Abstract
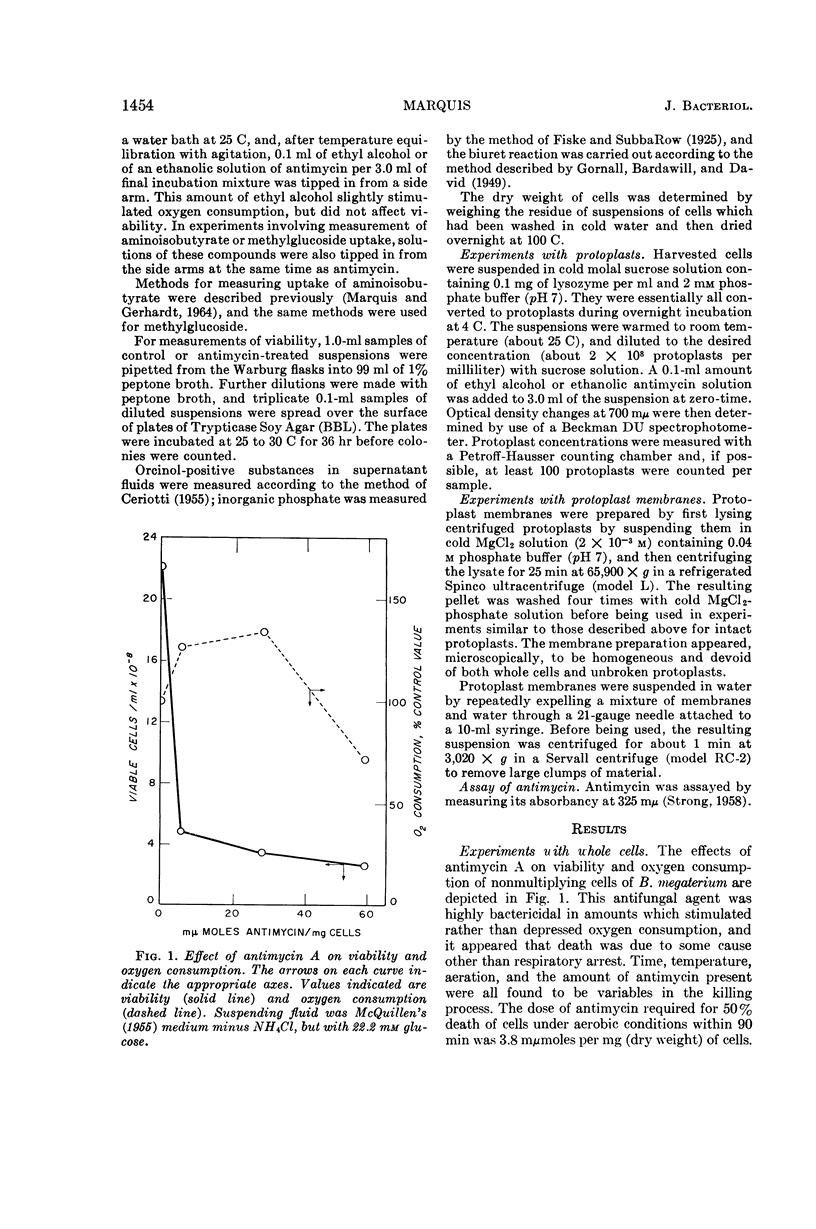
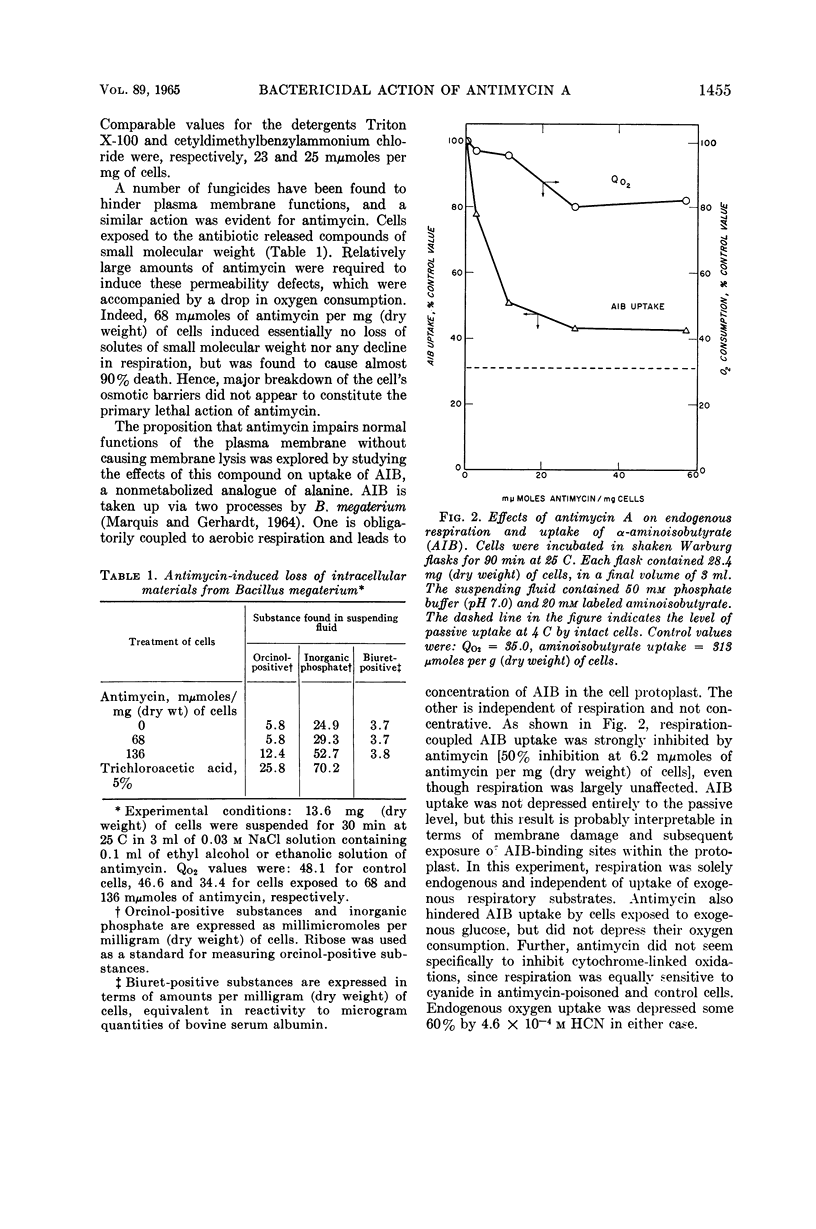
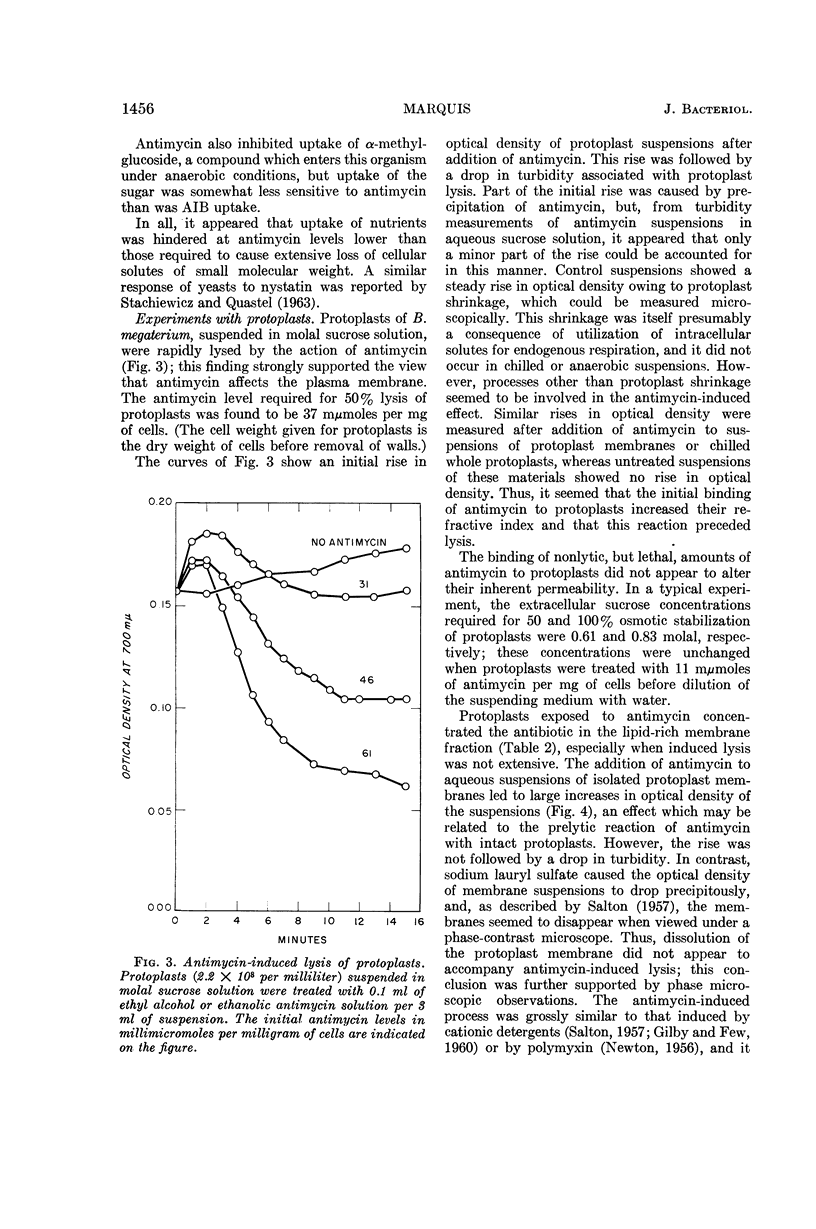
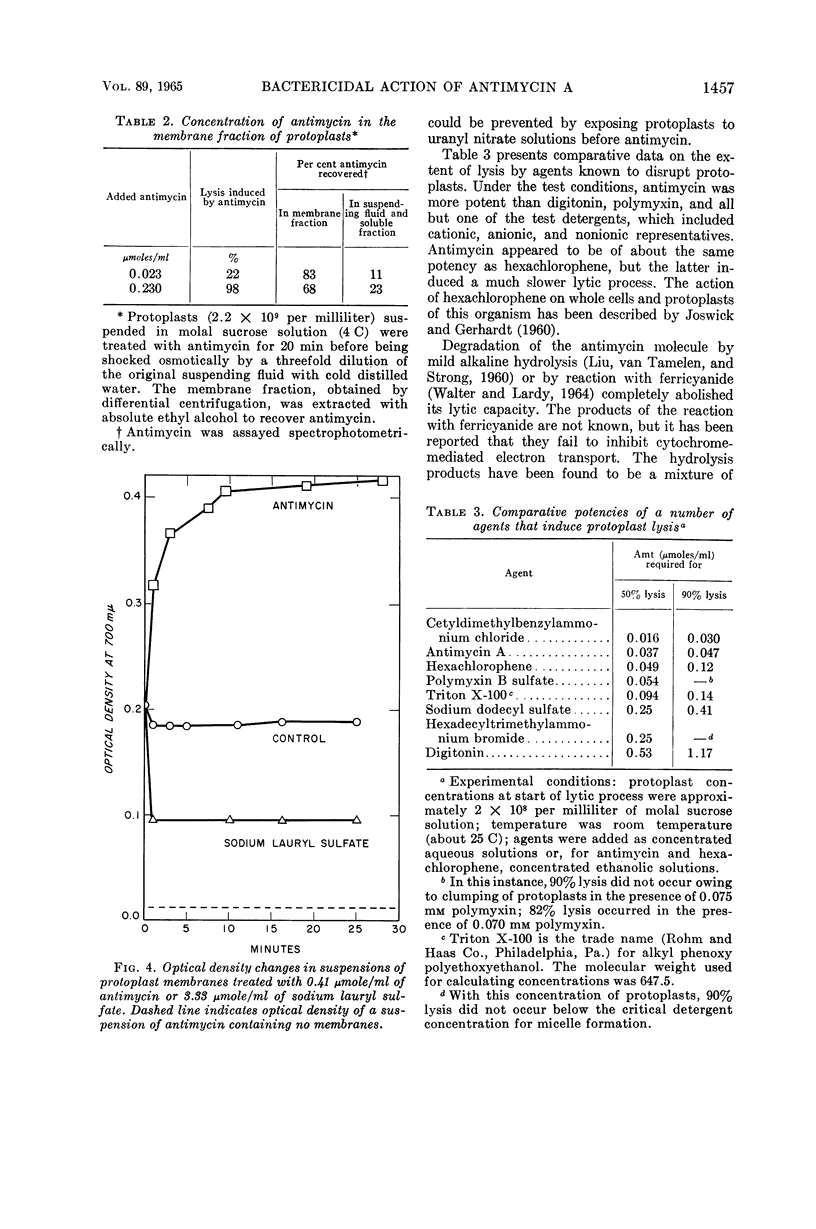
Marquis, Robert E. (University of Rochester, Rochester, N.Y.). Nature of the bactericidal action of antimycin A for Bacillus megaterium. J. Bacteriol. 89:1453–1459. 1965.—Antimycin A, a fungicidal antibiotic which specifically inhibits metabolic reduction of cytochrome c, was found to be lethal for Bacillus megaterium. However, the bactericidal action was correlated with a capacity of antimycin to hinder plasma-membrane functions other than cytochrome-mediated respiration. With conditions under which oxygen consumption was not appreciably depressed, antimycin almost completely inhibited concentrative uptake of both α-aminoisobutyrate and α-methylglucoside, and also caused death of cells. When present in amounts greater than those required for killing or for inhibition of nutrilite uptake, antimycin also induced extensive loss of inorganic phosphate and other substances from whole cells, inhibited aerobic respiration, and acted as a lytic agent for isolated protoplasts. The lytic potency of antimycin was greater, on a molar basis, than that