Abstract
Recent increases in marijuana smoking among the young adult population have been accompanied by the popularization of smoking marijuana as blunts instead of as joints. Blunts consist of marijuana wrapped in tobacco leaves, whereas joints consist of marijuana wrapped in cigarette paper. To date, the effects of marijuana smoked as joints and blunts have not been systematically compared. The current within-subject, randomized, double-blind, placebo-controlled study sought to directly compare the subjective, physiologic, and pharmacokinetic effects of marijuana smoked by these two methods. Marijuana blunt smokers (12 women; 12 men) were recruited and participated in a 6-session outpatient study. Participants were blindfolded and smoked three puffs from either a blunt or a joint containing marijuana with varying delta-9-tetrahydrocannabinol (THC) concentrations (0.0, 1.8, and 3.6%). Subjective, physiological (heart rate, blood pressure, carbon monoxide levels) and pharmacokinetic effects (plasma THC concentration) were monitored before and at specified time points for three hours after smoking. Joints produced greater increases in plasma THC and subjective ratings of marijuana intoxication, strength, and quality compared to blunts, and these effects were more pronounced in women compared to men. However, blunts produced equivalent increases in heart rate and higher carbon monoxide levels than joints, despite producing lower levels of plasma THC. These findings demonstrate that smoking marijuana in a tobacco leaf may increase the risks of marijuana use by enhancing carbon monoxide exposure and increasing heart rate compared to joints.
Keywords: Marijuana, THC, Joints, Blunt, Tobacco
1. INTRODUCTION
The rapid increase in marijuana smoking in the 1990’s (Compton et al., 2004) was accompanied by the popularization of blunts in contrast to more traditional methods, including joints and pipes. Joints consist of marijuana rolled in standard cigarette paper, whereas blunts are made by removing the tobacco from a cigar and replacing it with marijuana (Golub and Johnson, 1999; Soldz et al., 2003; Golub et al., 2005; Sifaneck et al., 2005; Ream et al., 2008). The cigar paper in which the marijuana is wrapped contains tobacco and nicotine that may interact with the cardiovascular and subjective effects of marijuana, an effect observed with combinations of transdermal nicotine patches and smoked marijuana (Penetar et al., 2005). Blunts may therefore produce a different set of effects and risks than marijuana smoked in cigarette paper,
Recent evidence suggests nicotinic contribution to the reinforcing, rewarding, anxiolytic, and physiologic effects of agonists that act at the CB1 receptor. Specifically, in rodents, nicotine has been shown to potentiate behavioral effects of THC on measures of locomotor activity, analgesia, and anxiety (Balerio et al., 2006; Valijent et al., 2002). The physiological effects of CB1 agonists are enhanced by nicotine (Valijent et al., 2002), as are the discriminative effects of THC (Solinas et al., 2007). Nicotine and nicotinic agonists have also been shown to increase the hypothesized positive affective components of CB1 agonists in rodents as demonstrated by enhanced conditioned place preference for a THC-paired environment as compared with THC alone (Valijent et al., 2002).
Combining tobacco with marijuana is a popular method of smoking in both North America and Europe; including methods of smoking that involve adding tobacco to joints (spliffs), ‘chasing’ marijuana with tobacco (smoking tobacco immediately after marijuana) (Ream et al., 2008), and blunts (Kelly, 2005). Anecdotally, blunt smoking is thought to produce greater intoxicating effects than joint smoking (Soldz et al., 2003). However, no studies to date have systematically investigated the effect of the tobacco leaf on marijuana’s subjective and physiologic effects.
The described within-subject, randomized, placebo-controlled study directly compared the subjective, physiologic, and pharmacokinetic effects of marijuana smoked as blunts compared to identical quantities of marijuana smoked in cigarette paper. Nontreatment seeking blunt smokers were recruited to take part in six 4-hour outpatient sessions. Volunteers were blindfolded and smoked a marijuana cigarette or blunt containing an equivalent quantity and strength of marijuana in a cigarette holder so that they could not feel differences between blunt and joint paper. Participants smoked according to verbal cues controlling the duration of inhalation and the amount of time the smoke was held in the lungs. Subjective measures, physiologic parameters (heart rate, blood pressure, expired carbon monoxide) and plasma levels of THC and nicotine were repeatedly measured before and after smoking.
2. METHODS
2.1. Participants
Normal, healthy volunteers ages 21–45 were recruited through newspaper advertisements and those who met inclusion/exclusion criteria after an initial phone screen were invited to the laboratory for further screening. Prior to enrollment, participants gave written informed consent, received a psychiatric and medical evaluation, and provided a detailed drug use and medical history. A total of 35 people signed consent and 24 participants completed the study. Twelve women (6 Black, 4 Hispanic, 2 mixed/other) average age of 25 ± 1 years, and twelve men (8 Black, 1 Hispanic, 1 White, 2 mixed/other) average age of 26 ± 3 years completed the study. Women reported smoking marijuana on average 6 ± 1.5 days a week, 2.3 ± 2 blunts per day and men reported smoking 6 ± 1.6 days a week, 1.6 ± 0.9 blunts per day. Seven women and ten men reported drinking alcohol weekly (2.6 ± 2.0 and 3.1 ± 2.0 days per week with 2.7 ± 1.4 and 2.9 ± 1.6 drinks per occasion, respectively). Four women and four men reported daily cigarette smoking (7.3 ± 2.5 and 5.3 ± 2.9 cigarettes per day respectively), although participants were not allowed to smoke during the sessions. An additional three participants completed the study, but their data were not used because their plasma THC levels were negligible under all THC concentration conditions, indicating that these participants did not inhale during the smoking procedure. Eight other participants were enrolled in the study but did not complete the study. Of these, blood was not able to be drawn from two, two were unreliable (arrived late or not at all for sessions), one reported a desire to stop smoking marijuana, one became pregnant, one did not follow the smoking procedure, and one became paranoid after marijuana smoking.
