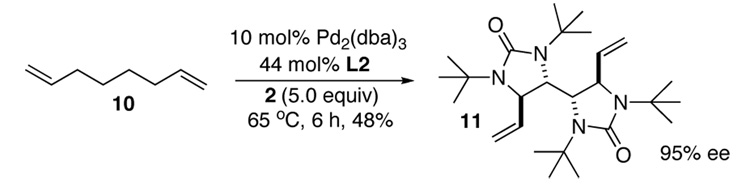Abstract
This paper describes a catalytic asymmetric diamination process for terminal olefins at allylic and homoallylic carbons via formal C-H activation using di-t-butyldiaziridinone as nitrogen source with catalyst generated from Pd2(dba)3 and chiral phosphorus amidite ligand. A wide variety of readily available terminal olefins can be effectively diaminated in good yields with high regio-, diastereo-, and enantioselectivities.
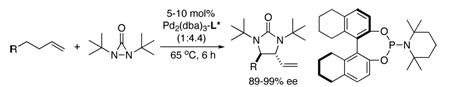
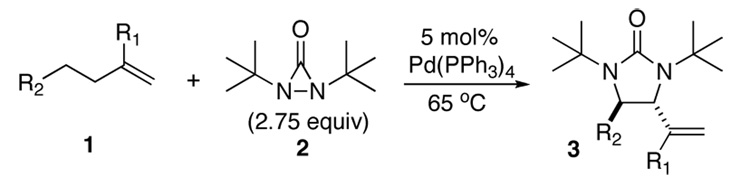
Metal-promoted diamination of olefins provides an effective approach to the synthesis of vicinal diamines, which are present in various biologically active molecules and are used as chiral control elements in asymmetric synthesis.1 Various diamination systems have been developed.1–7 Recently, we reported a Pd(0)-8,9 and Cu(I)10 -catalyzed regio- and stereoselective diamination of conjugated dienes and trienes using di-t-butyldiaziridinone (2)11 as nitrogen source. We have also shown that readily available terminal olefins can be diastereoselectively diaminated at allylic and homoallylic carbons via formal C-H activation with Pd(PPh3)4 (Scheme 1).12 Considering its synthetic potential, it is highly desirable to develop an asymmetric process for this diamination. Compared to asymmetric diamination of conjugated dienes as reported earlier,9a the current formal C-H diamination requires a catalyst system which will be able to effectively convert the terminal olefin into a conjugated diene in situ besides being enantioselective. Herein we wish to report our preliminary progress on this subject.
Scheme 1.
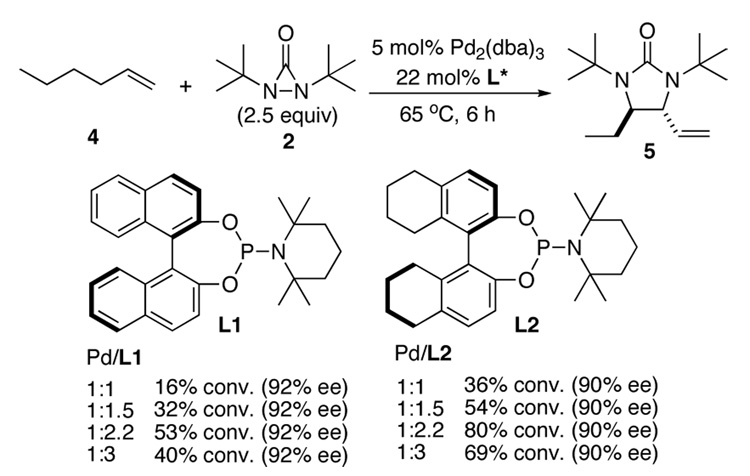
Asymmetric C-H diamination was initially investigated using 1-hexene (4) as substrate with slow addition of di-t-butyldiaziridinone (2) at 65 °C (Scheme 2) using phosphorus amidite ligand L1 (previously used for the asymmetric diamination of conjugated dienes).9a Ligand L1 gave 92% ee for 1-hexene (4) but with moderate conversion (Scheme 2). To search for more effective ligands, various commercially available or easily prepared chiral ligands13–14 were then examined for the diamination of 1-hexene under the conditions shown in Scheme 2. Studies show that conversions and ee’s of the diamination were highly dependent on the ligands used (Supporting Information). Overall, H8-BINOL-based phosphorus amidite ligand L2 gave the highest conversion for the reaction. Studies show that the reaction conversion was significantly influenced by the Pd/ligand ratio with 1:2.2 being optimal. 31P NMR studies also indicate that complexes with one Pd and two ligands were formed regardless of the Pd/ligand ratio (Supporting Information).
