Abstract
Bacteria use diverse small molecules for extra- and intracellular signaling. They scan small-molecule mixtures to access information about both their extracellular environment and their intracellular physiological status, and based on this information, they continuously interpret their circumstances and react rapidly to changes. Bacteria must integrate extra- and intracellular signaling information to mount appropriate responses to changes in their environment. We review recent research into two fundamental bacterial small-molecule signaling pathways: extracellular quorum-sensing signaling and intracellular cyclic dinucleotide signaling. We suggest how these two pathways may converge to control complex processes including multicellularity, biofilm formation, and virulence. We also outline new questions that have arisen from recent studies in these fields.
One major role of bacterial extracellular small-molecule signaling is in cell-cell communication (quorum sensing), which involves the production, release, and community-wide detection of molecules called autoinducers (1). Quorum sensing provides a mechanism for bacteria to monitor one another’s presence and to modulate gene expression in response to changes in population density. In the simplest scenario, accumulation of a threshold autoinducer concentration, which is correlated with increasing population density, initiates a signal transduction cascade that culminates in a population-wide alteration in gene expression. The synchronous response of bacterial populations to autoinducers confers a form of multicellularity to bacteria. Hence, many quorum sensing–controlled processes (e.g., bioluminescence, biofilm formation, virulence factor expression, antibiotic production, sporulation, and competence for DNA uptake) require the concerted action of numerous cells to be productive.
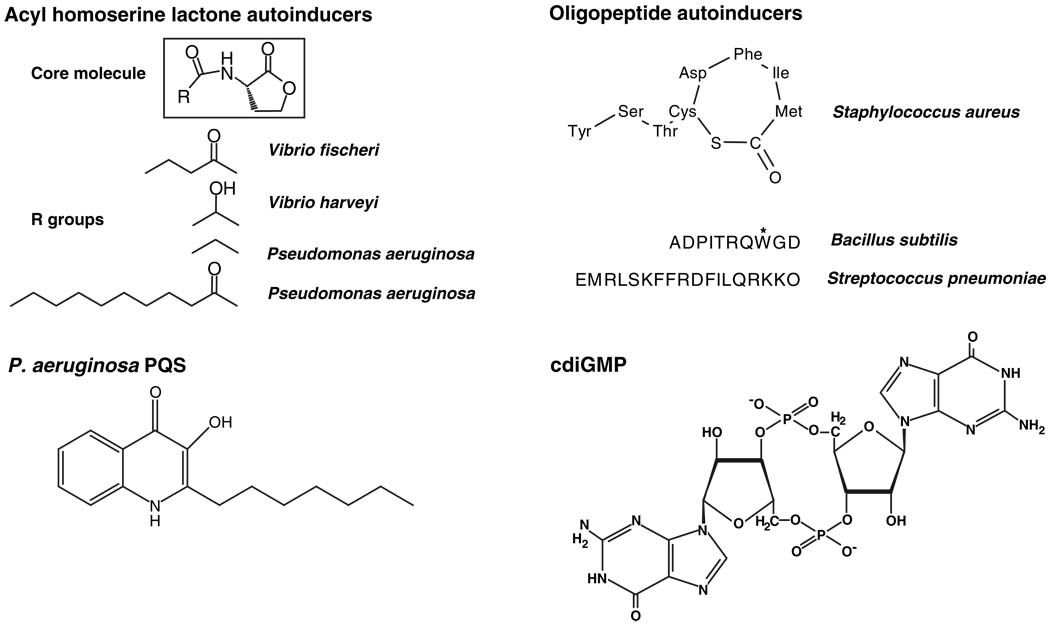
Two predominant types of small-molecule autoinducers, acyl homoserine lactones (AHLs) (2) and modified oligopeptides (3), are used by Gram-negative and Gram-positive bacteria, respectively (Fig. 1). AHLs are synthesized from S-adenosyl methionine (SAM) and particular fatty acyl carrier proteins by LuxI-type AHL synthases (4). AHL autoinducers all share the core homoserine lactone moiety, but distinct acyl side chains are incorporated into the signal molecules by the various LuxI-type enzymes (Fig. 1). Many AHLs cross membranes freely and are detected in the cytoplasm by LuxR-type proteins. Upon ligand binding, the LuxR-AHL complexes bind DNA promoter elements and activate transcription of quorum sensing–controlled genes (2). The specificity of the LuxR-AHL interaction is conferred by an acyl binding pocket in the LuxR protein, which precisely accommodates the acyl chain of its cognate AHL signal (5).
