Abstract
Sleep slow waves are the main phenomenon underlying NREM sleep. They are homeostatically regulated, they are thought to be linked to learning and plasticity processes and, at the same time, they are associated with marked changes in cortical information processing. Using transcranial magnetic stimulation (TMS) and high-density (hd) EEG we can measure slow waves, induce/measure plastic changes in the cerebral cortex and we can directly assess cortico-cortical information transmission. In this manuscript we review the results of recent experiments in which TMS/hd-EEG is used to demonstrate (i) a causal link between cortical plastic changes and sleep slow waves and (ii) a causal link between slow waves and the decreased ability of thalamocortical circuits to integrate information and to generate conscious experience during NREM sleep. The data presented here suggest a unifying mechanism linking slow waves, plasticity and cortical information integration; moreover, they suggest that TMS can be used as a non-pharmacological mean to controllably induce slow waves in the human cerebral cortex.
Keywords: sleep homeostasis, synaptic plasticity, consciousness, slow oscillations, bistability
Introduction
Sleep can be defined behaviorally as a state of reduced responsiveness to the environment that is readily reversible. According to this definition, sleep appears to be a universal phenomenon, being present in most, if not in all, species investigated, from Drosophila melanogaster to humans (Borbély & Achermann, 2000; Shaw et al., 2000; Tobler, 2000). While sleep is typically defined based on behavioral criteria, it's most striking and distinctive features can be found at the electrophysiological level. In fact, the introduction of continuous recordings of brain electrical activity (electroencephalogram, EEG) during sleep and wakefulness (Berger, 1929) has greatly enriched the study of sleep. Not only it has allowed to better distinguish waking from sleep but it also has led to the discovery of rapid eye-movement (REM) sleep as a specific state, different from non-REM (NREM) sleep (Aserinsky & Kleitman, 1953).
Falling asleep is a gradual phenomenon of progressive disconnection from the environment: we stop responding to stimuli and, to the extent that we remain conscious, our experiences become largely independent on the surrounding environment. After a few minutes in a transitional state, stage 1, subjects usually progress into stage 2, and, eventually, especially at the beginning of the night, into a period of stage 3. During stage 3, the process of awakening is drawn out, and subjects often remain confused for some time. This change in arousal threshold is accompanied by a dramatic change in the EEG, which shows high voltage, low frequency waves at around 1–2 Hz, which is why this stage is also known as slow wave sleep. The transition from the low-voltage, fast-frequency EEG observed during wakefulness to the characteristic EEG slow waves of NREM sleep is due to the occurrence of brief periods of hyperpolarization, called down states, in thalamocortical and cortical neurons. Down states are due to leakage potassium conductances that increase when ascending cholinergic neurons and other activating systems reduce their firing rate (for reviews see: (McCormick & Pape, 1990; Steriade et al., 1993; McCormick & Bal, 1997; Llinas & Steriade, 2006). As sleep deepens, down states become more frequent and synchronize through cortico-cortical and cortico-thalamo-cortical connections giving rise to the large waves that we record at the scalp level with the EEG. Human EEG recordings using 256 channels have revealed that the slow oscillation behaves as a traveling wave that sweeps across a large portion of the cerebral cortex (Massimini et al., 2004) including anterior, posterior cyngulum and parts of the default network (Riedner et al., submitted).
Slow waves and the underlying down-states represent the fundamental phenomenon of sleep and in the last 20 years we have learned a great deal about their mechanisms and their spatial-temporal dynamics. We also know that slow waves are homeostatically regulated, that they are, somehow, linked to learning processes and that during NREM sleep early in the night, when slow waves are most prominent, conscious experience is reduced (Stickgold et al., 2001). Indeed, homeostatic regulation, plasticity processes and changes in the level of consciousness during sleep appear as linked phenomena that have as a common denominator slow waves. In this review we will discuss several original insights on the complex relationships linking slow waves, sleep homeostasis, plasticity, and consciousness. We will do this by reviewing the results of experiments in which we used a novel technique, a combination of transcranial magnetic stimulation (TMS) and high-density (hd) EEG. This approach allows the investigator, at the same time, to interfere with and record the electrical activity of the human cerebral cortex and offers a tool to explore the causal relationships linking sleep homeostasis, synaptic plasticity, consciousness and slow waves. Specifically we employed TMS/hd-EEG to address the following questions:
Is there a link between the level of synaptic potentiation (or synaptic strength) and the generation of slow oscillations during sleep? To answer this question TMS (e.g. high-frequency stimulation, paired associative stimulation) is used to manipulate the strength of cortical synapses during wakefulness and while EEG is used to measure the effects of this manipulation on the subsequent sleep (Huber et al., 2007a; Huber et al., 2008). Such experiments directly test a recent hypothesis about the function of sleep – the synaptic homeostasis hypothesis – which proposes that increased sleep pressure is brought about by increased synaptic strength (Tononi & Cirelli, 2003; 2006).
Why does consciousness fade during sleep early in the night? Here, TMS/hd-EEG is used to measure directly the changes in cortical excitability and connectivity that occur upon falling asleep (Massimini et al., 2005; Massimini et al., 2007). These experiments are designed to specifically test a theoretical prediction claiming that the fading of consciousness during NREM sleep is due to a reduced ability of the brain to integrate information (Tononi, 2004).
Can we use TMS to increase sleep efficiency? Here, the ability of TMS to trigger sleep slow waves in sleeping humans is tested (Massimini et al., 2007). These experiments open the possibility of manipulating sleep, locally and globally, in a non-pharmacological way.
Questions
Is there a link between the level of synaptic strength and the generation of slow oscillations during sleep?
How our brain acquires and stores information has been a major focus of neuroscience in the past decades. Long-term potentiation (LTP) is the major candidate synaptic mechanism underlying learning and memory formation (Bliss & Lomo, 1973; Bliss & Collingridge, 1993; Whitlock et al., 2006). LTP refers to the process whereby the efficacy of communication between neurons is increased. This mechanism has been studied extensively at the cellular and molecular level in laboratory animals (Malenka & Nicoll, 1999; Abraham et al., 2002).