Scheme 2.
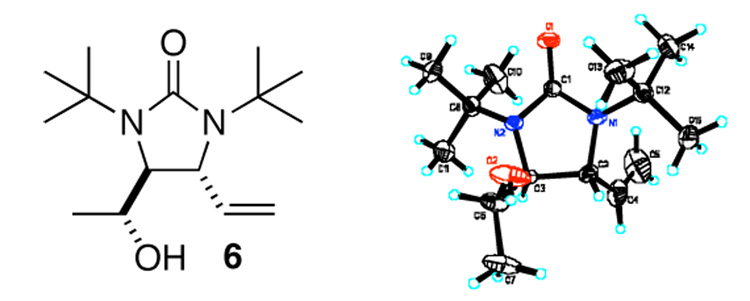
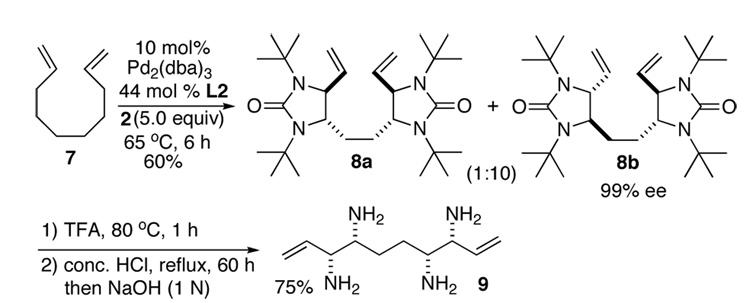
Asymmetric C-H diamination of various terminal olefins with Pd2(dba)3 and L2 was investigated. As shown in Table 1, all the diamination reactions occurred highly regio- and diastereoselectively at allylic and homoallylic carbons of terminal olefins, giving diamination products in good yields (50–85%) and high enantioselectivities (89–94% ee) (Table 1, entries 1–10). The olefin geometry for cis- and trans-1,5-undecadiene was maintained during the reaction (Table 1, entries 7 and 8). Both (R)- and (S)-5-(trimethylsiloxy)-1-hexene were also diaminated in good yields with high diastereoselectivities (Table 1, entries 11–12), indicating that the stereochemistry of diamination products was primarily determined by the chiral catalyst, and the stereogenic center of the substrate had only a small effect on diastereoselectivities. The diamination product in entry 11 was desilylated. The absolute configuration of the resulting alcohol (6) was determined to be (2R,3S,4R) by its X-ray structure (Figure 1). When 1,9-decadiene (7) was subjected to the diamination conditions (Scheme 3), bisdiamination compound 8b was obtained in 60% yield and 99% ee15 along with small amount of meso compound 8a.16 Treating 8b with CF3COOH, conc. HCl, and 1N aqueous NaOH led to the formation of optically active tetraamine 9 in 75% yield. When the reaction was carried out with 1,7-octadiene (10), compound 11 was obtained as a single diastereomer in 48% yield and 95% ee15 (Scheme 4). Since the bisdiaminations in Scheme 3 and Scheme 4 involved multiple transformations, thus a higher catalyst loading was needed.
Table 1.
Catalytic Asymmetric C-H Diamination of Terminal Olefinsa
| Entry | Substrate | Productb | Yield (%)c | ee (%)d |
|---|---|---|---|---|
 |
||||
| 1 | R = Et | 50 | 90 | |
| 2 | R =nC5H11 | 71 | 91 | |
| 3 | R = CH2Ph | 67 | 93e,f | |
| 4 | R = i-Pr | 51 | 94g | |
| 5 | R = Ph | 80 | 90e | |
| 6 |  |
80 | 92 | |
| 7 | 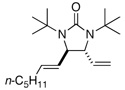 |
81 | 90g | |
| 8 | 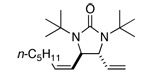 |
69 | 89 | |
| 9 | 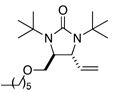 |
85 | 91 | |
| 10 | 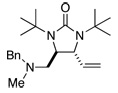 |
67 | 92 | |
| 11 | 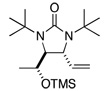 |
70 | 97:3h,i (dr) | |
| 12 | 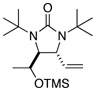 |
66 | 94:6h (dr) |
All reactions were carried out with olefin (0.80 mmol), 2 (2.0 mmol, 2.5 equiv), Pd2(dba)3 (0.04 mmol), and ligand L2 (0.176 mmol) at 65 °C for 6 h.
