Abstract
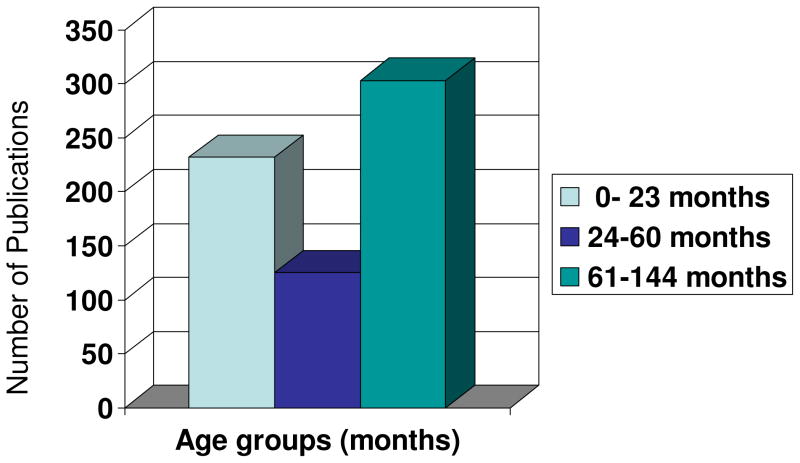
The pre-school years (i.e., 1–5 years of age) is a time of rapid and dramatic postnatal brain development, i.e., neural plasticity, and of fundamental acquisition of cognitive development i.e., working memory, attention and inhibitory control. Also, it is a time of transition from a direct maternal mediation/selection of diet-based nutrition to food selection that is more based on self-selection and self-gratification. However, there have been fewer published studies in pre-school children than in infants or school-aged children that examined the role of nutrition in brain/mental development (i.e., 125 studies vs. 232 and 303 studies, respectively during the last 28 years, Figure 1). This may arise because of age-related variability, in terms of individual differences in temperament, linguistic ability, and patterns of neural activity that may affect assessment of neural and cognitive development in pre-school children. In this review, we suggest several approaches for assessing brain function in children that can be refined. It would be desirable if the discipline developed some common elements to be included in future studies of diet and brain function, with the idea that they would complement more targeted measures based on time of exposure and understanding of data from animal models. Underlining this approach is the concepts of “window of sensitivity” during which nutrients may affect postnatal neural development: investigators and expert panels need to specifically look for region-specific changes and do so with understanding of the likely time window during which the nutrient was, or was not available. (244 words)
Keywords: Brain plasticity, children, nutrition, cognition, memory, neurogenesis, synaptogenesis
All life processes are subject to the influence of biological and nurturing factors and ultimately to their interplay. Brain growth and development and the functional outcome of these, behavior, are no exception. During embryonic, fetal and early postnatal life, genetic determinants specify the fate of neuronal progenitors and their migration to brain regions (1). These genetic determinants also modulate synaptic signal transmission and contribute to the establishment and maintenance of the central nervous system (2, 3). At the same time, environmental determinants play an equally critical role in shaping the neural configuration through postnatal synaptic “blooming and pruning” that incorporates ongoing experiences into the developing synaptic architecture of the brain (4). Some of these environmental determinants act by modifying gene expression through epigenetic mechanisms (5). In essence, an infant is born with the intrinsic capacity to learn, but how and what the infant learns is modulated by the environment.
What is the role of nutrition in this complex process? Nutrition is an environmental factor (6) as it represents access to resources from the environment (i.e., food and water), but in contrast to other environmental resources like medical care, education or experiences, nutrition can directly modify gene structure and mediate the expression of genetic factors by providing the specific molecules that enable genes to exert their potential or targeted effects on brain growth and development. The brain is a specialized tissue in which functionality depends upon the generation of electrical potentials and their conduction through long axonal components of cell-bodies and through the synaptic gaps between these cell-bodies. These special functions of brain are reflected in a higher need for certain nutrients such as choline, folic acid, iron, zinc and special fats (e.g. gangliosides, sphingolipids and docosahexaenoic acid (DHA)). Moreover, nutrition can have direct effects on gene expression in brain. Levi and Sanderson (7) described the epigenetic effects of nutrients, exerted by altering histone acetylation, and the effects of hypoglycemic diets on the genetic expression of neuronal factors. Additionally, nutrients can act as growth factors. For example retinoic acid, the active form of vitamin A, is involved in central nervous system morphogenesis and patterning (8). Some nutrients facilitate the incorporation of experiences into cognitive functions by being the basic structural components of neuronal cell-bodies and synapses. For example, evidence continues to accumulate suggesting that specific fatty acids like DHA are important for synaptogenesis particularly during the third trimester of human gestation (9). Thus, nutrition plays a critical role at the cross-roads of the biological and nurturing factors that mediate brain growth and development.
Our goal in this article is to examine the role of nutrition in postnatal brain and behavior development spanning the toddler and pre-school years (i.e., 1–5 years of age), identifying major gaps in our understanding of these processes and providing recommendations on how to fill these gaps. We will focus on this age range because this is a time of rapid and dramatic changes in the brain, i.e., brain plasticity, and it is a time for acquisition of fundamental cognitive and interpersonal skills (10, 11, 12, 13). During this time, children’s spoken vocabulary increases significantly; they gain greater motor coordination, and they are able to engage in tasks for slightly longer periods (14). Additionally, this age period is characterized by a time of transition from direct maternal control of infant nutrition to indirect maternal control in which infants do not procure their own nutrition, but they begin to assert increasing autonomy regarding what they eat. The toddler and preschool years are generally considered to be the most difficult phase of life to study because toddler performance is influenced by factors that are outside of experimental control such as emotional state, motivation, persistence, and comprehension of instructions. Thus, less research has been done in the toddler years (figure 1) not only because of this age-related variability, but because there has been a greater emphasis on measures of overall cognitive development like “IQ,” which is notably difficult to assess until elementary school years (15, 16, 17).
Figure 1.
Distribution of publications on nutrition and brain development stratified by age groups
The studies considered for inclusion in this analysis were identified in 3 separate searches of the MEDLINE (National Library of Medicine, Bethesda, MD) computerized bibliographic database spanning the years 1980–2008. The searches were completed on 7 June 2008. Each search was stratified by age group using the age divisions provided in PubMed: infants, birth - 23 months; Preschool Child, 2–5 years, and Child, 6–12 years. For each search, all articles that included the words: Brain/growth and development, or Mental Processes and nutrition, or diet and its derivatives in the title and in the key words were selected. There have been fewer studies published among pre-school-aged/toddlers children during the last 28 year than compared to studies conducted in infants and school aged children.
The role of nutrition in postnatal brain and behavior development
1. Nutrition as a mediator of the impact of Socioeconomic Status
In examining the role of nutrition on brain and behavioral development, it is important to recognize that human beings are not randomly assigned to specific conditions. Rather, the effects and outcomes of nutrition are almost always correlated to broader influences from environmental factors such as socio-economic status (SES), health, socio-behavioral factors and motivation (6). Among these correlates, SES usually emerges as the most salient factor explaining the influence of these other environmental factors on children’s brain development and general well being (18). In essence, SES is a proxy for a broad array of human activities such as education, social status and wealth that affect the ability of a family to purchase the goods and services that are essential for wellbeing. From this perspective, nutrition is an important mediator of the effects of SES on the child’s well-being. Bradley and Corwing (18 and references therein), in their review on how SES impacts on brain and mental development, emphasize the importance of the “nutrition pathway” proposed by Martorell (19) as the process through which low SES leads to inadequate dietary intakes, nutrient deficiency and eventually, morbidity and mortality. Food insecurity and malnutrition have been linked to nutrient deficiencies leading to learning and developmental deficits amongst the most vulnerable, infants and toddlers (20, 21). For example, studies have shown that nutrition mediates the impact of SES on the increased likelihood of neural tube defects caused by inadequate intake of folic acid during the first trimester of pregnancy (22), and on the prevalence of iron deficiency-mediated changes in brain function caused by inadequate intake of meats and vegetables rich in iron (23). Chronic undernutrition can deplete the energy resources of both parent and child, making the child more lethargic and less able to elicit attention from the parent and the parent becoming less sensitive and supportive of the child (24).