A recent hypothesis about the function of sleep – the synaptic homeostasis hypothesis (Tononi & Cirelli, 2003; 2006) - proposes that learning related increased synaptic strength is responsible for increased sleep pressure. It is well known, even from personal experience, that sleep pressure increases in proportion to the time spent awake. However, so far it is unknown what mechanism is responsible for the accumulation of sleep pressure during wakefulness and its release during sleep. What is known is that the regulation of sleep pressure is accurately reflected by the amount of slow wave activity (SWA, electroencephalographic (EEG) power in the low frequency range between 0.5 and 4.5 Hz) during non-rapid eye movement (NREM) sleep (Borbély, 1982; Borbély & Achermann, 2000). As repeatedly shown in both humans and mammals, SWA increases exponentially with the duration of prior wakefulness and decreases, also exponentially, during sleep, thus reflecting the accumulation of sleep pressure during wakefulness and its release during sleep. According to the synaptic homeostasis hypothesis, changes in synaptic strength induced during wakefulness, should result in a change of SWA during subsequent sleep. This can be tested experimentally. In the next few paragraphs, we will present our evidence linking changes in synaptic strength to local changes of SWA during sleep.
Numerous studies, in various mammalian species have shown that the level of SWA can be increased on a global level after sleep deprivation (Tobler, 2000). This global response to sleep deprivation is, however, modulated locally in a use dependent manner. For example, Kattler and co-workers provided in the 90ies some initial evidence that the level of SWA is regionally affected by previous activity (Kattler et al., 1994). In their study, a subject's hand was exposed to a vibratory stimulus for several hours before going to bed. During subsequent sleep the balance of SWA was shifted to the hemisphere contralateral to the stimulated hand. Similar results were obtained in the rat, in which unilateral whisker stimulation led to an asymmetry of sleep SWA (Vyazovskiy et al., 2000). However, the mechanisms underlying such use-dependent changes in SWA remain unknown. Thus, we set out to investigate whether a local manipulation of synaptic strength leads to a local change in SWA, as proposed by the synaptic homeostasis hypothesis. For the detection of such local changes we employed a high-density EEG system with 256 channels. Such a system allows for improved spatial resolution combined with the high temporal resolution characteristic of EEG recordings.
In a first study we aimed for a manipulation of synaptic strength by means of a specific learning task. We used a visuomotor learning task because its learning related activation as measured by PET studies was well localized (Ghilardi et al., 2000) and because of the availability of a kinematically equivalent motor control task. These key features of our visuomotor learning task enhanced the probability of finding a similar local change in EEG activity across subjects and allowed us to control for the mere use of a the visuomotor system as compared to the learning related induction of plastic changes within that system. The experiment was as follows: In the evening before going to bed, our subjects were trained on the task. In weekly intervals they performed either the learning or the motor control task. Immediately after the task our subjects were allowed to sleep and we recorded their brain activity by means of high-density EEG. We assessed local differences in SWA within the first half hour of sleep. Thus, after the visuomotor learning task we demonstrated that SWA was locally increased in a specific cortical region, but not after the kinematically equivalent motor control task that did not require learning (Huber et al., 2004). This local increase in SWA shared the characteristics of a global homeostatic response to increased sleep pressure (see (Borbély & Achermann, 2000): The largest increase of EEG power was found in the low frequency range and the increase subsided in the course of the first NREM sleep episode. Immediately after sleep the subjects were retested on the task to check for sleep dependent performance improvements. As expected, based upon the increasing number of reports of sleep dependent task performance improvements (Maquet, 2001; Stickgold, 2005; Born et al., 2006), our subjects improved on the task after sleep. Furthermore, the post sleep task performance improvement was positively correlated with the local increase of SWA. In summary, this experiment provided initial correlative evidence for a link between a learning-related change in synaptic strength and a change in sleep SWA.
A recent study obtained similar results when using a difficult declarative learning task. The task led to increased sleep SWA and spindle activity at left frontal locations during post-training sleep (Schmidt et al., 2006). The authors also found a positive correlation between the sleep EEG changes and changes in memory performance. This correlative evidence for a link between sleep SWA and waking performance is corroborated by studies trying to establish a causal link between the two. A first study showed that boosting slow oscillations by transcranial application of oscillating potentials has beneficial effects on the retention of hippocampus-dependent declarative memories (Marshall et al., 2006). In a second approach a reduction of slow waves by means of slow wave deprivation protocols was used to interfere with sleep dependent performance improvement. The successful reduction of slow-wave activity by means of acoustic stimuli prevented any sleep dependent performance improvements (Aeschbach et al., 2008); Landness et al., submitted).
If a learning related increase in synaptic strength results in an increase of SWA then a decrease in synaptic strength should lead to a decrease in SWA. Thus, in our second study we used short-term arm immobilization to induce a local reduction of synaptic strength (Huber et al., 2006). First, we showed that if a subject's arm is immobilized during the day, motor performance deteriorates. Modeling work implied that the performance deterioration was due to a reduction in the shoulder to elbow joint coordination (Moisello et al., 2008). We used somatosensory evoked potentials (SEPs) by means of electrical median nerve stimulation to determine changes in the responsiveness of the sensorimotor cortex. The observed amplitude reduction of SEPs after arm immobilization over contralateral sensorimotor cortex was indicative of a local reduction of synaptic strength. This finding was corroborated by a reduced amplitude of motor evoked potentials by means of TMS. Again, immediately after the immobilization our subjects were allowed to sleep and we recorded their hd-EEG. As expected, during subsequent sleep, SWA over the same cortical area was reduced (Huber et al., 2006).
In a third human study we used rTMS to directly induce potentiation in a non-physiological, but predictable, way. In animals in vivo, LTP is classically induced by high-frequency electrical stimulation (5–15 Hz) and assessed by recording changes in population responses to test stimuli (Bliss & Lomo, 1973). In humans, it has recently become possible to approximate this classic protocol non-invasively by combining transcranial magnetic stimulation (TMS) with hd-EEG (Esser et al., 2006): high-frequency electrical stimulation can be safely substituted by repetitive TMS (rTMS), while changes in cortical responses to test TMS pulses can be assessed with hd-EEG. As shown by Esser et al., 5-Hz rTMS conditioning results in increased amplitude of the EEG response to TMS pulses, indicative of potentiation of motor cortical circuits. Remarkably, during subsequent sleep we observed a prominent localized increase in SWA over affected motor areas (Huber et al., 2007a). Furthermore, we found a positive correlation between activity of the EEG response to TMS pulses and the increased SWA over ipsilateral premotor cortex.