The structures represent only proposed absolute configurations by analogy.
Isolated yield based on olefin.
The ee was determined by chiral-GC (Chiraldex B-DM column) unless otherwise stated.
The ee was determined by chiral HPLC (Chiralpak AD column) after the removal of t-butyl groups.
The (R,R) configuration was determined by comparing the optical rotation with the reported one (see ref. 9a).
The ee was determined by chiral-GC (Chiraldex B-DM column) after the removal of t-butyl groups.
The ratio was determined by achiral GC (VA-5MS column).
The (2R,3S,4R) configuration was determined by the X-ray structure of diamination product after the removal of TMS group.
Figure 1.
The X-ray structure of 6
Scheme 3.
Scheme 4.
In summary, a catalytic asymmetric allylic and homoallylic diamination for a variety of readily available terminal olefins has been successfully achieved using di-t-butyldiaziridinone (2) as nitrogen source with a catalyst generated from Pd2(dba)3 and H8-BINOL derived phosphorus amidite ligand L2, giving diamination products in good yields with high regio-, diastereo-, and enantioselectivities. For substrates bearing two terminal double bonds, four C-N bonds can be stereoselectively constructed in one step by formally replacing four sp3 C-H bonds.17 Compared to the asymmetric diamination of conjugated dienes, the current asymmetric diamination uses readily available terminal olefins without the need to prepare conjugated dienes. This advantage is even more apparent in the cases of bisdiaminations where the stereoselective preparation of sensitive conjugated tetraenes is not necessary. Further development of a more effective asymmetric catalytic process and expansion of the substrate scope as well as synthetic application are currently underway.
Supplementary Material
The experimental procedures, the ligand studies, the characterizations, the X-ray structures of L1, L2, and 6, and the data for determination of enantiomeric excess of diamination products along with the NMR spectra of compounds (79 pages).
Acknowledgment
We are grateful to the generous financial support from the Camille and Henry Dreyfus Foundation and the General Medical Sciences of the National Institutes of Health (GM083944-01).
References
- 1.For leading reviews, see: Lucet D, Gall TL, Mioskowski C. Angew. Chem. Int. Ed. 1998;37:2580. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2580::AID-ANIE2580>3.0.CO;2-L. ; (b) Mortensen MS, O’Doherty GA. Chemtracts: Organic Chemistry. 2005;18:555. [Google Scholar]; (c) Kotti SRSS, Timmons C, Li G. Chem. Biol. Drug. Des. 2006;67:101. doi: 10.1111/j.1747-0285.2006.00347.x. [DOI] [PubMed] [Google Scholar]
- 2.For examples of metal-mediated diaminations, see: Co: Becker PN, White MA, Bergman RG. J. Am. Chem. Soc. 1980;102:5676. Hg: Barluenga J, Alonso-Cires L, Asensio G. Synthesis. 1979:962. Mn: Fristad WE, Brandvold TA, Peterson JR, Thompson SR. J. Org. Chem. 1985;50:3647. Os: Chong AO, Oshima K, Sharpless KB. J. Am. Chem. Soc. 1977;99:3420. Muñiz K. Eur. J. Org. Chem. 2004:2243. Pd: Bäckvall J-E. Tetrahedron Lett. 1978:163. Tl: Aranda VG, Barluenga J, Aznar F. Synthesis. 1974:504.
- 3.For recent Cu(II)-mediated intramolecular diamination, see: Zabawa TP, Kasi D, Chemler SR. J. Am. Chem. Soc. 2005;127:11250. doi: 10.1021/ja053335v. ; Zabawa TP, Chemler SR. Org. Lett. 2007;9:2035. doi: 10.1021/ol0706713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.For Rh(II), Fe(III)-catalyzed diamination with TsNCl2, see: Li G, Wei H-X, Kim SH, Carducci MD. Angew. Chem. Int. Ed. 2001;40:4277. doi: 10.1002/1521-3773(20011119)40:22<4277::AID-ANIE4277>3.0.CO;2-I. ; Wei H-X, Kim SH, Li G. J. Org. Chem. 2002;67:4777. doi: 10.1021/jo0200769. [DOI] [PubMed] [Google Scholar]
- 5.For a recent Pd(II)-catalyzed intermolecular diamination of conjugated dienes, see: Bar GLJ, Lloyd-Jones GC, Booker-Milburn KI. J. Am. Chem. Soc. 2005;127:7308. doi: 10.1021/ja051181d.