Although this perspective offers an explanation, through nutrition, of the SES effects, it is important to recognize that nutrition is not the only pathway through which SES can affect brain and behavioral development; others include health care, housing, parenting and cognitively-stimulating play materials and social experiences (18). For example, children from low SES families are more likely to have endured high risk pregnancies that are associated with poor perinatal outcomes or are more likely to have suffered from chronic and debilitating disease during childhood and to have experienced more cognitive and behavioral disturbances than children from less stressed circumstances (6, 18). These children also are more likely to manifest symptoms of psychiatric disturbance, maladaptive functioning and low intellectual/academic achievements than children raised by high SES families (18). For this reason it is still difficult to determine the extent to which poor nutrition alone contributes to developmental problems because children who lack access to adequate nutrition also tend to lack access to these other resources. It is important that researchers control for various other mediators of SES when studying the effects of nutrition on brain as this increases the possibility of assessing the effect of nutrition per se.
There are notable advantages in conceptualizing nutrition as an important path by which SES affects cognitive development. For example, if this relationship is symmetrical, higher SES should be associated with better nutritional status and higher cognition. Johnston et al. (25) used height as a measure of overall nutritional history, and found a linear proportional association between increasing height, SES and IQ. Height in this population was a good proxy of nutritional status, but in other populations might be more closely related to the genetic potential of each individual (26). Brown and Pollitt (27) proposed that poor nutrition contributes to delay in intellectual development by causing “brain damage, enhancing the risk of illness, inducing lethargy and withdrawal or delayed physical growth.” Brain “damage” refers to relatively straightforward nutrient-induced structural or biochemical alterations. Illness as explained by Brown and Pollitt, delays the development of motor skills (e.g., crawling and walking) and thus, limits the child’s exposure to and exploration of the external environment (27). Similarly, delayed physical growth, lethargy and withdrawal would limit the child’s exploration of the external environment and the incorporation of new knowledge from external stimuli. Clearly, the causal relationship between nutrition and brain development is complex and there are various mechanisms whereby nutrition may influence brain development and behavior. Therefore, research that assesses the effect of nutrition interventions on brain development and behavior should delineate the outcomes that are to be measured and the specific mechanisms that are presumed to link the nutrition interventions to these specific outcomes.
2. Critical Periods vs. Windows of Sensitivity in Demarking the Essentiality of Nutrients in Postnatal Brain Development
In understanding the influence of nutrients and food-derived neurotrophic factors on brain and behavior development, it is important to realize that nutrients’ essentiality depends on the timing of their delivery in relation to critical periods during brain development (28, 29). A critical period typically encompasses a relatively narrow time-frame during which a particular brain region develops or in which a specific experience must occur. Prenatal development has well defined milestones or critical periods like neurulation (i.e., formation of the neural tube from which eventually evolves the central nervous system). For example, folic acid is essential for neural tube closure for a short period around 22 days human gestation (30). This timing relationship between nutrient availability and brain development is not only relevant to pre-natal development, but also to post-natal development. However, post-natal brain development milestones and timeframes are generally less well defined in onset; they are also broader and protracted in time. Thomas and Nelson (31) have characterized these periods of brain development during postnatal life as sensitive periods rather than critical periods because they are flexible and the time period in which they function is broader. For example, in the case of the visual and auditory cortex, the formation of experience-dependent synapses peaks about the fourth postnatal month, and is followed by a gradual retraction until the end of the preschool period (see Figure 1 (31)
The neural processes that are inherently important for postnatal brain development make less clear demarcating behavioral milestones. In early postnatal development there may be redundant axonal connectivity, which may modify vulnerability to damage in brain tissues (32). For example, infants have auditory responses in the temporal lobe as well as in the visual-cortex regions, whereas normal adults have them only in the temporal lobe regions (32, 33, 34). If there is an injury to either area in infancy, the redundancy of axonal connections can mitigate detrimental sensory loss compared to an injury in an adult (32). This intrinsic capacity of the brain to remodel itself, refer to as neural plasticity, is the result of overproduction and trimming of neuronal connections, which are associated with changes in synaptic processes, neurogenesis and myelination of axons (4, 35, 36). Most synaptic “blooming and pruning,” although varying by brain region, usually occurs postnatally (see Figure 2 (4)). The overproduction and trimming of neuronal connections allows the developing synaptic architecture of the brain to capture and incorporate experiences, giving rise to behavior as a manifestation of a coordinated neural network activity within a small space, i.e., the cranium. Pascual-Leone et al., note that this brain plasticity is the mechanism that supports development and learning, but also it can cause clinical disorders (35). Therefore, it is a challenge to demarcate behavioral milestones based on how these neural processes relate to the evolving anatomical organizations of the brain during childhood.
The neural processes and their timing during postnatal brain development have important implications for understanding the range and relative degree of severity of nutrient deficiencies. For example, nutrient deficiencies during the prenatal months usually cause irreversible effects on neurogenesis and synaptogenesis because these processes only occur during a specific programmed time in embryogenesis. In contrast, nutrient deficiencies during postnatal development may induce errors that are reversible because of neural plasticity. Moreover, changes in nutrient availability may occur and affect brain development at multiple separate time points across the postnatal life cycle. For example, iron deficiency may affect brain development and function in early infancy, during toddler’s years or in adolescence (29). Thus, the postnatal periods during which neural process occur can be labeled widows of sensitivity in the sense that they reflect an: “opportunity or exposure,” upon which nutrients or their lack of availability may exert an effect, rather than critical periods as in prenatal brain development.
In conceptualizing these periods as windows of sensitivity, it is important to recognize that other factors may exacerbate, confound or compensate for the effects of nutrients on the developing nervous systems. This approach has facilitated the estimation of risk assessment in developmental neurotoxicology (37). For example, the child’s environment influences not only the availability of nutrients but also modulates the effect that a nutrient may have on developmental outcomes. Because most nutrient deficiencies occur in poor (not experience enriched) environments, this may exacerbate the nutrient brain effects. On the other hand an enriched environment may mitigate the true effect of a nutrient intervention. Inherent to this concept of “window of sensitivity” are the effects and consequences of neural plasticity in brain development during postnatal life (4, 35, 36). The plasticity of the human brain may mitigate the effects of nutrient deficiencies on the brain by adapting or compensating in response to environmental pressures, physiological changes and experiences and thus, limiting the response to nutrient supplements. The challenge is to learn how nutrients modulate neural plasticity to achieve the best behavioral outcome. This would require that detection of postnatal nutrient brain effects be based on measurements that are highly reliable of the nutrient’s effect as well as to the brain outcome within the context of a window of sensitivity. These measures should include a combination of nutrient status assessment methods (i.e., biochemical and dietetic variables), brain measures that provide inference as to biochemistry, neurophysiology and behavior, as well as the inclusion of measures to control for the effects of other factors influencing brain development and neural plasticity such as age, gender and the presence or lack of an experience enriched environment or a stressful one.