Finally, our most recent study employed a paired associative stimulation protocol (PAS; (Classen et al., 2004), which is thought to produce both the potentiation and depression of a specific set of synapses based on spike dependent plasticity (Dan & Poo, 2004). The human PAS protocol uses a combination of direct cortical stimulation by means of TMS and peripheral electrical stimulation of the median nerve. According to Classen and co-workers, the interval between the two stimuli determines whether motor evoked potentials are potentiated or depressed (Stefan et al., 2000; Wolters et al., 2005). Such a protocol allowed us to study local changes in sleep SWA after local potentiation or depression of synapses using the same paradigm and with the exact same amount of stimulation, i.e. 90 pairs of stimuli. In a first step we used TMS/EEG to directly assess changes in cortical responsiveness after PAS (Huber et al., 2008). We found increased cortical responsiveness over sensorimotor cortex, in the long inter-stimulus interval condition where motor evoked potentials were shown to be increased (Classen et al., 2004). In contrast when comparing the short inter-stimulus interval condition to our sham control condition, and again in agreement with findings of changes in motor evoked potentials (Classen et al., 2004), we observed reduced cortical responsiveness over sensorimotor cortex. During subsequent sleep, SWA increased locally over sensory motor cortex in subjects whose cortical responsiveness increased and decreased in subjects whose cortical responsiveness decreased (Huber et al., 2008). In two recent studies the same PAS protocol was applied and both reported transient local changes in SWA during subsequent sleep (Bergmann et al., 2008; De Gennaro et al., 2008).
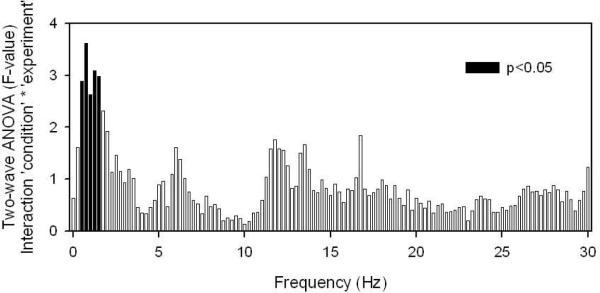
According to the synaptic homeostasis hypothesis changes in cortical responsiveness should be reflected in a change in SWA. To strengthen this point we performed an analysis of variance across all of our experiments introduced above. As shown in Figure 1, it is indeed activity within the SWA frequency range that is reacting most sensitively to experimental manipulations affecting synaptic plasticity. Within the SWA frequency range the effect is strongest between 0.75 and 1.5 Hz, which is around the frequency of slow oscillations (Steriade, 2000). Cross-correlating changes in EEG activity for each frequency bin reveals high correlations within the SWA frequency range and above 15 Hz (Figure 2). However, there is hardly any correlation between the activity change in the SWA frequency range and the activity change in the spindle frequency range. This is of particular interest in light of the proposed relationship between spindling and learning (Born et al., 2006). The discrepancy might be related to differences in the investigated memory system and/or topographical differences in the expression of task related changes of power in the spindle and slow-wave frequency range.
Figure 1.
Frequency specific EEG power change. For the analysis subjects of several experiments are pooled (n=62 in total: from Huber et al., 2004, visuomotor learning, 10; Huber et al., 2006, immobilization, 12; Huber et al., 2007; 5-Hz TMS, 10; Huber et al., 2008, PAS ISI 25, 17; PAS ISI 10, 12). For all subjects the change in EEG power in the manipulation condition compared to the control condition was averaged for the first half hour of NREM sleep within a region of interest. We applied a two-way ANOVA with factors `condition' (manipulation vs. control), `experiment' (visuomotor learning, immobilization, 5-Hz TMS, PAS ISI 25, PAS ISI 10) and interaction `condition' * `experiment'. No frequency bin reached significant for the factor `condition' and most frequency bins showed a significant experiment factor. The interaction is displayed in the figure and shows some frequency specific effect. Please note that statistics for frequency bins below 0.75 Hz might be affected by the fact that some of the data was high-pass filtered at 0.5 Hz.
Figure 2.

Cross-correlation of activity change. The change in EEG activity for all subjects included in Figure 1 was cross-correlated for every 0.25 Hz frequency bin. Correlation coefficients are color coded and the black arrow indicates significance level.
A relationship between sleep SWA and synaptic plasticity is also supported by animal studies. For example, a chronic lesion of the noradrenergic system in rats resulted in a reduced expression of molecular markers of synaptic potentiation, and a blunted SWA response (Cirelli et al., 2005). On the other hand, it was recently shown that the more rats explore the environment, the stronger is their cortical expression of brain-derived neurotrophic factor (BDNF), a major marker of neuronal plasticity during wakefulness, and the larger is the increase in SWA during the subsequent sleep period (Huber et al., 2007b). Furthermore, cortical unilateral microinjections of BDNF in awake rats resulted in a reversible increase of SWA during NREM sleep, whereas, microinjections, of a polyclonal anti-BDNF antibody or K252a, an inhibitor of BDNF TrkB receptors, led to a local SWA decrease during the following sleep period (Faraguna et al., 2008).