Participants were accepted into the study if they were healthy, as determined by a physical examination, electrocardiogram, and urine and blood chemistries. All participants had to currently smoke marijuana blunts at least twice a week as determined by urine toxicology and self-report. Participants were excluded if they used other drugs, with the exception of nicotine, alcohol, or caffeine as determined by urine toxicology and self-report. Those who reported heavy cigarette use (more than 10 cigarettes per day) were excluded in order to better assess changes in carbon monoxide levels due to laboratory smoking. Those who met Diagnostic and Statistical Manual (of Mental Disorders), fourth edition revised criteria for current or past Axis I psychopathology were excluded from the study. Women were excluded if they were pregnant or nursing. Volunteers were instructed that the study objective was to compare the effects of marijuana smoked in ‘blunts’ versus marijuana smoked in ‘joints’ and that they would receive both active and inactive marijuana during the study. Participants were admitted into the study only after written informed consent to participate was given. All study procedures were approved by the Institutional Review Board of the New York State Psychiatric Institute.
2.2. Drug
For experimental sessions, participants received either a marijuana blunt (0%, 1.8, 3.6% THC) or marijuana cigarette (0%, 1.8, 3.6% THC). Marijuana cigarettes were provided by the National Institute on Drug Abuse. Blunts were fabricated by cutting the bottom third off a Dutch Master® cigar, removing all of the cigar tobacco, and replacing it with all of the marijuana contained in a NIDA marijuana cigarette (ca. 800 mg). The order of dosing was randomized.
2.3. Study Design and General Procedure
A within-subject design was used in which all participants smoked all strengths of marijuana as joints and blunts on separate sessions. Primary dependent variables included subjective, pharmacokinetic, and physiologic effects of marijuana smoked as either blunts or joints. A total of 6 sessions were run over 2–5 weeks and doses and preparations (blunts and joints) were randomized across sessions. Sessions were separated by at least 48 hours, began at around 9 AM, were about 4 hours in duration, and took place at the New York State Psychiatric Institute. Data collected included subjective effects as assessed with two visual analog scales; the Marijuana Rating Form (MRF), a scale intended to assess the participants’ subjective ratings of the quality and strength of the marijuana, and a 50-item visual analog scale (VAS) measuring subjective mood and physical symptoms of the drug effect (see Haney et al., 2003 for description). Both subjective rating scales were visual analog scales, where participants indicated how they were feeling on a 100-mm line anchored with ‘not at all’ at the left end and ‘extremely’ at the right end, when prompted by a statement. Plasma THC and nicotine levels were analyzed to determine pharmacokinetic differences between the two preparations. Plasma nicotine levels were only obtained in the initial set of volunteers because nicotine was undetectable in 162 samples, regardless of smoking method or marijuana strength. To determine if marijuana strength or smoking method affected smoking behavior (i.e., inhalation), carbon monoxide levels were measured using a Bedfont Micro Smokerlyzer (Bedfont Scientific Ltd., Rochester, England) a breathalyzer that was calibrated regularly according to the manufacturers specifications. Expired carbon monoxide levels increase in direct proportion to the amount of marijuana smoked (Azorlosa et al., 1992). In addition, the amount of marijuana smoked as a function of strength and preparation was also assessed by weighing the marijuana after smoking. Heart rate and blood pressure were also monitored throughout the session to assess physiologic effects of marijuana.
2.4. Session Protocol
Participants were instructed to not eat breakfast prior to each session and to refrain from drinking alcohol 24 hours prior to the session. Participants were also told to not smoke marijuana or cigarettes after midnight the night before each session. Upon arrival to the laboratory, carbon monoxide levels were measured to confirm no recent smoking, breath alcohol levels were assessed, and use of illicit drugs other than marijuana was determined by a urine toxicology screen. If carbon monoxide levels indicated that the participant had smoked marijuana or a cigarette prior to arrival (5 ppm or higher) the session did not proceed and the volunteer was sent home. Pregnancy tests were also run before the first and fifth session for female participants. A standard breakfast was provided prior to the session, followed by insertion of a 20 gauge venous catheter (Quik-Cath®; Treavenol Laboratories, Deerfield IL, USA) into the arm for repeated blood withdrawal (6 mLs at each time point).