3. Defining Normal Post-Natal Brain Development
To demonstrate the effects of nutrients on brain development and behavior during infancy and childhood, an important first step is to define normal brain growth and to establish time windows of possible nutrient effects based on neurophysiology and behavioral changes. However, there is limited normative data on brain development and on specific milestones, especially during the toddler years. In addition, available data and brain development charts lack the complexity necessary to identify and link specific neurobiologic features with their underling respective cognitive and behavioral milestones in postnatal development. Thompson and Nelson (31) explained that this uncertainty exists because the best estimates of age-related differences in synaptic density are derived from human autopsy specimens, with sometimes only a few samples at any particular age. Additionally, the estimates of synaptic density represent static figures and do not indicate flux and rates of brain development. The National Institutes of Health MRI study of healthy brain development offers an opportunity to obtained reliable data on brain growth from a healthy cohort of infants and children (38). Preliminary results indicate that total cerebral volume peaks at age 14.5 years in boys and 11.5 years in girls, and that by six years of age, 95% of the brain volume has been achieved (38). Development in various brains areas can be charted with 95% confidence intervals in order to provide growth-curves of the normal changes in brain volume and of other brain regions. To what extent brain volume is a proxy for cognitive function has still to be determined. Nonetheless, theories of intelligence and cognition have proposed that a larger brain has a higher capacity to accommodate more neurons, axons and synapses (36). Comparing food-storing vs. non food-storing birds suggests that hippocampal size is proportionally correlated with memory function (39). In humans, the association is less clear as studies have varied in their methodologies of assessing memory. However, Van Petten in a meta-analysis of 33 clinical studies demonstrated a significant proportional correlation between hippocampal volume and memory performance (40). Therefore, the development of charts that integrate data on the change in volume of the hippocampus and other brain regions in combination with neurobiological information and behavioral milestones is likely to be helpful in assessing the effects of nutrients.
Strategies for Measuring Nutrient-Induced Structural and Behavioral Alterations
1. Determining Mechanistic Pathways
Access to brain tissue is necessarily limited in human studies, making experimental models important. By using in vitro models or in vivo animal models, the effects of nutrition can be explored by linking nutritional deficiencies to structural and/or functional alterations in neural maturation and to alterations in growth and behavior (28, 29). An important advantage of using these models is that they can facilitate screening for possible neurotrophic agents, nutraceuticals and nutrients that affect neurogenesis and synaptogenesis. This can be accomplished by using neural progenitor cells in primary cell culture, or by using neuronalcell lines derived from rodents or humans (1, 41). These models facilitate the use of molecular biological tools to study gene-nutrient interactions, gene expression, proteomic and metabolic changes associated with exposure to nutrients. Ideally, in vivo models could lead to identification of a gene that is associated with a behavior change. This approach has been used to assess the developmental neurobehavioral toxicity of lead across species and in determining the validity of these models in providing inference to human behavior (42). These in vivo experiments can also help identify a window of sensitivity to nutrients for optimizing a brain function. The ultimate goal of this approach is to provide evidence of and describe a plausible mechanistic pathway explaining the nutrient-induced structural alteration or biochemical alteration leading to a behavioral alteration, which should be established sequentially and closely linked among structural, functional and behavioral brain outcomes (28).
Moreover, in vivo models based on comparing deficient vs. sufficient states, are useful in providing a comparison between the extreme intakes, low vs. high, and thus in determining a range of the nutrient intake that can maximize brain-related benefits. For example, manipulating the dose of choline in the diet to provide a high dose (4x normal diet), during pregnancy increased the offspring pup’s ability to use relational cues to navigate a maze compared to those pups from dams on a standard diet (43). These effects of choline could not be reversed by changing dietary choline after the critical window of sensitivity, and may be permanent because of epigenetic modifications in the switches that control gene expression (44) and that these gene expression changes result in the formation and survival of more neurons in brain (45). These experiments in model systems provide a mechanistic basis for examining the effects of this nutrient in humans. In fact, there is human data that suggests that this nutrient influences brain development Californian women who consumed pre-conceptionly less than 290 mg/day (lowest quartile) of choline in the diet had 4-fold increased risk of having an infant with a neural tube defect (NTD) than did women in the highest diet intake quartile (intakes > 498 mg/day of choline) (46). The results from these studies suggest that there may be windows of time in human development when choline intakes could be increased to enhance brain development.. However, these experimental models by themselves do not provide the information necessary to determine nutrient requirements in the population; other approaches are necessary.
Although animal models provide insights into the mechanisms by which nutrients affect brain development and performance, inferences on nutrient levels and their extrapolation to human populations are difficult because these animal species develop and mature at varying rates different from humans. This difference has important implications for extrapolation of these data to human populations. Though the biological processes are similar in rodents and humans, it is obvious that the human brain is more complex and sophisticated than is the rodent brain. To help understand the difference itself and be able to extrapolate this information, neuroinformatics has been developed. This is an analytical approach that combines neuroscience, evolutionary science, statistical modeling and computer science (47). This analysis relates numeric values assigned to at least 10 mammalian species so that the results can help to integrate data in the neurodevelopmental literature across laboratory species and extrapolate them more accurately to humans. Finally, laboratory animals are usually genetically homogeneous, while humans are not, which further limits generalizations. Confirmatory information from human studies is greatly valued for substantiating these mechanisms, but these studies are difficult for the reasons we have already discussed. Recent advances in technology may facilitate more mechanistic studies in humans.
Available technologies in neuroscience include, but are not limited to measuring event-related brain potentials (ERPs), magnetic resonance imaging (MRI), functional magnetic resonance imaging fMRI and magnetic resonance spectroscopy (MRS). These non-invasive methods for measuring brain size and activity during cognitive processing hold promise for identifying the neural sub-processes involved in complex cognitive, motor, or perceptual tasks. They can be time-linked to the stimulus onset (e.g., the presentation of a word, a sound, or an image), and have been used in infants and children with some success. fMRI can be used to map changes in brain hemodynamics that correspond to mental operations (48) and it is possible to observe the structures that participate in specific brain functions. Magnetic Resonance Spectroscopy (MRS) permits the characterization of biochemistry in brain tissue by using the signal from protons to determine the concentration of brain metabolites such as N-acetyl aspartate, choline, creatine and lactate in the tissue examined, and it has been used in infants and toddlers (49). MRI was used in studies linking brain structural changes associated with hypoglycemia vs. hyperglycemia with cognitive functions (50). Within the diabetic group, children with one or more severe hypoglycemic episodes showed less grey matter volume at the left temporal-occipital region, whereas those with episodes of severe hyperglycemia showed less grey matter volume in the posterior cortical area (50). These structures are associated with brain performance related to the episodic memory system and higher-order visuospatial functions. A subsequent study of a similar population assessed the effects of a severe episode of hypoglycemia vs. hyperglycemia on cognitive development (51). Early, frequent severe hypoglycemia was associated with decreased delayed recall of explicitly learned information, whereas severe hyperglycemia decreased estimated verbal intelligence (51). These studies demonstrated how brain structural changes could be linked with cognitive functions by using MRI studies of brain region volume in combination with cognitive test of intelligence, memory and processing speed (50, 51). Another example of this linkage is the use of ERP studies to show that infants of diabetic mothers have impairments in memory from birth through 8 months of age that are consistent with alterations in mechanistic pathways of memory observed in animal models of perinatal iron deficiency (52). For a basic review of the strengths and weaknesses of these methods as well as their integration see Lee and Chamberlain (53).
2. Using Cognitive Function to Assess Effects of Nutrition on Development
Given the fundamental role of nutrients in supporting all aspects of structural and functional development, nutritional deficits may have quite specific effects on development. However, research that looks at broad outcomes rather than specific underlying abilities may lack the focus that would be needed in order to document such specific effects. To illustrate this point, we will review recent research on psychological development in children who have deficient levels of iron, and use these data to explore the degree to which relevant principles of neuroscience and developmental psychology have been applied.