Together, these results suggest that local sleep homeostasis, as reflected by sleep SWA, is directly related to synaptic plasticity. In addition, a series of papers in computo, in the rat, and in humans, has provided additional evidence that the slope of slow waves in the sleep EEG may serve as a marker of synaptic strength (Esser et al., 2007; Riedner et al., 2007; Vyazovskiy et al., 2007), and that the slope of slow waves is high after periods of wakefulness and decreases in the course of sleep. According to the synaptic homeostasis hypothesis (Tononi & Cirelli, 2003; 2006), SWA homeostasis is not only a reflection of local synaptic changes, but is also serves a functional purpose, namely a generalized decrease in synaptic strength that recalibrates neural circuits to levels that are sustainable in terms of energy consumption, space requirements in the neuropil, supply of proteins, lipids and other cellular constituents, and that permit further learning by desaturating synapses. So far, there is only limited evidence that SWA during sleep is not just a reflection of the previous waking history, but rather plays an active role. The most direct evidence for a link between the homeostatic regulation of sleep and the homeostatic regulation of synaptic strength comes from a study be Vyazovskiy et al., (Vyazovskiy et al., 2008) showing, using a combination of electrophysiological and molecular approaches, that wakefulness appears to be associated with net synaptic potentiation, whereas sleep favours global synaptic depression, thereby preserving an overall balance of synaptic strength. The net synaptic potentiation during wakefulness is in agreement with the increase in intracortical facilitation found in a human study that combined EEG and TMS (De Gennaro et al., 2007).
Why does consciousness fade during sleep early in the night?
One way to understand the relationships between the brain and consciousness is to consider conditions in which consciousness is globally reduced and ask what has changed in the brain. Sleep reminds us everyday that consciousness is something that can come and go, grow and shrink, depending on how our brain is functioning. Everyone is familiar with the impression of nothingness that lies in between our falling into and awakening from dreamless sleep. In the laboratory, subjects are awakened during different stages of sleep and asked to report “anything that was going through your mind just before waking up” (see (Fagioli, 2002; Nielsen, 2004) for a review). A very reproducible finding is that a number of awakenings from NREM sleep, especially early in the night when EEG slow waves are prevalent, can yield short reports or no report whatsoever. In fact, the first NREM episode of the night is the only phase of adult life during which healthy human subject may deny that they were experiencing anything at all (Pivik & Foulkes, 1968; Suzuki et al., 2004).
Understanding what changes in the brain upon falling into NREM sleep early in the night may help us in identifying what is really necessary and sufficient for the brain to give rise to conscious experience. The relationships between sleep and consciousness are indeed interesting and puzzling. It was first thought that the fading of consciousness during sleep was due to the brain shutting down. However, while metabolic rates decrease in some cortical areas, the thalamocortical system remains active also during slow wave sleep, with mean firing rates comparable to those of quiet wakefulness (Steriade et al., 2001). It was also hypothesized that sensory inputs are blocked during sleep and that they are necessary to sustain conscious experience. However, we now know that, even during deep sleep, sensory signals continue to reach the cerebral cortex (Kakigi et al., 2003) where they are processed subconsciously (Portas et al., 2000). Gamma activity and synchrony have been viewed as possible correlates of consciousness and they were found to be low in NREM sleep (Cantero et al., 2004; He et al., 2008). However, they were equally low in REM sleep, when subjective experience is usually vivid, and they can be high in anesthesia (Vanderwolf, 2000). Moreover, intracellular recordings show that gamma activity persists during NREM sleep, and other studies report that gamma coherence is a local phenomenon that does not change between wakefulness and sleep (Bullock et al., 1995). Large-scale synchrony in the alpha and theta band may also correlate with conscious perception during wakefulness, but synchrony in these frequency bands actually increases during NREM sleep (Duckrow & Zaveri, 2005). Why, then, does consciousness fade?
A recently formulated theory, the integrated information theory of consciousness (Tononi, 2004), may help addressing this question experimentally. According to the IITC, what is critical for consciousness is not necessarily firing rates, sensory input, specific frequency bands, or synchronization per se but rather the amount of integrated information generated by a system. Thus, the brain substrate of consciousness is thought to be a complex of neural elements, presumably located within the thalamocortical system, which is endowed with the following two properties: i) information— the system has a large repertoire of available states so that, when it enters a specific state, it rules out a large number of alternative states and therefore generates a large amount of information; ii) integration— the system cannot be decomposed into a collection of independent subsystems so that, when it enters a specific state, it generates information as a whole. An exhaustive measure of complexes and the associated value of integrated information is currently feasible in simple artificial networks, but it is a daunting proposition in a complex biological system, such as the human brain. Nonetheless, the theory makes clear-cut predictions that can be addressed experimentally at least at a gross level. Specifically, the fading of consciousness during early NREM sleep should be associated with either a reduction of integration within the main thalamocortical complex (for example, it could break down into causally independent modules) or a reduction of information (the repertoire of available states might shrink), or both. The theory also suggests that, to evaluate integrated information, it is not enough to observe activity levels or patterns of temporal correlations among distant brain regions (functional connectivity). Instead, the ability to integrate information among distributed cortical regions must be examined from a causal perspective; one must employ a perturbational approach (effective connectivity) and examine to what extent cortical regions can interact causally as a whole (integration) to produce responses that are specific for that particular perturbation (information). One should probe effective connectivity by directly stimulating the cortex to avoid possible subcortical filtering and gating, and ideally one should do so in humans, as only with humans do we know that consciousness is reduced during early NREM sleep. If the prediction of the IITC are correct, during wakefulness a direct cortical perturbation will results in a global and specific pattern of activation while, during NREM sleep it will produce either a local response (loss of integration) or a broad and non-specific reaction (loss of information).
In a series of recent experiments (Massimini et al., 2005; Ferrarelli et al., 2007), we employed a combination of navigated transcranial magnetic stimulation (TMS) and high-density electroencephalography (hd-EEG) to measure noninvasively and with good spatiotemporal resolution the brain response to the direct perturbation of selected brain regions. Using a 60- channel TMS-compatible EEG amplifier, we recorded TMS-evoked brain responses while subjects, lying with eyes closed on a reclining chair, progressed from wakefulnessto deep NREM sleep. Thanks to noise masking and other procedures, subjects were unaware of TMS.
Figure 3A shows the response obtained after stimulation of rostral premotor cortex in one subject during wakefulness and NREM sleep (modified from (Massimini et al., 2005). The black traces represent the voltage recorded from all scalp electrodes, the cortical currents associated with the main peaks of activity are depicted below. The circles on the cortical surface indicate the site of stimulation, while the cross highlights the location of maximal cortical activation. In these experiments, TMS, applied at an intensity corresponding to motor threshold, triggered, during wakefulness, a series of low-amplitude, high-frequency (25–30 Hz) waves of activity associated with cortical activations that propagated along long-range ipsilateral and transcallosal connections. The exact same stimulation, applied 15 minutes later, during NREM sleep stages 3–4, resulted in a very different picture. TMS triggered a large, low-frequency wave that, associated with a strong cortical activation that did not propagate to connected brain regions and dissipated rapidly. Thus, during NREM sleep the cortical area directly engaged by TMS preserved its reactivity but behaved as an isolated module. Based on these findings we concluded that the fading of consciousness during certain stages of sleep may be related to a breakdown in cortical effective connectivity and to a lack of integration between thalamocortical circuits.