of digitonin, hexachlorophene, polymyxin B, and all but one of a number of test detergents. Protoplasts concentrated antimycin primarily in or on the plasma membrane, and the refractive index of isolated protoplast membranes rose sharply as a result of antimycin binding. In all, antimycin-induced lysis appeared not to include dissolution of the protoplast membrane similar to that produced by dodecyl sulfate. Rather, the lytic process seemed more akin to that induced by cationic detergents or by polymyxin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CERIOTTI G. Determination of nucleic acids in animal tissues. J Biol Chem. 1955 May;214(1):59–70. [PubMed] [Google Scholar]
- GILBY A. R., FEW A. V. Lysis of protoplasts of Micrococcus lysodeikticus by ionic detergents. J Gen Microbiol. 1960 Aug;23:19–26. doi: 10.1099/00221287-23-1-19. [DOI] [PubMed] [Google Scholar]
- KINSKY S. C. COMPARATIVE RESPONSES OF MAMMALIAN ERYTHROCYTES AND MICROBIAL PROTOPLASTS TO POLYENE ANTIBIOTICS AND VITAMIN A. Arch Biochem Biophys. 1963 Aug;102:180–188. doi: 10.1016/0003-9861(63)90169-3. [DOI] [PubMed] [Google Scholar]
- KINSKY S. C. Nystatin binding by protoplasts and a particulate fraction of Neurospora crassa, and a basis for the selective toxicity of polyene antifungal antibiotics. Proc Natl Acad Sci U S A. 1962 Jun 15;48:1049–1056. doi: 10.1073/pnas.48.6.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARQUIS R. E., GERHARDT P. RESPIRATION-COUPLED AND PASSIVE UPTAKE OF ALPHA-AMINOISOBUTYRIC ACID, A METABOLICALLY INERT TRANSPORT ANALOGUE, BY BACILLUS MEGATERIUM. J Biol Chem. 1964 Oct;239:3361–3371. [PubMed] [Google Scholar]
- McQUILLEN K. Bacterial protoplasts. I. Protein and nucleic acid metabolism in protoplasts of Bacillus megaterium. Biochim Biophys Acta. 1955 Jul;17(3):382–390. doi: 10.1016/0006-3002(55)90387-5. [DOI] [PubMed] [Google Scholar]
- NEWTON B. A. The properties and mode of action of the polymyxins. Bacteriol Rev. 1956 Mar;20(1):14–27. doi: 10.1128/br.20.1.14-27.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMACHANDRAN S., GOTTLIEB D. Mode of action of antibiotics. II. Specificity of action of antimycin A and ascosin. Biochim Biophys Acta. 1961 Oct 28;53:396–402. doi: 10.1016/0006-3002(61)90451-6. [DOI] [PubMed] [Google Scholar]
- REIF A. E. Succinoxidase inhibition studies. II. Mechanism of the binding of a naphthoquinone and of antimycin A by serum albumin. Arch Biochem Biophys. 1953 Dec;47(2):396–407. doi: 10.1016/0003-9861(53)90476-7. [DOI] [PubMed] [Google Scholar]