Iron is necessary for normal neurodevelopment (29), and its deficiency is widespread in infants and young children. Although animal studies have demonstrated that iron deficiency alters myelination, monoamine neurotransmitter synthesis, and hippocampal energy metabolism (29), iron deficiency is a particularly complicated topic in the human because effects may result from deficiency during various stages of the life cycle and thus, effects of iron supplementation would be expected to differ depending upon the the supplemented individual’s stage of development (29, 54 and references within).
As summarized in Table 1, Zhou et al. (55) provided iron supplements for anemic pregnant women in Australia and found no effects on the child’s IQ at 4 years of age. Lind et al. (56) provided daily iron supplementation to Indonesian infants from 6 to 12 months and found no effect on mental development on the Bayley (a standardized assessment of general intelligence) at 12 months but found some improvement in motor development. Black et al. (57) report comparable results in Bangladesh, with no effect of iron supplementation at either 6 or 12 months. Lozoff et al. (58) treated Costa Rican infants, 12 to 23 months of age, and after 3 months of treatment, the children whose anemia and iron deficiency were corrected had higher mental and motor test scores on the Bayley. Logan et al. (59) reviewed studies that used a randomized placebo or iron treatment with children younger than 3 years, and found only a single effective study: long-term iron treatment (4 months) improved mental and motor performance on the Bayley (60). More recently, Akman et al. (61) examined iron deficient children aged 6–30 months and found that differences on the Bayley and the Denver Developmental Screening Test were ameliorated after 3 months of iron treatment. These studies suggest that iron supplementation must be continued for a long duration to have an effect. Furthermore, regarding the locus of supplementation effects, Metallinos-Katsaras et al. (62) provided iron supplements for anemic Greek children 3–4 year olds and found improvement in selective attention and other cognitive skills. This latter result is particularly interesting in the present context because iron can influence dopamine metabolism, which can affect attention and memory as well as other cognitive systems (63). Finally, in the Gonazlez et al. (64) study that compared 4–10 year old healthy children with low versus normal visuomotor ability and IQ, higher serum ferritin level (an index of iron) was correlated with visuomotor ability.
Table 1.
Effects of Iron Supplementation in Young Children
| Study | Supplementation | Outcome Measure | Effect |
|---|---|---|---|
| Zhou et al. (55) | Iron supplements for anemic pregnant women | IQ at 4 years | No effect |
| Lind et al. (56) | Daily iron supplementation to infants 6–12 months | Bayley at 12 months. | No effect on mental, some improvement in motor |
| Black et al. (57) | Daily iron supplementation to infants 6–12 months | Bayley at 6 & 12 months. | No effect |
| Idjradinata & Pollitt (60) | Daily iron for 4 months | Bayley at 12–18 months | Developmental delays were reversed. |
| Lozoff et al. (58) | Daily iron for 3 months | Bayley at 12–23 months | Developmental delays were reversed. |
| Akman et al. (61) | Daily iron for 3 months | Bayley and Denver at 6–30 months | Developmental delays were reversed |
| Metallinos-Katsaras et al. (62) | Daily iron for 3 months | Computerized tests of cognitive function at 3–4 years | Improved performance. |
To summarize, iron supplementation for an appropriate duration can have positive effects on measures of general cognitive function as well as some specific abilities, but most research to date has focused on broad measures of general cognitive functioning that are not focused on specific effects of a nutrient. One salient aspect of the research investigating nutritional influences of iron on cognitive development in toddlers and preschool children is that most studies have used a standardized assessment of general intelligence as the primary outcome of interest. Intelligence has been an important construct for over a century because it is a strong predictor of school-related outcomes but this statement applies most directly to children who are 5 years of age or older. Moreover, the “intelligence” measured by any particular test reflects the test maker’s particular theory of intelligence, which can vary quite significantly across time and across cultures. Intelligence tests for young children are based on highly predictable age-related changes in specific relevant behaviors. For example, almost all human infants have some comprehension of words by their 8th month, some production of words by their 12th month, and produce two-word combinations by their 18th month (65). Comparisons of performance on age-appropriate tasks is the underlying basis for tests of general intelligence in a developmental context (e.g., the Bayley Scales of Infant Development, the Mullen Scales of Early Learning, the Denver Developmental Screening Test), and this approach has been quite useful when the goal is to identify children whose development is ahead of or behind their peers. The main limitation of this approach is that it provides no insight into the underlying abilities that influence the child’s performance, which would be particularly problematic if a nutrient has a relatively specific effect on neural development.
A more sensitive approach to assessing cognitive development is to identify and measure specific aspects of cognitive ability. Most intelligence tests provide subtest-scores to reflect distinctions such as mental versus motor ability, or separate skills such as memory, problem solving, or verbal ability, but these subtest-scores emerge from a relatively simplistic testing context in which an examiner interacts with the child using various play-oriented materials. A more potent strategy for assessing specific aspects of cognitive ability is to use laboratory procedures in which an aspect of cognitive ability can be measured in various contexts using an array of outcome variables that include not only overt behavior but also more subtle behaviors such as reaction time and eye movements as well as physiological responses such as changes in heart rate or evoked electrical potentials. Given the goal of assessing nutritional effects on cognitive development in children in the 1- to 5-year range, attention and memory are two obvious candidates for specific focus.
Attention
Attention refers to the broad array of processes that direct an organism’s sensory focus. For example, endogenous attention refers to the internal, volitional process through which sensory focus is directed toward external stimuli and can be contrasted with related aspects of the term “attention” (e.g., maintaining alertness, orienting toward compelling external stimuli). The emergence of endogenous control of attention during the toddler and preschool years allows children to accrue important information about their surroundings and to engage in the dynamic social interactions that form the basis for interpersonal relationships.
Several procedures have been developed to measure endogenous attention in toddlers and preschool-aged children. Focused attention can be assessed using behavioral ratings of attentiveness while the child is playing with toys in the context of a specific distraction. For example, Brown et al. (66) placed toys in front of 1- to 3-year-olds for 45 seconds and coded videotapes for duration of attention and number of periods of attention. In some procedures, focused attention is assessed in the context of a competing stimulus. For example, Kannass et al. (67) presented multiple toys to 31-months-olds and measured aspects of looking and inattention. To assess vulnerability to distraction, 5-sec video clips were presented at random intervals while the child was playing. As would be expected, older children become less vulnerable to distraction.
A second aspect of endogenous attention is the ability to monitor a stimulus stream for the occurrence of a specific target. This ability is the common denominator across a wide array of so-called “continuous performance tasks.” For example, Weissberg et al. (68) tested children as young as 2.5 years on a task that required pushing a button upon detecting a target. Results indicated improvement in target detection reaction time with age, and also established strong reliability for the task. Scerif et al. (69) taught 2- and 3-year-olds to touch the large circles in an array that included varying number of circles of varying sizes. Older toddlers improved in their speed of search on correct responses, their efficient choice of sequential targets, and their accuracy.
Finally, combining focused attention and monitoring leads to an interesting paradigm that captures aspects of each. In a gap-overlap task, the stimulus presentation is engineered to allow an explicit comparison between the ability to orient toward a peripheral target per se and the ability to orient toward a peripheral target in the context of having to disengage attention from an ongoing target. Heffelfinger et al. (70) tested 14–60 month olds using a task in which a stimulus was presented on a central monitor, with a subsequent stimulus presented on one of 2 monitors on either side of the central monitor. In the gap condition, the stimulus on the central monitor was extinguished before the onset of the peripheral stimulus, so the only challenge for the child was to reorient visual attention to the peripheral target. In the overlap condition, the stimulus on the central monitor remained visible while the peripheral stimulus was presented, thus requiring the child to disengage attention from one target and refocus on an alternative target. Hellelfinger et al. found that reaction time to look at the peripheral stimulus differentiated control and cocaine exposed toddlers (70).