Before marijuana administration, the subjective-effects questionnaires were completed, heart rate and blood pressure were measured using a Sentry II vital signs monitor (Model 6100: NBS Medical Services, Costa Mesa CA), and a baseline balancing task was completed. Blood was drawn (6 mLs) about 30 minutes prior to and 3, 6, 10, 15, 30, 60, 120, and 180 minutes after marijuana administration, centrifuged, and plasma was removed then decanted into a polystyrene tube and stored in a freezer (−30° C) and later analyzed by the Department of Behavioral Endocrinology of the New York State Psychiatric Institute. Levels of Δ9 -THC were quantified using capillary gas chromatography-mass spectroscopy (GC/MS) with a procedure that utilized the negative chemical ionization of the derivitized compounds and deuterated internal standards with selected ion monitoring and methane/ammonia as the reactant gas. The between-run imprecision of the assay was approximately 8% at 7.5 ng/mL THC. Plasma nicotine was quantified by gas chromatography fitted with a nitrogen-phosphorous detector using an alkaline solvent extraction and N-ethyl-nornicotine as an internal standard (limit of nicotine detection, 0.5 ng/mL; limit of nicotine quantification, 1 ng/mL) (Davis, 1986). Subjective measures and vitals (heart rate and blood pressure) were assessed 15, 30, 45, 60, 90, 120, 150, and 180 minutes after marijuana administration. Carbon monoxide levels were measured 10, 30, 60, and 180 minutes after smoking. Timing of each measurement was scheduled to capture the full timecourse of marijuana’s effects and to allow for consistent intervals between each event. At the end of each session (about 3 hours after smoking) participants were free to go home once sobriety was determined using field sobriety and balancing tasks.
2.5. Marijuana Administration
Joints and blunts were given to blindfolded participants in plastic cigarette holders in order to block visual and tactile cues associated with each preparation. Both joints and blunts were stored frozen in an airtight container and humidified at room temperature for 24h prior to the session. During the smoking procedure, the experimenter held the cigarette and instructed participants to ‘inhale’ (5s), ‘hold smoke in lungs’ (10s) and ‘exhale’. Participants smoked three puffs, with a 40 second interval between puffs. At the end of the session, participants were asked to judge whether they had received a blunt or a joint.
2.6. Data Analysis
To determine if participants were able to differentiate between marijuana smoked as blunts or joints, percentage of correct guesses at the end of the session were calculated according to strength of marijuana and preparation. Standard error for the percentages were calculated as the square-root of the expected probability (p = 1) multiplied by p minus the proportion of correct guesses (q) divided by the total number of guesses (n) (refer to Moore and McCabe, 1993). Repeated measures analyses of variance (ANOVA) with planned comparisons were implemented to compare marijuana smoked as blunts and joints for each marijuana strength condition and to compare active doses to placebo marijuana. For all dependent variables, values were averaged across session time points (excluding pre-dosing/baseline values). Dependent variables included subjective measures, as assessed with the VAS and MRF scales, heart rate, blood pressure, plasma-THC levels, and carbon monoxide levels. Blunts and joints were weighed after smoking to determine how much marijuana was smoked under each condition. Because the cigar paper used for the blunts was heavier than the cigarette paper used for the joints, weights of blunts and joints containing active marijuana were analyzed as percent of weights of blunts and joints containing placebo marijuana. Repeated measures ANOVA with planned comparisons were implemented to compare weights of blunts and joints after smoking to see if there was an effect of the smoking preparation on the amount of marijuana smoked. Post-smoking weights of blunts and joints from two participants (one male and one female) were excluded due to missing data. In addition to comparisons determined according to the entire group, the effects of marijuana strength and smoking method were characterized separately for men and women; the sample-size in the current study did not afford enough statistical power to determine a between-subject comparison of effects in men and women. Results were considered statistically significant when p values were equal to or less than 0.05 using Huynh-Feldt corrections.
3. RESULTS
Participants were not able to reliably detect whether they had received a blunt or joint across sessions, regardless of strength of marijuana smoked. Participants smoking all strengths of marijuana as blunts correctly guessed the preparation 46 ± 15% of the time. When marijuana was smoked as joints, participants guessed correctly 46 ± 15%, 67 ± 12%, and 54 ± 14% of the time when placebo, 1.8%, and 3.6% marijuana was administered, respectively.
3.1. Plasma THC levels
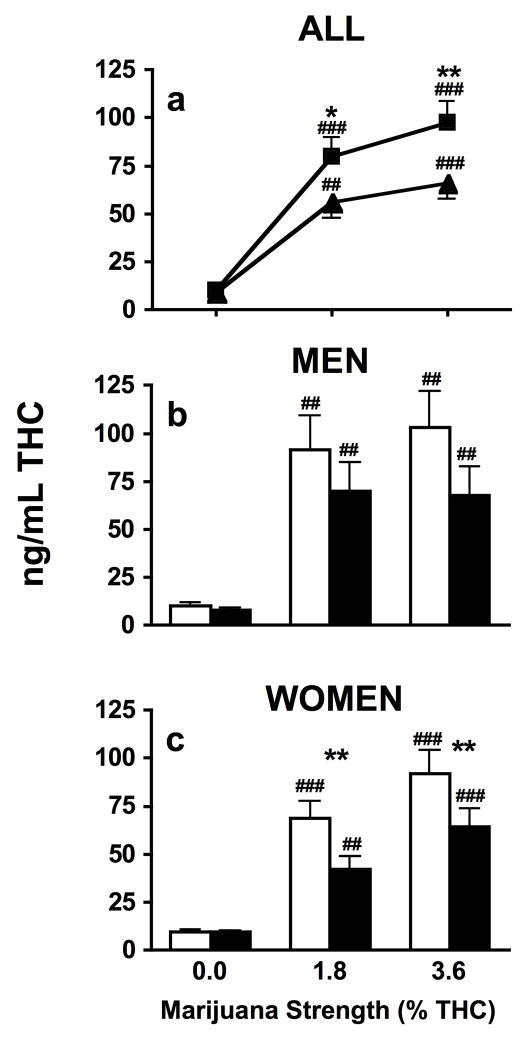
Average baseline plasma THC levels were 13.9 ± 0.4 ng/mL, with plasma baseline THC levels of 12.4 ± 0.49 ng/mL for men and 15.4 ± 0.43 ng/mL for women. Figure 1, which portrays average plasma THC levels across all time points as a function of marijuana strength and preparation, shows both active strengths of marijuana produced greater plasma THC levels than inactive marijuana for both smoking methods (p ≤ 0.01). THC plasma levels were significantly higher when active marijuana was smoked as joints compared to blunts (p ≤ 0.05). Analyzing the data according to sex revealed no significant difference in plasma THC levels according to smoking method for any marijuana strength among men. However, among women joints increased plasma THC levels for both active strengths of marijuana compared to blunts (p ≤ 0.01).