Fig. 1.
Small-molecule bacterial signals. Representative structures of autoinducer molecules used in bacterial cell-cell communication, and of the intracellular signaling molecule cdiGMP. The asterisk on the tryptophan residue of the Bacillus subtilis oligopeptide autoinducer represents an isoprenyl modification.
Gram-positive bacterial oligopeptide auto-inducers range from 5 to 17 amino acids in length (Fig. 1) and are often posttranslationally modified by the incorporation of lactone and thiolactone rings, lanthionines, and isoprenyl groups. Oligopeptide autoinducers are detected by membrane-bound two-component signaling proteins, and signal transduction occurs by a phosphorylation cascade (6). Like AHLs, different oligopeptide autoinducers often contain subtle variations, which confer signaling specificity because of the discriminatory properties of their cognate receptors. Some bacteria release and detect multiple AHLs or multiple oligo-peptides that control distinct sets of target genes (1).
These categories of signals are not comprehensive because several other small-molecule quorum-sensing autoinducers have recently been discovered. Among these, two discoveries (PQS and AI-2) are especially interesting.
The first, 2-heptyl-3-hydroxy-4-quinolone (PQS, for Pseudomonas quinolone signal) (Fig. 1) (7, 8), is produced by the opportunistic pathogen Pseudomonas aeruginosa, a colonizer of the lungs of people with cystic fibrosis (CF) (9). These infections, in which the bacteria are presumed to exist in biofilms, can persist for decades, are recalcitrant to antibiotic treatment, and are a major cause of mortality in CF patients. Together with two well-studied AHL auto-inducers, PQS functions as a quorum-sensing signal to control a battery of genes required for virulence factor expression and biofilm formation (10, 11). PQS is quite hydrophobic, obscuring any obvious mechanism for it to act as an extracellular signal; however, an exciting new study shows that a specialized vesicular transport mechanism conveys the PQS signal between P. aeruginosa cells (12). The PQS signal and other quinolones/quinolines are packaged into endogenously produced membrane vesicles that traffic the molecules between the bacterial cells. The vesicles are proposed to be crucial for efficient information transfer between P. aeruginosa cells existing in biofilms in CF sputum. Consistent with this mechanism, mutants that do not produce the vesicles do not exhibit quorum sensing–mediated communication.
P. aeruginosa produces 55 quinolones/quinolines, and although the initial steps in their biosynthesis are identical, the terminal steps are unique to each entity. For example, in the case of PQS, the product of pqsH catalyzes the final biosynthetic step. Membrane vesicle formation does not occur in a P. aeruginosa pqsH mutant even though the other 54 quinolones/quinolines are still produced. Addition of exogenous PQS restores vesicle formation to the pqsH mutant, and surprisingly, also to a pqsA mutant that is defective in production of all quinolones/quinolines. Together these experiments suggest that PQS is the critical quinolone both for signaling and for vesicle formation (12).
The P. aeruginosa membrane vesicles fuse with recipient cells, and their cargo is delivered internally, so it seems that the membrane vesicles protect the quinolones/quinolines from degradation in the environment and may also facilitate mass delivery of these molecules to neighboring cells. Additionally, many of the P. aeruginosa quinolones/quinolines have antibiotic activity against Gram-positive cells (8), so when the vesicles are delivered to a competing bacterial species, this mode of trafficking and internal delivery of contents could boost the antibacterial efficacy of quinolones/quinolines.