Figure 3.
(A) NREM sleep reduces cortical integration in humans. EEG voltages and current densities are shown from a representative subject in which the premotor cortex was stimulated with transcranial magnetic stimulation (TMS) (black arrow). During waking (top), stimulation evokes EEG responses first near the stimulation site (black circle; the white cross is the site of maximum evoked current) and then, in sequence, at other cortical locations. During deep sleep (bottom), the stimulus-evoked response remains local, indicating a loss of cortical integration. (B) Sleeping reduces cortical information carrying capacity in humans. (Top) During waking, stimulation over the mesial parietal cortex produces a specific, sequential pattern of activation. (Bottom) During sleep, the same stimulation produces a broad reaction that spreads, like an oil-spot, from the stimulation site to most of the cortex. This response is not specific and indicates a loss of differentiation and information capacity in cortical circuits. Black traces represent averaged voltage potentials recorded at all electrodes arranged in a butterfly plot, below estimated current density on the cortical surface is displayed in absolute scale.
What prevents the emergence of a specific long-range pattern of activation during sleep? As described above, during NREM sleep, cortical neurons are depolarized and fire tonically just as in quiet wakefulness, but these depolarized up-states are interrupted by short, hyperpolarized down-states when neurons remain silent (Sanchez-Vives & McCormick, 2000). This alternation involves large populations of cortical neurons and is reflected in the EEG as high-amplitude slow oscillations. The transition from up- to down-states appears to be due to depolarization-dependent potassium currents that increase with the amount of prior activation. Perhaps, because of this bistability of cortical networks during NREM sleep (Hill & Tononi, 2005), any local activation, whether occurring spontaneously or induced by TMS, will eventually trigger a local down-state, or a localized increase in inhibition (Esser et al., unpublished results) that prevents further propagation of activity. If bistability is a key mechanism underlying both the occurrence of slow waves and the alteration of information transmission during NREM, then it must be possible to trigger full-fledged slow waves with TMS.
We tested this prediction by probing systematically the reactivity of the sleeping brain by applying TMS at different intensities in different areas (Massimini et al., 2007). Increasing TMS intensity resulted in progressively larger responses that met the detection criteria for typical spontaneous slow waves when strong pulses (>130% of motor threshold) were applied over sensory-motor cortex during sleep. Spatially, the TMS-evoked slow oscillation was associated with a broad and non-specific response: cortical currents spread, like an oil-spot, from the stimulated site to the rest of the brain (Fig. 2B, lower panel). The same stimulation, applied during wakefulness, resulted, instead, in a long-range differentiated pattern of cortical activation (Fig. 2B, upper panel). Thus, while during wakefulness corticothalamic circuits react to a direct perturbation with a complex pattern of activation, during NREM sleep the only possible response is a stereotypical slow wave, that can be local or global, depending on stimulation intensity.
Altogether, these TMS-EEG measurements are in agreement with theoretical predictions and suggest that the sleeping brain, despite being active and reactive, loses its ability of entering states that are both integrated and differentiated: it either breaks down in causally independent modules, or it bursts into an explosive and non-specific response. In no case, during NREM sleep, did TMS result in a balanced, long-range differentiated pattern of activation. The TMS-EEG perturbational approach also suggests that intrinsic bistability in thalamocortical networks, the key mechanism responsible for the occurrence of the spontaneous slow oscillations of sleep, may be the reason why information integration is impaired in early NREM sleep.
Can we use TMS to increase sleep efficiency?
SWA is considered a reliable indicator of sleep need that increases with time awake, decreases during sleep, and may mediate the restorative function of slow-wave sleep. As discussed above, SWA is linked to the induction of cortical plastic changes, because it increases locally after a learning task and is positively correlated with post-sleep performance improvement. Certainly, the possibility of directly enhancing SWA in a reliable and controlled fashion using a non-pharmacological mean, such as electric or magnetic stimulation, may have relevant application. Recently, transcranial direct current stimulation during sleep at the frequency of the slow oscillation (SO) was reported to enhance declarative memory, although in that study brain activity could not be recorded during stimulation. Using TMS/hd-EEG, we were able to demonstrate that it is possible to trigger reliably slow waves in humans that resemble in all aspects spontaneously occurring slow waves (Fig. 4). Similar to a cardiac pacemaker, each and every TMS pulse, delivered during NREM sleep with the appropriate parameters, triggered a full-fledged slow wave that started under the stimulator and spread to the rest of the brain. TMS-triggered slow waves matched the period-amplitude detection criteria for spontaneous ones and were also associated with a significant modulation of spindle density during the positive-going phase of the oscillation. More importantly, the reliable triggering of slow waves with each and every TMS pulse resulted in a substantial increase in SWA both locally (up to eight times) and globally (two times) over the scalp. TMS-evoked slow waves and enhancement of SWA could be obtained during all stages of NREM sleep (stages 2, 3, and 4), but not during wakefulness, a state during which thalamocortical circuits are not bistable. In fact, while TMS cannot directly induce sleep, it can exploit the underlying bistability of sleep to regularly trigger slow oscillations on the background of a seemingly stable EEG. Indeed, the most dramatic effect was observed when TMS was applied during stage 2. In this case, the repetitive triggering of individual slow waves produced a sudden electrophysiological transition to deep slow wave sleep. However, despite the striking resemblance between the evoked and the spontaneous electrophysiological patterns of sleep, we still do not know whether evoked slow waves have the same beneficial impact on the brain of spontaneously occurring ones. In order to demonstrate this, one should show that 1) inducing long trains of slow waves with TMS decreases the “need” for spontaneous SWA and 2) that enhancing slow waves with TMS has a significant behavioural effect in terms of performance improvement. Unfortunately, the current TMS/hd-EEG set-up has several limitations that prevent the application of long trains of TMS in a sleeping subject. Among these limitations are stimulator overheating and the fact that, in order to maintain an optimal stimulation target, the subject needs to be partially restrained in a supine position, inevitably affecting the quality of sleep over the whole night. A possible way to explore the practical applications of TMS in slow wave induction would be to design and build a helmet, integrating, for example, a water-cooled stimulator and few EEG leads that a subject can comfortably wear for the entire night. Only in this way it will be conceivable to compare the effects on sleep homeostasis and behaviour of a regular night of sleep with the ones of a night of “magnetically induced” slow waves. For now, we know that bypassing the thalamic gate and stimulating directly the cerebral cortex with TMS can turn, at any time, the potential for a slow wave, that is, the bistability underlying NREM sleep, into a full-blown electrical oscillation.