Figure 1.
Plasma THC levels when marijuana was smoked as joints and blunts averaged across all post-smoking time points analyzed for all participants (panel a) (joints, ν; blunts, σ), and men (panel b) and women (panel c) analyzed separately (joints, □; blunts, ■). Significant differences between joints and blunts indicated as follows: *, p ≤ 0.05; **, p ≤ 0.01. Significant differences between placebo and active marijuana is indicated as follows: #, p ≤ 0.05; ##, p ≤ 0.01.
3.2. Subjective Effects
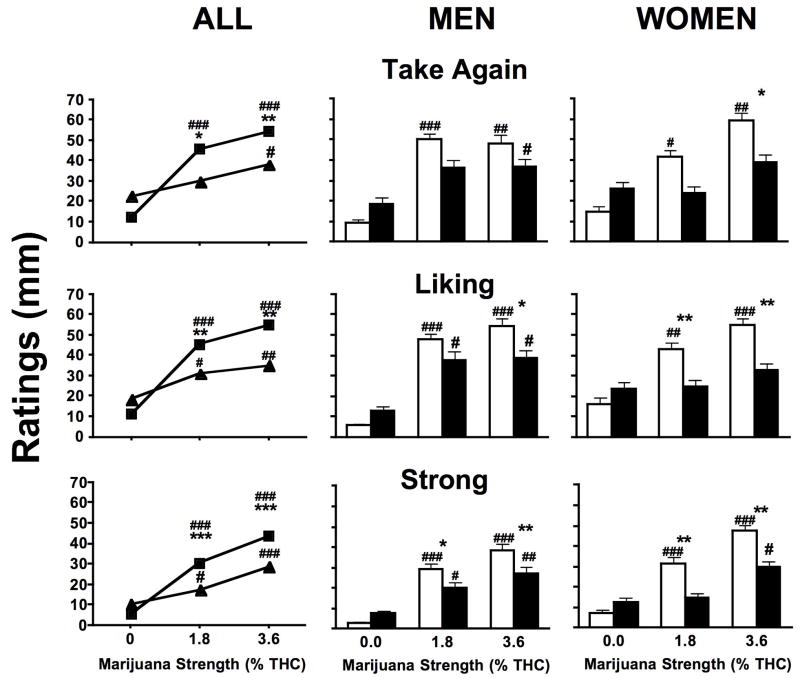
Figure 2 portrays MRF ratings of ‘Take Again’, ‘Liking’, and ‘Strong’ as a function of marijuana strength and smoking method for the whole sample and separately for men and women. Both active strengths of marijuana increased ratings of these subjective effects compared to placebo for joints (p ≤ 0.0001), and the highest strength of marijuana increased ratings of all these subjective effects when smoked as blunts (p ≤ 0.05). Joints produced higher ratings of ‘Take Again’ (p ≤ 0.01), ‘Liking’ (p ≤ 0.01), and ‘Strong’ (p ≤ 0.0001) than blunts when active marijuana was smoked. Independent analyses of men and women revealed that men reported joints to increase ratings of ‘Liking’ (p ≤ 0.05) for the highest marijuana strength and ‘Strong’ for both active strengths compared to blunts (p ≤ 0.05). Among women, joints increased ratings of ‘Take Again’ (p ≤ 0.05) for the highest marijuana strength and ‘Liking’ (p ≤ 0.01) and ‘Strong’ (p ≤ 0.01) for both active strengths compared to blunts.
Figure 2.
Subjective MRF ratings of ‘Take Again’, ‘Liking’, and ‘Strong’ averaged across all post-smoking time points when marijuana was smoked as joints and blunts analyzed for all participants (joints, ν; blunts, σ), and for men and women analyzed separately (joints, □; blunts, ■). Significant differences between joints and blunts indicated as follows: *, p ≤0.05; **, p ≤ 0.01; ***, p ≤ 0.001. Standard error of the means are smaller than some of symbols depicting averages and are therefore not visible in all figures. Significant differences between placebo and active marijuana is indicated as follows: #, p ≤ 0.05; ##, p ≤ 0.01; ###, p ≤ 0.001.
