Scholar]
- 21.Sheiko SS, Sumerlin BS, Matyjaszewski K. Prog Polym Sci. 2008;33:759–785. [Google Scholar]
- 22.Zhang MF, Müller AHE. J Polym Sci Part A Polym Chem. 2005;43:3461–3481. [Google Scholar]
- 23.Xia Y, Kornfield JA, Grubbs RH. Macromolecules. 2009;42:3761–3766. [Google Scholar]
- 24.Moad G, Rizzardo E, Thang SH. Aus J Chem. 2005;58:379–410. [Google Scholar]
- 25.Lai JT, Filla D, Shea R. Macromolecules. 2002;35:6754–6756. [Google Scholar]
- 26.Choi TL, Grubbs RH. Angew Chem Int Ed. 2003;42:1743–1746. doi: 10.1002/anie.200250632. [DOI] [PubMed] [Google Scholar]
- 27.Maurer JJ, Eustace DJ, Ratcliffe CT. Macromolecules. 1987;20:196–202. [Google Scholar]
- 28.Pradip, Maltesh C, Somasundaran P, Kulkarni RA, Gundiah S. Langmuir. 1991;7:2108–2111. [Google Scholar]
- 29.Lee HI, Boyce JR, Nese A, Sheiko SS, Matyjaszewski K. Polymer. 2008;49:5490–5496. [Google Scholar]
- 30.Boydston AJ, Holcombe TW, Unruh DA, Fréchet JMJ, Grubbs RH. J Am Chem Soc. 2009;131:5388–5389. doi: 10.1021/ja901658c. [DOI] [PMC free article] [PubMed] [Google Scholar]