Figure 4.
TMS during sleep triggers slow waves that resemble spontaneously occurring ones. (A, Upper) The signal recorded from a channel (Cz) located under the stimulator during two TMS-ON blocks over a background of spontaneous NREM sleep (single-subject data). Each TMS-ON block consisted of 40 stimuli at 0.8 Hz. The stimulation site (hot spot) is marked by a yellow cross on the cortical surface (the colored area represent the overall electric field induced by TMS). The red boxes (expanded below) include the slow waves triggered at the beginning and at the end of one block. Spontaneously occurring slow waves recorded from the same subject a few minutes later are depicted underneath. (B) TMS-evoked and spontaneous slow waves, recorded from all channels, were detected based on period-amplitude criteria and averaged on the negative peak. TMS-evoked and spontaneous slow waves had similar shape. (C) The average signal recorded from Cz was bandpass filtered (0.25–4 Hz) in the top trace. In the middle and bottom traces the corresponding single trials were filtered in the spindles frequency range (12–15 Hz) and rectified (rms). The positive wave of the TMS-evoked slow wave was associated with an increase in spindle amplitude.
Concluding remarks
The results of the experiments summarized in the first section of this review suggest that learning and synaptic potentiation during wakefulness bring about an increase of SWA during subsequent sleep. This effect is probably due to the fact that stronger synapses and neural connections within cortical network result in a higher level of synchronization and in turn in larger slow wave. There is good evidence suggesting that slow waves during NREM sleep reflect synaptic strength and may be responsible for synaptic downscaling. Slow waves during sleep may, actually, represent an ideal activity pattern to induce synaptic depression (see (Tononi & Cirelli, 2006). Supporting this idea are the findings that i) TMS protocols using low frequency (< 1 Hz) stimulation cause depression of motor evoked potentials (e.g. (Chen, 2000), that, ii) simulation of the slow oscillation firing pattern leads to synaptic depression in the slice (Czarnecki et al., 2007) and that, iii) in rats, the slope of cortico-cortical evoked potentials, a typical indicator of synaptic strength, decreases proportionally to the amount of previous SWA. Slow waves are, thus, homeostatically regulated based on the amount of total synaptic weight that has been accumulated during previous wakefulness and, in turn, may promote synaptic downscaling, a process that prevents the metabolic and computational problems associated with synaptic overload.
However, a necessary condition for slow waves to occur is bistability in thalamocortical circuits. This “permissive” factor comes into play only when brainstem activating systems reduce their firing rate upon falling asleep. Coherently, as shown in the second part of this review, TMS, even when delivered with the appropriate parameters, can not trigger a slow wave if the subject is awake. While bistability is necessary for slow waves and synaptic downscaling to occur, it also interferes with the normal way the brain processes information. Indeed, TMS during NREM sleep fails to evoke a long-range, specific pattern of activation and result in a stereotypical local or global slow wave. Hence, due to bistability, the inescapable occurrence of a down-state after any increase in cortical activity may prevent thalamocortical circuit from producing responses that are, at the same time, integrated and differentiated. In this sense, the reduction in the level of consciousness that we experience during NREM sleep may be seen as the price we pay for bistability, slow waves and their beneficial effects.
Future applications of TMS in the field of sleep research are suggested in the third part of this review. Here it is shown that it is possible to trigger full-fledged slow waves, and to deepen NREM sleep, by an electrophysiological point of view, by applying non-invasively rhythmic pulses with the appropriate parameters. Testing the viability of TMS as a nonpharmacological mean to increase sleep efficiency requires, however, further technical developments and experiments. Besides manipulating sleep, TMS in combination with EEG, might also be used to measure directly sleep efficiency in humans. Indeed, if synaptic downscaling is the essential function of NREM sleep, the amplitude and the slope of the early electrical response to a direct cortical perturbation (a reliable indicator of synaptic strength in vitro and in vivo), measured with TMS-EEG before and after a night of sleep, may provide a reliable and straightforward indicator of the effect of sleep on cortical circuits.