- 6.For recent Pd(II) and Ni-catalyzed intramolecular diamination of olefins, see: Streuff J, Hövelmann CH, Nieger M, Muñiz K. J. Am. Chem. Soc. 2005;127:14586. doi: 10.1021/ja055190y. ; (b) Muñiz K, Streuff J, Hövelmann CH, Núñez A. Angew. Chem. Int. Ed. 2007;46:7125. doi: 10.1002/anie.200702160. [DOI] [PubMed] [Google Scholar]; (c) Muñiz K. J. Am. Chem. Soc. 2007;129:14542. doi: 10.1021/ja075655f. [DOI] [PubMed] [Google Scholar]; (d) Muñiz K, Hövelmann CH, Streuff J. J. Am. Chem. Soc. 2008;130:763. doi: 10.1021/ja075041a. [DOI] [PubMed] [Google Scholar]
- 7.For leading references on asymmetric diamination using bisimidoosmium as reagent, see: Muñiz K, Nieger M. Synlett. 2003:211. ; Muñiz K, Nieger M. Chem. Commun. 2005:2729. doi: 10.1039/b502150b. [DOI] [PubMed] [Google Scholar]
- 8.(a) Du H, Zhao B, Shi Y. J. Am. Chem. Soc. 2007;129:762. doi: 10.1021/ja0680562. [DOI] [PubMed] [Google Scholar]; (b) Xu L, Du H, Shi Y. J. Org. Chem. 2007;72:7038. doi: 10.1021/jo0709394. [DOI] [PubMed] [Google Scholar]
- 9.For an asymmetric process, see: Du H, Yuan W, Zhao B, Shi Y. J. Am. Chem. Soc. 2007;129:11688. doi: 10.1021/ja074698t. ; Xu L, Shi Y. J. Org. Chem. 2008;73:749. doi: 10.1021/jo702167u. [DOI] [PubMed] [Google Scholar]
- 10.Yuan W, Du H, Zhao B, Shi Y. Org. Lett. 2007;9:2589. doi: 10.1021/ol071105a. [DOI] [PubMed] [Google Scholar]
- 11.Greene FD, Stowell JC, Bergmark WR. J. Org. Chem. 1969;34:2254. [Google Scholar]
- 12.Du H, Yuan W, Zhao B, Shi Y. J. Am. Chem. Soc. 2007;129:7496. doi: 10.1021/ja072080d. [DOI] [PubMed] [Google Scholar]
- 13.For leading references on BINOL-based phosphorus amidite ligands, see: de Vries AHM, Meetsma A, Feringa BL. Angew. Chem. Int. Ed. 1996;35:2374. ; (b) Sewald N, Wendisch V. Tetrahedron: Asymmetry. 1998;9:1341. [Google Scholar]; (c) Arnold LA, Imbos R, Mandoli A, de Vires AHM, Naasz R, Feringa BL. Tetrahedron. 2000;56:2865. [Google Scholar]
- 14.For leading references on H8-BINOL-based phosphorus amidite ligands, see: Zeng Q, Liu H, Cui X, Mi A, Jiang Y, Li X, Choi MCK, Chan ASC. Tetrahedron: Asymmetry. 2002;13:115. ; Duursma A, Minnaard AJ, Feringa BL. Tetrahedron. 2002;58:5773. [Google Scholar]
- 15.The ee was determined by chiral HPLC (Chiralcel OD column) after the removal of t-butyl groups.
- 16.A 1:1 mixture of 8a and 8b was obtained using Pd(PPh3)4 (see: ref. 12).
- 17.For leading reviews on C-H amination, see: Espino CG, Du Bois J. Chapter 17. In: Evans PA, editor. Modern Rhodium-Catalyzed Organic Reactions. Weinheim, Germany: WILEY-VCH; 2005. ; (b) Davies HML, Long MS. Angew. Chem. Int. Ed. 2005;44:3518. doi: 10.1002/anie.200500554. [DOI] [PubMed] [Google Scholar]; (c) Dick AR, Sanford MS. Tetrahedron. 2006;62:2439. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The experimental procedures, the ligand studies, the characterizations, the X-ray structures of L1, L2, and 6, and the data for determination of enantiomeric excess of diamination products along with the NMR spectra of compounds (79 pages).