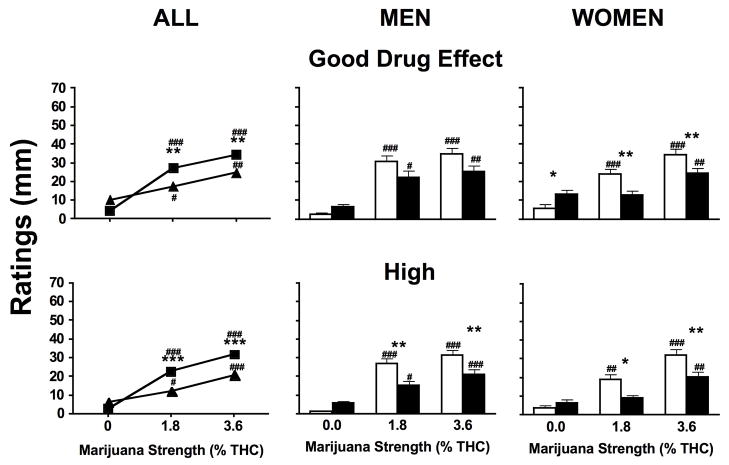
Figure 3 portrays subjective ratings as a function of marijuana strength and preparation for ‘Good Drug Effect’ and ‘High’ for the whole sample and separately for men and women. Both active strengths of marijuana increased ratings of these subjective effects compared to placebo for both joints and blunts (p ≤ 0.05). When active marijuana was smoked as joints ratings of ‘Good Drug Effect’ (p ≤ 0.01) and ‘High’ (p ≤ 0.0001) were greater compared to when active marijuana was smoked as blunts. Among men, joints increased ratings of ‘High’ (p ≤ 0.01) for both active strengths of marijuana compared to blunts. Among women, joints increased subjective ratings of ‘Good Drug Effect’ and ‘High’ for both active strengths of marijuana compared to blunts (p ≤ 0.05). However, blunts increased subjective ratings of ‘Good Drug Effect’ when inactive marijuana was smoked compared to joints (p ≤ 0.05)
Figure 3.
Subjective VAS ratings of ‘Good Drug Effect’ and ‘High’ averaged across all post-smoking time points when marijuana was smoked as joints and blunts analyzed for all participants (joints, ν; blunts, σ), and men and women analyzed separately (joints, □; blunts, ■). Significant differences between joints and blunts indicated as follows: *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001. Significant differences between placebo and active marijuana is indicated as follows: #, p ≤ 0.05; ##, p ≤ 0.01; ###, p ≤ 0.001.
3.3. Heart Rate, Carbon Monoxide, and Marijuana Weight
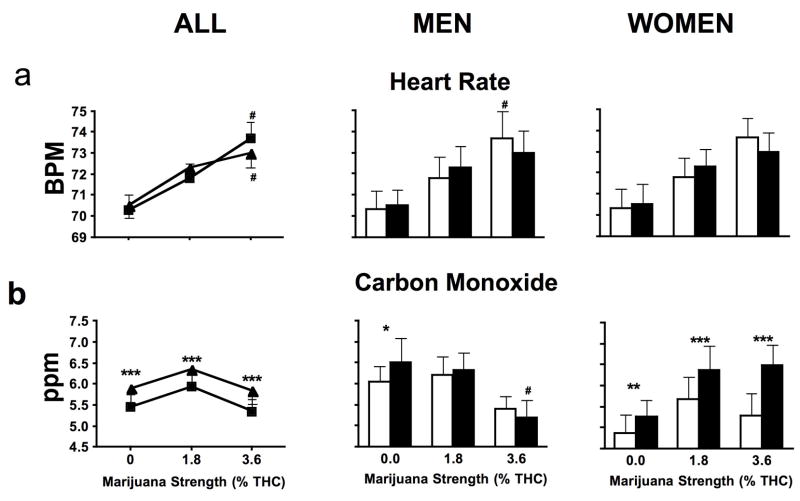
Figure 4 illustrates the effects of marijuana smoked as blunts and joints on heart rate and expired carbon monoxide. The highest strength of marijuana increased heart rate compared to inactive marijuana for both joints and blunts (p ≤ 0.05). However no statistical difference in smoked marijuana’s cardiovascular effects was observed between the two smoking preparations (Figure 4a). Greater expired carbon monoxide levels were detected when blunts were smoked compared to joints for all marijuana strengths tested (p ≤ 0.01) (Figure 4b). Among men, higher carbon monoxide levels were detected after inactive marijuana was smoked as blunts compared to joints (p ≤ 0.05), whereas women demonstrated greater expired carbon monoxide levels for all strengths of marijuana smoked as blunts compared to joints (p ≤ 0.01).
Figure 4.
Heart rate (panel a) and expired carbon monoxide (panel b) averaged across all post-smoking time points when marijuana was smoked as joints and blunts for all participants (joints, ν; blunts, σ), and men and women analyzed separately (joints, □; blunts, ■). Significant differences between joints and blunts indicated as follows: *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001. Significant differences between placebo and active marijuana is indicated as follows: #, p ≤ 0.05.
Figure 5 depicts the difference in blunt and joint weights after smoking according to smoking method and marijuana strength. Post-smoke weights from blunts and joints containing active marijuana were analyzed as proportion of post-smoke weight from the placebo blunts and joints in order to take into account the differences in the weight of the cigarette and cigar paper (refer to 2.6. Data Analysis). For the highest strength of marijuana a greater proportion of the blunts was smoked compared to the joints demonstrated by proportionally heavier post-smoking weights of the joints compared to blunts (p ≤ 0.05). Independent analyses according to sex demonstrated that there were no differences in weights of blunts and joint among men, while women smoked a greater proportion of the blunts compared to joints for the highest marijuana strength, as indicated by heavier post-smoke weights of joints compared to blunts (p ≤ 0.01).