The development of endogenous attention in young children is likely to be an important fundamental cognitive skill that enables children to accomplish critical competencies such as learning language and establishing social relations. We know very little about the development of endogenous attention other than obvious commonsense conclusions about increases in endogenous attention capacity during this age range. Measures of focused attention and monitoring, and possibly the gap-overlap paradigm will enable researchers to tap and explore this important domain.
Memory
Memory implies the encoding, storage, and retrieval of information, which is very important from a developmental perspective because the capacity to hold information and process it supports various higher level accomplishments such as language, categorization, and social cognition. Several paradigms have been developed to assess memory in young children. For example, in a deferred imitation paradigm, the child watches the examiner model a sequence of actions performed with a set of objects. If the child performs the modeled sequence after a delay of minutes, hours, or days, this behavior implies that the original presentation was encoded, stored, and retrieved. Children in the 1–3 year age range are able to imitate a sequence that they saw as long ago as several months (71, 72, 73), with notable improvement in storage and retrieval as children get older.
Working memory refers to the ability to hold information “on line,” use it to accomplish a goal, and then discard it. Examples include holding a phone number in mind long enough to dial the number, or remembering the words of a sentence long enough to make sense of the sentence. The capacity to hold information in working memory emerges during the first year (74) and working memory capacity continues to improve during childhood (74). Older children can be given task instructions and they can provide verbal responses or well-trained motor responses. Unfortunately, the toddler and pre-school age range is more difficult to work with and tasks must be designed to systematically challenge memory within the context of an engaging game-like task. For example, in a hide and find task (75), the child watches the experimenter hide a desired object at one of several possible locations. The experimenter then engages the child’s attention to break his or her fixation on the hiding location. After a timed delay, the child is allowed to search for the object. If the child finds the object, the child’s working memory capacity is sufficient to span that delay and distinguish among that number of alternative locations. If the child searches incorrectly, it is assumed that the child’s working memory capacity has been exceeded.
Short-term working memory is a relatively straightforward construct that has been successfully measured in young children using variations of the delayed-response task. Results from these studies suggest a monotonic increase in parameters such as capacity and durability. And, working memory, which has been linked to development of prefrontal cortex, has been widely investigated in the context of typical and atypical development. Short-term working memory would certainly appear to be a prime target as an index of how nutrition affects cognitive development in young children.
Many other cognitive abilities can be assessed in the 1–5 year age range (e.g., categorization, problem solving, counting) and a complete evaluation of nutritional influences on development would require data from this broader spectrum. Endogenous attention and short-term working memory have been our focus here for several reasons. First, these two constructs provide a fundamental, underlying basis for acquiring and using information that supports a wide array of broader abilities that emerge in the 1–5 year age range such as language and social interaction. Second, extrapolation from research on adults and animal models suggests specific neural mechanisms associated with endogenous attention and short-term working memory. Finally, endogenous attention and short-term working memory have relatively obvious behavioral manifestations and can be assessed within a convenient time frame as opposed to constructs that entail more general ability (e.g., problem solving) or that reflect processing over a broad time frame (e.g., long term memory). Researchers who explore nutritional effects in the 1–5 year range will need a broad and deep toolbox, but endogenous attention and short-term memory are good tools to have on top.
Using Brain and Behavioral Outcomes to Assess Nutrient Requirements
Better methods for characterizing the functional changes in brain that are associated with diet could set the foundation for revising and improving dietary recommendations. Carefully characterized functional phenotypes are used by the Institute of Medicine USA Food and Nutrition Board as the basis for estimating human nutrient requirements (76). For example, the dietary requirement for iron is based on the amount of iron that must be consumed to prevent the functional phenotype of anemia (77). The expert panels that make these estimations examine human data (supported by more extensive animal studies) on various functional phenotypes related to a nutrient, and then choose the function that is most sensitive to the nutrient (i.e., the organ function that is abnormal after the smallest increment or decrement in dietary intake) to set the recommended intake or upper limit of recommended intake. If behavioral effects of iron deficiency were the most sensitive phenotype of brain dysfunction in iron deficiency (Lozoff reports that these are apparent before anemia (78)), this brain function change would be used to set the recommended dietary intake. Conversely, if supplemental iron intake above the current recommended amount optimizes the functional brain phenotype, the recommendation likely should consider this higher iron level as optimal dietary intake. Behavioral phenotype has been rarely used to assess dietary intake requirements because there is not enough human data in the published literature that is based on comparable methodology. It is much easier to measure anemia than it is to measure brain function.
In this review, we suggest several approaches that can be refined for assessing brain function in children. It would be desirable if the discipline developed some common elements to be included in future studies of diet and brain function, because these elements would complement more targeted measures based on time of exposure and understanding of data from animal models. Studies that only use gross measures such as IQ and which lump nutrient exposures across broad swatches of time, are unlikely to generate useable data for setting nutrient recommendations. When more sophisticated brain phenotyping methods are applied to nutrition-related questions, human data will accrue that could allow expert panels to use brain phenotype when setting diet recommendations. However, there are other complications that need to be addressed before this strategy becomes common place. As discussed earlier, the effects of nutrients on brain development may only occur during specific sensitive windows in brain development. Folic acid only alters spinal cord closure during a few days in embryonic development (79). Dietary choline may only alter brain development if varied during the few days during development when neural progenitor cells are programmed to divide and migrate to specific areas of brain (45). This programmed window for neurogenesis is not uniform within brain: it occurs earlier in the cerebellum than in the hippocampus, and earlier in the hippocampus than in the cortex (80). The consequence of this variability is that the characterization of the behavioral, anatomical or biochemical brain phenotype takes considerable understanding of brain development, and investigators and expert panels must specifically look for region-specific changes and must do so with an understanding of the likely time window during which the nutrient was or was not available. The “window of sensitivity” approach is likely to extend beyond brain development. Epigenetic marking of DNA and histones in response to diet also occurs during specific windows of sensitivity during development (5). These marks set the “switches” that turn many genes on and off, and may be the major underlying mechanism whereby early life nutrition has lifelong effects (81).
Summary
There is no aspect of our physical or psychological existence that is not affected in some way by nutrition. A profound lack of nutrition would obviously have a negative influence on all aspects of development, and such effects of malnutrition are well documented (29, 82). But, moving beyond this general truism, an important goal for research is to reveal specific links between the intake level of particular nutrients and specific behavioral outcomes.
We have discussed the complex role that nutrition plays in postnatal brain and behavior development during the pre-school years. Nutrition and nutrients not only represent environmental resources, but also can have epigenetic effects modifying the influence of biological and nurturing factors. We have highlighted some of the gaps in our understanding of this role and have provided some recommendations for defining this role. We hope that these perspectives help build a momentum and motivate further research from the interaction among neuroscientists, developmental psychologists and nutritional scientists.
From a research perspective, attention has mainly been focused on problems caused by deficits in nutrition or nutrients. In contrast, we know relatively little about the effects of above-normal exposure to necessary substances (83), but this topic is of considerable importance. For example, as noted earlier, research with rodents has demonstrated that pups whose uterine environment has supplemental choline have notable enhancement of memory capacity throughout life (43) and it would certainly be feasible to apply this intervention in humans. Ethical considerations preclude providing humans with nutrients at levels above the normal range without a solid scientific basis, but it is interesting to ponder the possible salubrious effects of supplemental doses of various micronutrients.