References
- Abraham WC, Logan B, Greenwood JM, Dragunow M. Induction and experience-dependent consolidation of stable long-term potentiation lasting months in the hippocampus. J Neurosci. 2002;22:9626–9634. doi: 10.1523/JNEUROSCI.22-21-09626.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aeschbach D, Cutler AJ, Ronda JM. A role for non-rapid-eye-movement sleep homeostasis in perceptual learning. J Neurosci. 2008;28:2766–2772. doi: 10.1523/JNEUROSCI.5548-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aserinsky E, Kleitman N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science. 1953;118:273–274. doi: 10.1126/science.118.3062.273. [DOI] [PubMed] [Google Scholar]
- Berger H. On the Electroencephalogram of Man. Archiv Psychiatrie. 1929;87:527–570. [Google Scholar]
- Bergmann TO, Molle M, Marshall L, Kaya-Yildiz L, Born J, Roman Siebner H. A local signature of LTP- and LTD-like plasticity in human NREM sleep. The European journal of neuroscience. 2008;27:2241–2249. doi: 10.1111/j.1460-9568.2008.06178.x. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbély AA. A two process model of sleep regulation. Human Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- Borbély AA, Achermann P. Homeostasis of human sleep and models of sleep regulation. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. W. B. Saunders; Philadelphia: 2000. pp. 377–390. [Google Scholar]
- Born J, Rasch B, Gais S. Sleep to remember. Neuroscientist. 2006;12:410–424. doi: 10.1177/1073858406292647. [DOI] [PubMed] [Google Scholar]
- Bullock TH, McClune MC, Achimowicz JZ, Iragui-Madoz VJ, Duckrow RB, Spencer SS. Temporal fluctuations in coherence of brain waves. Proc Natl Acad Sci U S A. 1995;92:11568–11572. doi: 10.1073/pnas.92.25.11568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantero JL, Atienza M, Madsen JR, Stickgold R. Gamma EEG dynamics in neocortex and hippocampus during human wakefulness and sleep. NeuroImage. 2004;22:1271–1280. doi: 10.1016/j.neuroimage.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Chen R. Studies of human motor physiology with transcranial magnetic stimulation. Muscle & nerve. 2000;9:S26–32. doi: 10.1002/1097-4598(2000)999:9<::aid-mus6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Huber R, Gopalakrishnan A, Southard TL, Tononi G. Locus ceruleus control of slow-wave homeostasis. J. Neurosci. 2005;25:4503–4511. doi: 10.1523/JNEUROSCI.4845-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classen J, Wolters A, Stefan K, Wycislo M, Sandbrink F, Schmidt A, Kunesch E. Paired associative stimulation. Supplements to Clinical neurophysiology. 2004;57:563–569. [PubMed] [Google Scholar]
- Czarnecki A, Birtoli B, Ulrich D. Cellular mechanisms of burst firing-mediated long-term depression in rat neocortical pyramidal cells. J Physiol. 2007;578:471–479. doi: 10.1113/jphysiol.2006.123588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan Y, Poo MM. Spike timing-dependent plasticity of neural circuits. Neuron. 2004;44:23–30. doi: 10.1016/j.neuron.2004.09.007. [DOI] [PubMed] [Google Scholar]
- De Gennaro L, Fratello F, Marzano C, Moroni F, Curcio G, Tempesta D, Pellicciari MC, Pirulli C, Ferrara M, Rossini PM. Cortical plasticity induced by transcranial magnetic stimulation during wakefulness affects electroencephalogram activity during sleep. PLoS ONE. 2008;3:e2483. doi: 10.1371/journal.pone.0002483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gennaro L, Marzano C, Veniero D, Moroni F, Fratello F, Curcio G, Ferrara M, Ferlazzo F, Novelli L, Concetta Pellicciari M, Bertini M, Rossini PM. Neurophysiological correlates of sleepiness: a combined TMS and EEG study. NeuroImage. 2007;36:1277–1287. doi: 10.1016/j.neuroimage.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Duckrow RB, Zaveri HP. Coherence of the electroencephalogram during the first sleep cycle. Clin Neurophysiol. 2005;116:1088–1095. doi: 10.1016/j.clinph.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Esser SK, Hill S, Tononi G. Sleep homeostasis and cortical synchronization: I. Modeling the effects of synaptic strength on sleep slow waves. Sleep. 2007;30:1617–1630. doi: 10.1093/sleep/30.12.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser SK, Huber R, Massimini M, Peterson MJ, Ferrarelli F, Tononi G. A direct demonstration of cortical LTP in humans: A combined TMS/EEG study. Brain Res Bull. 2006;69:86–94. doi: 10.1016/j.brainresbull.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Fagioli I. Mental activity during sleep. Sleep Med Rev. 2002;6:307–320. doi: 10.1053/smrv.2001.0214. [DOI] [PubMed] [Google Scholar]
- Faraguna U, Vyazovskiy VV, Nelson AB, Tononi G, Cirelli C. A causal role for brain-derived neurotrophic factor in the homeostatic regulation of sleep. J Neurosci. 2008;28:4088–4095. doi: 10.1523/JNEUROSCI.5510-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarelli F, Huber R, Peterson MJ, Massimini M, Murphy M, Riedner BA, Watson A, Bria P, Tononi G. Reduced sleep spindle activity in schizophrenia patients. The American journal of psychiatry. 2007;164:483–492. doi: 10.1176/ajp.2007.164.3.483. [DOI] [PubMed] [Google Scholar]
- Ghilardi MF, Ghez C, Dhawan V, Moeller J, Mentis M, Nakamura T, Antonini A, Eidelberg D. Patterns of regional brain activation associated with different forms of motor learning. Brain Res. 2000;871:127–145. doi: 10.1016/s0006-8993(00)02365-9. [DOI] [PubMed] [Google Scholar]
- He BJ, Snyder AZ, Zempel JM, Smyth MD, Raichle ME. Electrophysiological correlates of the brain's intrinsic large-scale functional architecture. Proc Natl Acad Sci U S A. 2008;105:16039–16044. doi: 10.1073/pnas.0807010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill S, Tononi G. Modeling sleep and wakefulness in the thalamocortical system. Journal of neurophysiology. 2005;93:1671–1698. doi: 10.1152/jn.00915.2004. [DOI] [PubMed] [Google Scholar]
- Huber R, Esser SK, Ferrarelli F, Massimini M, Peterson MJ, Tononi G. TMS-induced cortical potentiation during wakefulness locally increases slow wave activity during sleep. PLoS ONE. 2007a;2:e276. doi: 10.1371/journal.pone.0000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Ferrarelli F, Riedner BA, Peterson MJ, Tononi G. Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nature neuroscience. 2006;9:1169–1176. doi: 10.1038/nn1758. [DOI] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- Huber R, Maatta S, Esser SK, Sarasso S, Ferrarelli F, Watson A, Ferreri F, Peterson MJ, Tononi G. Measures of cortical plasticity after transcranial paired associative stimulation predict changes in electroencephalogram slow-wave activity during subsequent sleep. J Neurosci. 2008;28:7911–7918. doi: 10.1523/JNEUROSCI.1636-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R, Tononi G, Cirelli C. Exploratory behavior, cortical BDNF expression, and sleep homeostasis. Sleep. 2007b;30:129–139. doi: 10.1093/sleep/30.2.129. [DOI] [PubMed] [Google Scholar]