Figure 5.
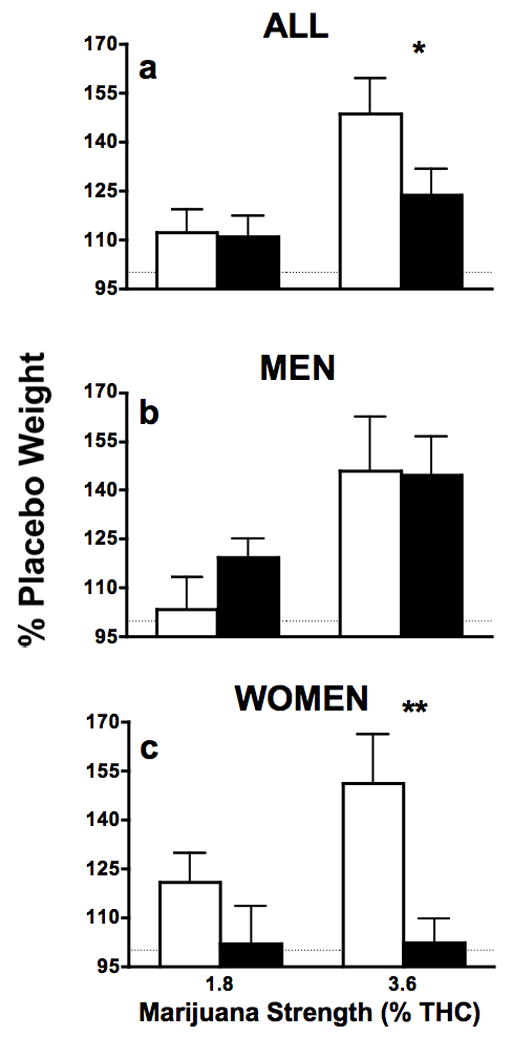
Weights of blunts and joints after smoking depicted as percent of placebo post-smoking weight for all participants, and men and women analyzed separately (joints, □; blunts, ■). Significant differences between joints and blunts indicated as follows: *, p ≤ 0.05; **, p ≤ 0.01.
4. DISCUSSION
The results reported herein demonstrate that under double-blind conditions, marijuana produces a distinct pattern of effects when smoked as blunts as compared to joints. Blindfolded participants were not able to differentiate whether they were smoking blunts or joints, demonstrating that there were no olfactory, tactile, or gustatory cues that reliably distinguished the two preparations, thereby minimizing the potential influence of expectancy effects. Under the current experimental conditions, marijuana smoked as joints produced greater increases in plasma THC and intoxicating effects compared to marijuana smoked as blunts. Despite differences in THC plasma levels afforded by the two preparations, active marijuana smoked as blunts produced equivalent increases in heart rate and greater increases in carbon monoxide levels than active marijuana smoked in joints. The likely explanation for the lower THC levels with the blunts is procedural: To decrease the tactile cues associated with the two preparations, blunts and joints were placed in cigarette holders, yet the cigar paper was thicker than the cigarette paper, making it more difficult to inhale the marijuana in the blunts than the marijuana in the joints.
Consistent with an earlier report (Heishman et al., 1989), there was evidence that participants titrated their marijuana exposure: participants smoked less as the marijuana potency increased. Thus, post-smoking weights of the joints and blunts containing the highest strength of marijuana were greater than those containing the lower strengths of marijuana. Despite this dose-related titration, THC plasma levels still increased as a function of marijuana strength and were accompanied by increases in subjective ratings of marijuana intoxication. The lawful relationship between plasma THC levels and subjective ratings of intoxication is also reflected in the highest strength of marijuana smoked as joints and blunts. When smoked as joints, the highest marijuana strength afforded plasma THC levels that were almost two-times higher than when smoked as blunts (100 ng/mL vs. 50 ng/mL), the same magnitude of difference in the subjective ratings of marijuana intoxication observed under these conditions. These findings are consistent with previous reports demonstrating that subjective ratings of marijuana and dronabinol vary as a function of THC plasma levels and are dependent upon both dose and time after smoking and dronabinol administration (Haney et al., 2003; Wachtel et al., 2002; Heishman et al., 1990).
In the past, heart rate has been reported to reliably increase as a function of cannabis strength (Haney, 2007; Haney et al., 2005; Hart et al., 2005; McDonald et al., 2003), and to be a predictor of marijuana intoxication. However, in the current study, differences in THC plasma levels and subjective ratings of intoxication were observed between the two preparations, whereas both methods produced similar increases in heart rate. The cigar leaf that was used to construct the blunt did not provide detectable levels of plasma nicotine, so it is unlikely that nicotine contributed to the cardiovascular and subjective effects of blunts. The equivalent increases in heart rate produced by the two smoking methods is probably due to the enhanced carbon monoxide levels produced by blunt smoking compared to joint smoking. Carbon monoxide effectively decreases blood oxygen carrying capacity and consequently results in a number of hemodynamic responses, including increases in heart rate (see Penney, 1988 for review). Carbon monoxide also contributes to cardiovascular and pulmonary disease. The elevated carbon monoxide levels observed after blunt smoking may be representative of other toxic gasses and particulates found in tobacco smoke that may contribute to increased risk of smoking blunts, including carcinogens such as phenols, nitrosamines, aldehydes, volatile hydrocarbons (Baker, 2006).