Research on the use of nutritional supplements to remediate deficits is difficult for various reasons. One issue is that nutritional influences can be short term or long term. For example, we can observe the immediate effect of a high-glucose snack, the day-long effect of having a poor quality breakfast, or the day-to-day effect of iron supplementation. From a long-term perspective, nutritional effects can occur in utero and last for the entire life span. For example, early experiences such as sub-optimal nutrition or exposure to teratogens have been linked to a wide array of long-term outcomes including taste preference, intelligence, obesity, and cardiac function (81) through various neural mechanisms (84). It is also possible for nutritional effects to occur later in life and have relatively short-term effects on behavior and for these reasons it is important that period of sensitivity be determined. To accomplish this goal, research on nutritional influences must use an array of designs and strategies to capture both short-term and long-term outcomes.
Acknowledgments
Dr. Zeisel is supported by grants from the National Institutes of Health (DK55865, AG09525) and by the UNC Clinical Nutrition Research Unit (DK56350).
The authors acknowledge no conflict of interest or personal interest/gain in any company/organization, or having received any financial support from any industry-related organization in the preparation of this manuscript currently or at the time that the manuscript was prepared. FJR was an employee of Mead Johnson Nutrition, and presently he works for Abbott Nutrition, Singapore.
Dr. Zeisel is supported by grants from the National Institutes of Health (DK55865, AG09525) and by the UNC Clinical Nutrition Research Unit (DK56350).
Footnotes
All the authors contributed intellectually and practically to the development and preparation of the manuscript; more specifically, FJR contributed to the concept and content of the paper, preparation of the manuscript, and its editing; SJR contributed to the content of the paper, preparation and editing, and SHZ contributed to the content of the paper, preparation and editing.
Contributor Information
Francisco J. Rosales, Global Research and Development, Mead Johnson Nutritionals, Evansville, Indiana
J. Steven Reznick, Psychology Department, University of North Carolina at Chapel Hill.
Steven H. Zeisel, Nutrition Research Institute at Kannapolis, Department of Nutrition, School of Public Health and School of Medicine, University of North Carolina at Chapel Hill
List of references
- 1.Hsu YC, Lee DC, Chiu IM. Neural stem cells, neural progenitors, and neurotrophic factors. Cell Transplant. 2007;16:133–50. [PubMed] [Google Scholar]
- 2.Heng JI, Moonen G, Nguyen L. Neurotransmitters regulate cell migration in the telencephalon. Eur J Neurosci. 2007;26:537–46. doi: 10.1111/j.1460-9568.2007.05694.x. [DOI] [PubMed] [Google Scholar]
- 3.Nakajima K, Tohyama Y, Maeda S, Kohsaka S, Kurihara T. Neuronal regulation by which microglia enhance the production of neurotrophic factors for GABAergic, catecholaminergic, and cholinergic neurons. Neurochem Int. 2007;50:807–20. doi: 10.1016/j.neuint.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Levitt P. Structural and functional maturation of the developing primate brain. J Pediatr. 2003;143:S35–45. doi: 10.1067/s0022-3476(03)00400-1. [DOI] [PubMed] [Google Scholar]
- 5.Zeisel SH. Importance of methyl donors during reproduction. Am J Clin Nutr. 2009;89(2):673S–7S. doi: 10.3945/ajcn.2008.26811D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bryan J, Osendarp S, Hughes D, Calvaresi E, Baghurst K, van Klinken JW. Nutrients for cognitive development in school-aged children. Nutr Rev. 2004;62:295–306. doi: 10.1111/j.1753-4887.2004.tb00055.x. [DOI] [PubMed] [Google Scholar]
- 7.Levi RS, Sanderson IR. Dietary regulation of gene expression. Curr Opin Gastroenterol. 2004;20:139–42. doi: 10.1097/00001574-200403000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Parada C, Gato A, Bueno D. All-trans retinol and retinol-binding protein from embryonic cerebrospinal fluid exhibit dynamic behaviour during early central nervous system development. Neuroreport. 2008;19:945–50. doi: 10.1097/WNR.0b013e3283021c94. [DOI] [PubMed] [Google Scholar]
- 9.Jacobson JL, Jacobson SW, Muckle G, et al. Beneficial effects of a polyunsaturated fatty acid on infant development: evidence from the inuit of arctic Quebec. J Pediatr. 2008;152:356–64. doi: 10.1016/j.jpeds.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Livesay PJ, Morgan GA. The development of response inhibition in 4- and 5-year-old children. Aust J Psychol. 1991;43:133–137. [Google Scholar]
- 11.Kochanska G, Coy KC, Murray KT. The development of self-regulation in the first four years of life. Child Dev. 2001;72:1091–1111. doi: 10.1111/1467-8624.00336. [DOI] [PubMed] [Google Scholar]
- 12.Zelazo PD, Frye D, Rapus T. An age-related dissociation between knowing rules and using them. Cog De. 1996;11:37–63. [Google Scholar]
- 13.Burrage MS, Ponitz CC, McCready EA, et al. Age- and schooling-related effects on executive functions in young children: a natural experiment. Child Neuropsychol. 2008;14:510–24. doi: 10.1080/09297040701756917. [DOI] [PubMed] [Google Scholar]
- 14.Sakai KL. Language acquisition and brain development. Science. 2005;310:815–9. doi: 10.1126/science.1113530. [DOI] [PubMed] [Google Scholar]
- 15.Kranzler JH. Assessment of children and youth from culturally and linguistically diverse backgrounds with mental chronometric techniques. Percept Mot Skills. 1998;86:321–2. doi: 10.2466/pms.1998.86.1.321. [DOI] [PubMed] [Google Scholar]
- 16.Kranzler JH. Educational policy issues related to the use and interpretation of intelligence tests in the schools. School Psyc Rev. 1997;26:150–163. [Google Scholar]
- 17.Canivez GL, Watkins MW. Long-term stability of the Wechsler Intelligence Scale for Children-Third Edition. Psycholl Assess. 1998;10:285–291. [Google Scholar]
- 18.Bradley RH, Corwyn RF. Socioeconomic status and child development. Annu Rev Psychol. 2002;53:371–99. doi: 10.1146/annurev.psych.53.100901.135233. [DOI] [PubMed] [Google Scholar]
- 19.Martorell R. The interrelation of diet and infectious disease in P.E.M. In: Green LS, Johnston FE, editors. Social and Biological Predictors of Nutritional Status, Physical Growth, and Neurological Development. New York, NY: Academic Press; 1980. pp. 188–213. [Google Scholar]
- 20.Cook JT, Frank DA, Casey PH, et al. A brief indicator of household energy security: associations with food security, child health, and child development in US infants and toddlers. Pediatrics. 2008;122:e867–75. doi: 10.1542/peds.2008-0286. [DOI] [PubMed] [Google Scholar]
- 21.Weinreb L, Wehler C, Perloff J, et al. Hunger: its impact on children’s health and mental health. Pediatrics. 2002;110:e41. doi: 10.1542/peds.110.4.e41. [DOI] [PubMed] [Google Scholar]