- Kakigi R, Naka D, Okusa T, Wang X, Inui K, Qiu Y, Tran TD, Miki K, Tamura Y, Nguyen TB, Watanabe S, Hoshiyama M. Sensory perception during sleep in humans: a magnetoencephalograhic study. Sleep medicine. 2003;4:493–507. doi: 10.1016/s1389-9457(03)00169-2. [DOI] [PubMed] [Google Scholar]
- Kattler H, Dijk D-J, Borbély AA. Effect of unilateral somatosensory stimulation prior to sleep on the sleep EEG in humans. J. Sleep Res. 1994;3:159–164. doi: 10.1111/j.1365-2869.1994.tb00123.x. [DOI] [PubMed] [Google Scholar]
- Llinas RR, Steriade M. Bursting of thalamic neurons and states of vigilance. Journal of neurophysiology. 2006;95:3297–3308. doi: 10.1152/jn.00166.2006. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation--a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Maquet P. The role of sleep in learning and memory. Science. 2001;294:1048–1052. doi: 10.1126/science.1062856. [DOI] [PubMed] [Google Scholar]
- Marshall L, Helgadottir H, Molle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- Massimini M, Ferrarelli F, Esser SK, Riedner BA, Huber R, Murphy M, Peterson MJ, Tononi G. Triggering sleep slow waves by transcranial magnetic stimulation. Proc Natl Acad Sci U S A. 2007;104:8496–8501. doi: 10.1073/pnas.0702495104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimini M, Ferrarelli F, Huber R, Esser SK, Singh H, Tononi G. Breakdown of cortical effective connectivity during sleep. Science. 2005;309:2228–2232. doi: 10.1126/science.1117256. [DOI] [PubMed] [Google Scholar]
- Massimini M, Huber R, Ferrarelli F, Hill S, Tononi G. The sleep slow oscillation as a traveling wave. J Neurosci. 2004;24:6862–6870. doi: 10.1523/JNEUROSCI.1318-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Bal T. Sleep and arousal: thalamocortical mechanisms. Annu Rev Neurosci. 1997;20:185–215. doi: 10.1146/annurev.neuro.20.1.185. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Pape HC. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J Physiol. 1990;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisello C, Bove M, Huber R, Abbruzzese G, Battaglia F, Tononi G, Ghilardi MF. Short-term limb immobilization affects motor performance. Journal of motor behavior. 2008;40:165–176. doi: 10.3200/JMBR.40.2.165-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen TA. Chronobiological features of dream production. Sleep Med Rev. 2004;8:403–424. doi: 10.1016/j.smrv.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Pivik T, Foulkes D. NREM mentation: relation to personality, orientation time, and time of night. Journal of consulting and clinical psychology. 1968;32:144–151. doi: 10.1037/h0025489. [DOI] [PubMed] [Google Scholar]
- Portas CM, Krakow K, Allen P, Josephs O, Armony JL, Frith CD. Auditory processing across the sleep-wake cycle: simultaneous EEG and fMRI monitoring in humans. Neuron. 2000;28:991–999. doi: 10.1016/s0896-6273(00)00169-0. [DOI] [PubMed] [Google Scholar]
- Riedner BA, Vyazovskiy VV, Huber R, Massimini M, Esser S, Murphy M, Tononi G. Sleep homeostasis and cortical synchronization: III. A high-density EEG study of sleep slow waves in humans. Sleep. 2007;30:1643–1657. doi: 10.1093/sleep/30.12.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nature neuroscience. 2000;3:1027–1034. doi: 10.1038/79848. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Peigneux P, Muto V, Schenkel M, Knoblauch V, Munch M, de Quervain DJ, Wirz-Justice A, Cajochen C. Encoding difficulty promotes postlearning changes in sleep spindle activity during napping. J Neurosci. 2006;26:8976–8982. doi: 10.1523/JNEUROSCI.2464-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287:1834–1837. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123(Pt 3):572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- Steriade M. Corticothalamic resonance, states of vigilance and mentation. Neuroscience. 2000;101:243–276. doi: 10.1016/s0306-4522(00)00353-5. [DOI] [PubMed] [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. Journal of neurophysiology. 2001;85:1969–1985. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272–1278. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- Stickgold R, Malia A, Fosse R, Propper R, Hobson JA. Brain-mind states: I. Longitudinal field study of sleep/wake factors influencing mentation report length. Sleep. 2001;24:171–179. doi: 10.1093/sleep/24.2.171. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Uchiyama M, Tagaya H, Ozaki A, Kuriyama K, Aritake S, Shibui K, Tan X, Kamei Y, Kuga R. Dreaming during non-rapid eye movement sleep in the absence of prior rapid eye movement sleep. Sleep. 2004;27:1486–1490. doi: 10.1093/sleep/27.8.1486. [DOI] [PubMed] [Google Scholar]
- Tobler I. Phylogeny of sleep regulation. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. Saunders; Philadelphia: 2000. pp. 72–81. [Google Scholar]
- Tononi G. An information integration theory of consciousness. BMC neuroscience. 2004;5:42. doi: 10.1186/1471-2202-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res. Bull. 2003;62:143–150. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med. Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Vanderwolf CH. Are neocortical gamma waves related to consciousness? Brain Res. 2000;855:217–224. doi: 10.1016/s0006-8993(99)02351-3. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy V, Borbély AA, Tobler I. Unilateral vibrissae stimulation during waking induces interhemispheric EEG asymmetry during subsequent sleep in the rat. J. Sleep Res. 2000;9:367–371. doi: 10.1046/j.1365-2869.2000.00230.x. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nature neuroscience. 2008;11:200–208. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Riedner BA, Cirelli C, Tononi G. Sleep homeostasis and cortical synchronization: II. A local field potential study of sleep slow waves in the rat. Sleep. 2007;30:1631–1642. doi: 10.1093/sleep/30.12.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Wolters A, Schmidt A, Schramm A, Zeller D, Naumann M, Kunesch E, Benecke R, Reiners K, Classen J. Timing-dependent plasticity in human primary somatosensory cortex. J Physiol. 2005;565:1039–1052. doi: 10.1113/jphysiol.2005.084954. [DOI] [PMC free article] [PubMed] [Google Scholar]