Characterizing the effects of marijuana smoked as joints and blunts according to sex revealed similar trends between men and women, although there were more significant differences in the effects of joints and blunts in women than in men. In women, joints provided significantly greater plasma THC than blunts for both active doses of marijuana and produced significantly greater effects on several subjective ratings of marijuana quality and intoxication (‘Take Again’, ‘Liking’, and ‘Good Drug Effect’) for women but not for men. Additionally, blunts produced significantly higher carbon monoxide levels across all marijuana conditions in women, while in men, the difference was only significant under placebo marijuana conditions. In men, there was an orderly relationship between marijuana strength, titration and carbon monoxide: less marijuana was smoked as its strength increased, reflected in both dose-related decreases in carbon monoxide and increases in the weight of the unsmoked marijuana. For women, however, the effect was less clear. Women smoked less of the joint as potency increased, but they did not titrate their blunt smoking. Since women smoked a greater proportion of the blunt compared to the joint for both active strengths of marijuana, they therefore had higher carbon monoxide levels from the blunts. These data suggest that the factors influencing patterns of marijuana smoking may vary between men and women. This is consistent with data from cigarette smokers. Factors influencing cigarette smoking (e.g., nicotine dose, stimuli paired with cigarettes) have been shown to vary between men and women (e.g., Perkins et al., 2002).
The findings from this study demonstrate that under the described smoking procedure, marijuana smoked as blunts produces equivalent cardiovascular effects as when smoked in cigarette paper, yet affords lower THC plasma levels and consequently lower subjective ratings of intoxications then joints. It is unknown if the cigar paper would provide physiologically relevant nicotine plasma levels under more extended durations of smoking, data that would be relevant given that blunts are usually smoked under extended durations. Additionally, this study suggests that males and females have distinct patterns of marijuana smoking. However, the small sample size prohibited a between-subject analysis to verify a statistically significant sex difference. Furthermore, menstrual phase was not tracked among women. Given that the effects of stimulants have been shown to vary based upon menstrual phase (for review, see Evans, 2007), THC’s effects may also vary according to menstrual phase.
This study is the first controlled investigation to report the effects of marijuana smoked as blunts, a method of smoking that is gaining popularity and seems to be preferred to conventional smoking methods including joints and pipe smoking (see Introduction). Using identical smoking procedures, marijuana smoked as joints affords greater THC plasma levels and subjective intoxicating effects compared to blunts, yet blunts produce equal increases in heart rate and greater increases in carbon monoxide levels than joints. Thus, smoking marijuana in a tobacco leaf increases the risks of marijuana smoking by enhancing carbon monoxide exposure and heart rate compared to marijuana smoked in paper, risks that may be especially marked in women.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Azorlosa JA, Heishman SJ, Stitzer ML, Mahaffey JM. Marijuana smoking: effect of varying delta 9-tetrahydrocannabinol content and number of puffs. J Pharmacol Exp Ther. 1992;261:114–22. [PubMed] [Google Scholar]
- Baker RR. Smoke generation inside a burning cigarette: Modifying combustion to develop cigarettes that may be less hazardous to health. Progress in Energy and Combustion Science. 2006;32:373–385. [Google Scholar]
- Balerio GN, Aso E, Maldonado R. Role of the cannabinoid system in the effects induced by nicotine on anxiety-like behaviour in mice. Psychopharmacology. 2006;184:504–13. doi: 10.1007/s00213-005-0251-9. [DOI] [PubMed] [Google Scholar]
- Boileau I, Dagher A, Leyton M, Welfeld K, Booij L, Diksic M, Benkelfat C. Conditioned dopamine release in humans: a positron emission tomography [11C]raclopride study with amphetamine. J Neurosci. 2007;27:3998–4003. doi: 10.1523/JNEUROSCI.4370-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton WM, Grant BF, Colliver JD, Glantz MD, Stinson FS. Prevalence of marijuana use disorders in the United States: 1991–1992 and 2001–2002. JAMA. 2004;291:2114–2121. doi: 10.1001/jama.291.17.2114. [DOI] [PubMed] [Google Scholar]
- Davis RA. The determination of nicotine and cotinine in plasma. J Chromatogr Sci. 1986;24:134–141. doi: 10.1093/chromsci/24.4.134. [DOI] [PubMed] [Google Scholar]
- Evans SM. The role of estradiol and progesterone in modulating the subjective effects of stimulants in humans. Exp Clin Psychopharmacol. 2007;15:418–26. doi: 10.1037/1064-1297.15.5.418. [DOI] [PubMed] [Google Scholar]