- SHOCKMAN G. D., LAMPEN J. O. Inhibition by antibiotics of the growth of bacterial and yeast protoplasts. J Bacteriol. 1962 Sep;84:508–512. doi: 10.1128/jb.84.3.508-512.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STACHIEWICZ E., QUASTEL J. H. Amino acid transport in yeast and effects of nystatin. Can J Biochem Physiol. 1963 Feb;41:397–407. [PubMed] [Google Scholar]
- SZULMAJSTER J. BIOCHIMIE DE LA SPOROG'EN'ESE CHEZ B. SUBTILIS. Bull Soc Chim Biol (Paris) 1964;46:443–481. [PubMed] [Google Scholar]
- VERNON L. P., MANGUM J. H. Cytochromes of Bacillus megaterium and Bacillus subtilis. Arch Biochem Biophys. 1960 Sep;90:103–104. doi: 10.1016/0003-9861(60)90618-4. [DOI] [PubMed] [Google Scholar]
- WALTER P., LARDY H. A. EFFECT OF ANTIMYCIN A ON OXIDATIVE PHOSPHORYLATION WITH FERRICYANIDE AS ELECTRON ACCEPTOR. Biochemistry. 1964 Jun;3:812–816. doi: 10.1021/bi00894a015. [DOI] [PubMed] [Google Scholar]
- WEIBULL C., BERGSTROM L. The chemical nature of the cytoplasmic membrane and cell wall of Bacillus megaterium, strain M. Biochim Biophys Acta. 1958 Nov;30(2):340–351. doi: 10.1016/0006-3002(58)90059-3. [DOI] [PubMed] [Google Scholar]