- 22.Wasserman CR, Shaw GM, Selvin S, Gould JB, Syme SL. Socioeconomic status, neighborhood social conditions, and neural tube defects. Am J Public Health. 1998;88:1674–80. doi: 10.2105/ajph.88.11.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oski FA. Iron deficiency in infancy and childhood. N Engl J Med. 1993;329:190–3. doi: 10.1056/NEJM199307153290308. [DOI] [PubMed] [Google Scholar]
- 24.Valenzuela M. Maternal sensitivity in a developing society: the context of urban poverty and infant chronic undernutrition. Dev Psychol. 1997;33:845–55. doi: 10.1037//0012-1649.33.5.845. [DOI] [PubMed] [Google Scholar]
- 25.Johnston FE, Low SM, de Baessa Y, MacVean RB. Interaction of nutritional and socioeconomic status as determinants of cognitive development in disadvantaged urban Guatemalan children. Am J Phys Anthropol. 1987;73:501–6. doi: 10.1002/ajpa.1330730412. [DOI] [PubMed] [Google Scholar]
- 26.Deaton A. Height, health, and development. Proc Natl Acad Sci. 2007;104:13232–7. doi: 10.1073/pnas.0611500104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown JL, Pollitt E. Malnutrition, poverty and intellectual development. Sci Am. 1996;274:38–43. doi: 10.1038/scientificamerican0296-38. [DOI] [PubMed] [Google Scholar]
- 28.Rao R, Georgieff MK. Early nutrition and brain development. The effects of early adversity on neurobehavioral development. In: Nelson CA, editor. Minnesota Symposium on Child Psychology. Vol. 31. Hillsdale, NJ: Erlbaum Associates; 2000. pp. 1–30. [Google Scholar]
- 29.Georgieff MK. Nutrition and the developing brain: nutrient priorities and measurement. Am J Clin Nutr. 2007;85:614S–620S. doi: 10.1093/ajcn/85.2.614S. [DOI] [PubMed] [Google Scholar]
- 30.Czeizel A, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med. 1992;327:1832–35. doi: 10.1056/NEJM199212243272602. [DOI] [PubMed] [Google Scholar]
- 31.Thompson RA, Nelson CA. Developmental science and the media. Early brain development. Am Psychol. 2001;56(1):5–15. doi: 10.1037/0003-066x.56.1.5. [DOI] [PubMed] [Google Scholar]
- 32.Neville H, Bavelier D. Human brain plasticity: evidence from sensory deprivation and altered language experience. Prog Brain Res. 2002;138:177–88. doi: 10.1016/S0079-6123(02)38078-6. [DOI] [PubMed] [Google Scholar]
- 33.Collignon O, Voss P, Lassonde M, Lepore F. Cross-modal plasticity for the spatial processing of sounds in visually deprived subjects. Exp Brain Res. 2009;192:343–58. doi: 10.1007/s00221-008-1553-z. [DOI] [PubMed] [Google Scholar]
- 34.Huttenlocher PR, de Courten C. The development of synapses in striate cortex of man. Hum Neurobiol. 1987;6:1–9. [PubMed] [Google Scholar]
- 35.Pascual-Leone A, Amedi A, Fregni F, Merabet LB. The plastic human brain cortex. Annu Rev Neurosci. 2005;28:377–401. doi: 10.1146/annurev.neuro.27.070203.144216. [DOI] [PubMed] [Google Scholar]
- 36.Mercado E., 3rd Neural and cognitive plasticity: from maps to minds. Psychol Bull. 2008;134(1):109–37. doi: 10.1037/0033-2909.134.1.109. [DOI] [PubMed] [Google Scholar]
- 37.Adams J, Barone S, Jr, LaMantia A, Philen R, Rice DC, Spear L, Susser E. Workshop to identify critical windows of exposure for children’s health: neurobehavioral work group summary. Environ Health Perspect. 2000;108:535–44. doi: 10.1289/ehp.00108s3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30:718–29. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 39.Hampton RR, Sherry DF, Shettleworth SJ, Khurgel M, Ivy G. Hippocampal volume and food-storing behavior are related in parids. Brain Behav Evol. 1995;45:54–61. doi: 10.1159/000113385. [DOI] [PubMed] [Google Scholar]
- 40.Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: review and meta-analysis. Neuropsychologia. 2004;42(10):1394–413. doi: 10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 41.Niculescu MD, Craciunescu CN, Zeisel SH. Gene expression profiling of choline-deprived neural precursor cells isolated from mouse brain. Brain Res Mol Brain Res. 2005;134:309–22. doi: 10.1016/j.molbrainres.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 42.Davis JM, Otto DA, Weil DE, Grant LD. The comparative developmental neurotoxicity of lead in humans and animals. Neurotoxicol Teratol. 1990;12(3):215–29. doi: 10.1016/0892-0362(90)90093-r. [DOI] [PubMed] [Google Scholar]
- 43.Meck WH, Williams CL. Metabolic imprinting of choline by its availability during gestation: implications for memory and attentional processing across the lifespan. Neurosci Biobehav Rev. 2003;27(4):385–99. doi: 10.1016/s0149-7634(03)00069-1. [DOI] [PubMed] [Google Scholar]
- 44.Niculescu MD, Craciunescu CN, Zeisel SH. Dietary choline deficiency alters global and gene-specific DNA methylation in the developing hippocampus of mouse fetal brains. FASEB J. 2006;20:43–9. doi: 10.1096/fj.05-4707com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Craciunescu CN, Albright CD, Mar MH, Song J, Zeisel SH. Choline availability during embryonic development alters progenitor cell mitosis in developing mouse hippocampus. J Nutr. 2003;133:3614–8. doi: 10.1093/jn/133.11.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shaw GM, Carmichael SL, Yang W, Selvin S, Schaffer DM. Periconceptional dietary intake of choline and betaine and neural tube defects in offspring. Am J Epidemiol. 2004;160(2):102–9. doi: 10.1093/aje/kwh187. [DOI] [PubMed] [Google Scholar]
- 47.Clancy B, Kersh B, Hyde J, Darlington RB, Anand KJ, Finlay BL. Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinformatics. 2007;5(1):79–94. doi: 10.1385/ni:5:1:79. [DOI] [PubMed] [Google Scholar]
- 48.Thomas KM, Tseng A. Functional MRI methods in developmental cognitive neuroscience. In: Nelson CA, Luciana M, editors. Handbook of Developmental Cognitive Neuroscience. 2. Cambridgw, MA: MIT Press; 2008. pp. 311–23. [Google Scholar]
- 49.Smith LM, Chang L, Yonekura ML, et al. Brain proton magnetic resonance spectroscopy and imaging in children exposed to cocaine in utero. Pediatrics. 2001;107(2):227–31. doi: 10.1542/peds.107.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perantie DC, Wu J, Koller JM, Lim A, Warren SL, Black KJ, Sadler M, White NH, Hershey T. Regional brain volume differences associated with hyperglycemia and severe hypoglycemia in youth with type 1 diabetes. Diabetes Care. 2007;30:2331–7. doi: 10.2337/dc07-0351. [DOI] [PubMed] [Google Scholar]
- 51.Perantie DC, Lim A, Wu J, Weaver P, Warren SL, Sadler M, White NH, Hershey T. Effects of prior hypoglycemia and hyperglycemia on cognition in children with type 1 diabetes mellitus. Pediatr Diabetes. 2008;9:87–95. doi: 10.1111/j.1399-5448.2007.00274.x. [DOI] [PubMed] [Google Scholar]