- Flaten MA, Blumenthal TD. Caffeine-associated stimuli elicit conditioned responses: an experimental model of the placebo effect. Psychopharmacology. 1999;145:105–12. doi: 10.1007/s002130051038. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Haney M. Conditioned effects of environmental stimuli paired with smoked cocaine in humans. Psychopharmacology. 2000;149:24–33. doi: 10.1007/s002139900340. [DOI] [PubMed] [Google Scholar]
- Golub AL, Johnson BD. Cohort changes in illegal drug use among arrestees in Manhattan: from the Heroin Injection Generation to the Blunts Generation. Subst Use Misuse. 1999;34:1733–63. doi: 10.3109/10826089909039425. [DOI] [PubMed] [Google Scholar]
- Golub A, Johnson BD, Dunlap E. The growth in marijuana use among American youths during the 1990s and the extent of blunt smoking. J Ethn Subst Abuse. 2005;4:1–21. doi: 10.1300/J233v04n03_01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M. Opioid antagonism of cannabinoid effects: differences between marijuana smokers and nonmarijuana smokers. Neuropsychopharmacology. 2007;32:1391–403. doi: 10.1038/sj.npp.1301243. [DOI] [PubMed] [Google Scholar]
- Haney M, Bisaga A, Foltin RW. Interaction between naltrexone and oral THC in heavy marijuana smokers. Psychopharmacology. 2003;166:77–85. doi: 10.1007/s00213-002-1279-8. [DOI] [PubMed] [Google Scholar]
- Haney M, Rabkin J, Gunderson E, Foltin RW. Dronabinol and marijuana in HIV(+) marijuana smokers: acute effects on caloric intake and mood. Psychopharmacology. 2005;181:170–8. doi: 10.1007/s00213-005-2242-2. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Vosburg SK, Comer SD, Foltin RW. Reinforcing effects of oral Delta9-THC in male marijuana smokers in a laboratory choice procedure. Psychopharmacology. 2005;18:237–43. doi: 10.1007/s00213-005-2234-2. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Huestis MA, Henningfield JE, Cone EJ. Acute and residual effects of marijuana: profiles of plasma THC levels, physiological, subjective, and performance measures. Pharmacol Biochem Behav. 1990;37:561–5. doi: 10.1016/0091-3057(90)90028-g. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Stitzer ML, Yingling JE. Effects of tetrahydrocannabinol content on marijuana smoking behavior, subjective reports, and performance. Pharmacol Biochem Behav. 1989;34:173–9. doi: 10.1016/0091-3057(89)90369-9. [DOI] [PubMed] [Google Scholar]
- Kelly BC. Bongs and blunts: notes from a suburban marijuana subculture. J Ethn Subst Abuse. 2005;4:81–97. doi: 10.1300/J233v04n03_04. [DOI] [PubMed] [Google Scholar]
- McDonald J, Schleifer L, Richards JB, de Wit H. Effects of THC on behavioral measures of impulsivity in humans. Neuropsychopharmacology. 2003;28:1356–65. doi: 10.1038/sj.npp.1300176. Epub 2003 Apr 30. [DOI] [PubMed] [Google Scholar]
- Moore DS, McCabe GP. Introduction to the practice of statistics. 2. Freeman; New York: 1993. [Google Scholar]
- Penetar DM, Kouri EM, Gross MM, McCarthy EM, Rhee CK, Peters EN, Lukas SE. Transdermal nicotine alters some of marihuana’s effects in male and female volunteers. Drug Alcohol Depend. 2005;79:211–223. doi: 10.1016/j.drugalcdep.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Penney DG. Hemodynamic response to carbon monoxide. Environ Health Perspect. 1988;77:121–30. doi: 10.1289/ehp.8877121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Jacobs L, Sanders M, Caggiula AR. Sex differences in the subjective and reinforcing effects of cigarette nicotine dose. Psychopharmacology. 2002;163:194–201. doi: 10.1007/s00213-002-1168-1. [DOI] [PubMed] [Google Scholar]
- Ream GL, Benoit E, Johnson BD, Dunlap E. Smoking tobacco along with marijuana increases symptoms of cannabis dependence. Drug Alcohol Depend. 2008;95:199–208. doi: 10.1016/j.drugalcdep.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sifaneck SJ, Johnson BD, Dunlap E. Cigars-for-blunts: choice of tobacco products by blunt smokers. J Ethn Subst Abuse. 2005;4:23–42. doi: 10.1300/J233v04n03_02. [DOI] [PubMed] [Google Scholar]
- Soldz S, Huyser DJ, Dorsey E. The cigar as a drug delivery device: youth use of blunts. Addiction. 2003;98:1379–86. doi: 10.1046/j.1360-0443.2003.00492.x. [DOI] [PubMed] [Google Scholar]
- Solinas M, Scherma M, Tanda G, Wertheim CE, Fratta W, Goldberg SR. Nicotinic facilitation of delta9-tetrahydrocannabinol discrimination involves endogenous anandamide. J Pharmacol Exp Ther. 2007;321:1127–34. doi: 10.1124/jpet.106.116830. [DOI] [PubMed] [Google Scholar]
- Valjent E, Mitchell JM, Besson MJ, Caboche J, Maldonado R. Behavioural and biochemical evidence for interactions between Delta 9-tetrahydrocannabinol and nicotine. Br J Pharmacol. 2002;135:564–78. doi: 10.1038/sj.bjp.0704479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachtel SR, El Sohly MA, Ross SA, Ambre J, de Wit H. Comparison of the subjective effects of Delta (9)-tetrahydrocannabinol and marijuana in humans. Psychopharmacology. 2002;161:331–9. doi: 10.1007/s00213-002-1033-2. [DOI] [PubMed] [Google Scholar]