- 52.deRegnier RA, Long JD, Georgieff MK, Nelson CA. Using event-related potentials to study perinatal nutrition and brain development in infants of diabetic mothers. Dev Neuropsychol. 2007;31:379–96. doi: 10.1080/87565640701229524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee N, Chamberlain L. Neuroimaging and psychophysiological measurement in organizational research: an agenda for research in organizational cognitive neuroscience. Ann N Y Acad Sci. 2007;1118:18–42. doi: 10.1196/annals.1412.003. [DOI] [PubMed] [Google Scholar]
- 54.Beard J. Recent evidence from human and animal studies regarding iron status and infant development. J Nutr. 2007;137:524s–530s. doi: 10.1093/jn/137.2.524S. [DOI] [PubMed] [Google Scholar]
- 55.Zhou SJ, Gibson RA, Crowther CA, Baghurst P, Makrides M. Effect of iron supplementation during pregnancy on the intelligence quotient and behavior of children at 4 y of age: long-term follow-up of a randomized controlled trial. Am J Clin Nutr. 2006;83:1112–7. doi: 10.1093/ajcn/83.5.1112. [DOI] [PubMed] [Google Scholar]
- 56.Lind T, Lönnerdal B, Stenlund H, Gamayanti IL, Ismail D, Seswandhana R, Persson LA. A community-based randomized controlled trial of iron and zinc supplementation in Indonesian infants: effects on growth and development. Am J Clin Nutr. 2004;80:729–36. doi: 10.1093/ajcn/80.3.729. [DOI] [PubMed] [Google Scholar]
- 57.Black MM, Baqui AH, Zaman K, et al. Iron and zinc supplementation promote motor development and exploratory behavior among Bangladeshi infants. Am J Clin Nutr. 2004;80:903–10. doi: 10.1093/ajcn/80.4.903. [DOI] [PubMed] [Google Scholar]
- 58.Lozoff B, Brittenham GM, Wolf AW, et al. Iron deficiency anemia and iron therapy effects on infant developmental test performance. Pediatrics. 1987;79:981–95. [PubMed] [Google Scholar]
- 59.Logan S, Martins S, Gilbert R. Iron therapy for improving psychomotor development and cognitive function in children under the age of three with iron deficiency anaemia. Cochrane Database Syst Rev. 2001;(2):CD001444. doi: 10.1002/14651858.CD001444. [DOI] [PubMed] [Google Scholar]
- 60.Idjradinata P, Pollitt E. Reversal of developmental delays in iron-deficient anaemic infants treated with iron. Lancet. 1993;34:1–4. doi: 10.1016/0140-6736(93)92477-b. [DOI] [PubMed] [Google Scholar]
- 61.Akman M, Cebeci D, Okur V, Angin H, Abali O, Akman AC. The effects of iron deficiency on infants’ developmental test performance. Acta Paediatr. 2004;93:1391–6. [PubMed] [Google Scholar]
- 62.Metallinos-Katsaras E, Valassi-Adam E, Dewey KG, Lönnerdal B, Stamoulakatou A, Pollitt E. Effect of iron supplementation on cognition in Greek preschoolers. Eur J Nutr. 2004;58:1532–42. doi: 10.1038/sj.ejcn.1602005. [DOI] [PubMed] [Google Scholar]
- 63.Beard JL. Iron deficiency alters brain development and functioning. J Nutr. 2003;133:1468S–1472S. doi: 10.1093/jn/133.5.1468S. [DOI] [PubMed] [Google Scholar]
- 64.González HF, Malpeli A, Etchegoyen G, et al. Acquisition of visuomotor abilities and intellectual quotient in children aged 4–10 years: relationship with micronutrient nutritional status. Biol Trace Elem Res. 2007;120:92–101. doi: 10.1007/s12011-007-8023-5. [DOI] [PubMed] [Google Scholar]
- 65.Fenson L, Marchman VA, Thal D, Dale P, Reznick JS, Bates E. User’s Guide and Technical Manual. 2. Baltimore, MD: Paul H. Brookes Publishing Company; 2007. The MacArthur-Bates Communicative Development Inventories. [Google Scholar]
- 66.Brown JH, Johnson MH, Paterson SJ, Gilmore R, Longhi E, Karmiloff-Smith A. Spatial representation and attention in toddlers with Williams syndrome and Down syndrome. Neuropsychologia. 2003;41:1037–46. doi: 10.1016/s0028-3932(02)00299-3. [DOI] [PubMed] [Google Scholar]
- 67.Kannass KN, Oakes LM, Shaddy DJ. A longitudinal investigation of the development of attention and distractibility. J Cog Dev. 2006;7:381–409. [Google Scholar]
- 68.Weissberg R, Ruff HA, Lawson KR. The usefulness ofreaction time tasks in studying attention and organization of behavior in young children. Devel Behav Pedia. 1990;11:59–64. [PubMed] [Google Scholar]
- 69.Scerif G, Cornish K, Wilding J, Driver J, Karmiloff-Smith A. Visual search in typically developing toddlers and toddlers with Fragile X or Williams syndrome. Dev Sci. 2004;7:116–130. doi: 10.1111/j.1467-7687.2004.00327.x. [DOI] [PubMed] [Google Scholar]
- 70.Heffelfinger AK, Craft S, White DA, Shyken J. Visual attention in preschool children prenatally exposed to cocaine: Implications for behavioral regulation. J Int Neuropsychol Soc. 2002;8:12–21. [PubMed] [Google Scholar]
- 71.Meltzoff AN. What infant memory tells us about infantile amnesia: long-term recall and deferred imitation. J Exp Child Psychol. 1995;59:497–515. doi: 10.1006/jecp.1995.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Klein PJ, Meltzoff AN. Long-term memory, forgetting and deferred imitation in 12-month-old infants. Dev Sci. 1999;2:102–13. doi: 10.1111/1467-7687.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liston C, Kagan J. Brain development: memory enhancement in early childhood. Nature. 2002;419:896. doi: 10.1038/419896a. [DOI] [PubMed] [Google Scholar]
- 74.Reznick JS, Morrow JD, Goldman BD, Snyder J. The onset of working memory in infants. Infancy. 2004;6:145–54. [Google Scholar]
- 75.Kagan J, Hamburg M. The enhancement of memory in the first year. J Genet Psychol. 1981 Mar;138:3–14. doi: 10.1080/00221325.1981.10532837. [DOI] [PubMed] [Google Scholar]
- 76.Food and Nutrition Board Institute of Medicine. Dietary Reference Intakes: A risk assessment model for establishing upper intake levels for nutrients. Washington, D.C: National Academy Press; 1998. [PubMed] [Google Scholar]
- 77.Food and Nutrition Board Institute of Medicine. Dietary Reference Intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, D.C: National Academies Press; 2001. [PubMed] [Google Scholar]
- 78.Lozoff B, Clark KM, Jing Y, Armony-Sivan R, Angelilli ML, Jacobson SW. Dose-response relationships between iron deficiency with or without anemia and infant social-emotional behavior. J Pediatr. 2008;152:696–702. 702, 31–3. doi: 10.1016/j.jpeds.2007.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rush D. Periconceptional folate and neural tube defect. Am J Clin Nutr. 1994;59:511S–515S. doi: 10.1093/ajcn/59.2.511S. [DOI] [PubMed] [Google Scholar]
- 80.Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- 81.Godfrey KM, Barker DJ. Fetal programming and adult health. Public Health Nutr. 2001;4:611–24. doi: 10.1079/phn2001145. [DOI] [PubMed] [Google Scholar]
- 82.Bhutta ZA. Micronutrient needs of malnourished children. Curr Opin Clin Nutr Metab Care. 2008;11:309–314. doi: 10.1097/MCO.0b013e3282fbf5a0. [DOI] [PubMed] [Google Scholar]
- 83.Aggett P. Evidence based nutrition and health claims on foods: a renaissance? Matern Child Nutr. 2006;2:65–6. doi: 10.1111/j.1740-8709.2006.00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dauncey MJ, Bicknell RJ. Nutrition and neurodevelopment: mechanisms of developmental dysfunction and disease in later life. Nutr Res Rev. 1999;12:231–253. doi: 10.1079/095442299108728947. [DOI] [PubMed] [Google Scholar]