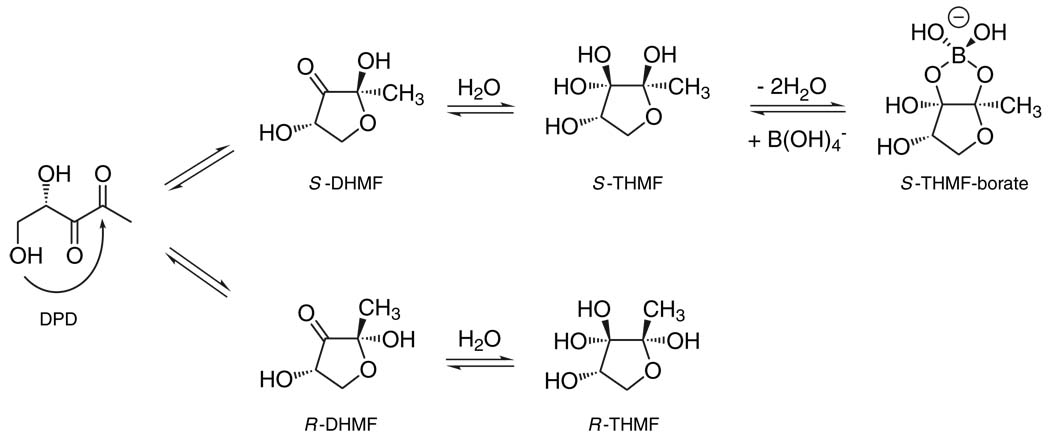
The second autoinducer that we highlight is AI-2. It is produced and detected by a wide variety of bacteria and is proposed to enable interspecies communication (1). The AI-2 synthases, called LuxS, all produce the molecule 4,5-dihydroxy-2,3-pentanedione (DPD), which undergoes a variety of spontaneous rearrangements (13). Different species of bacteria recognize distinctly rearranged DPD moieties (Fig. 2), which allows bacteria to respond to AI-2 derived from their own DPD and also to that produced by other bacterial species (13, 14). Some bacteria, including Escherichia coli and Salmonella enterica serovar Typhimurium, produce and consume AI-2 (15). Examination of gene expression in mixtures of different species of bacteria shows that when E. coli produces AI-2, nearby bacterial species initiate quorum sensing–controlled behaviors in response to cumulative cell number. By contrast, consumption of AI-2 by E. coli causes neighboring species to underestimate population density, and hence they fail to initiate or incorrectly terminate quorum sensing (16). Pro- and anti-AI-2–mediated interactions could occur in natural niches, and furthermore, eukaryotes could profit from these signaling manipulations by evolving particular associations with bacterial species that use or interfere with AI-2–mediated communication. Such associations may be important for the maintenance of the normal human gut microflora and in bacterial disease.
Fig. 2.
AI-2: an interconverting family of extracellular signal molecules. The precursor molecule, DPD, undergoes various rearrangements and additional reactions to form distinct biologically active AI-2 signal molecules. The Vibrio harveyi AI-2 (S-THMF-borate) is produced by the upper pathway, and the Salmonella enterica serovar Typhimurium AI-2 (R-THMF) is produced by the lower pathway (13, 14).
Other prokaryote-prokaryote and eukaryote-prokaryote mechanisms for interference with AHL and oligopeptide signaling have been reported. For example, different strains of Staphylococcus aureus produce similar oligo-peptide autoinducers that stimulate their own quorum-sensing cascades while cross-inhibiting oligopeptide-mediated signaling in other strains (17). Many Bacillus species release an enzyme, AiiA, that cleaves the lactone rings from AHLs, rendering them impotent (18). The alga Delisea pulchra coats its surface with a mixture of halogenated furanones that are structurally similar to AHLs. The furanones are internalized by bacteria, bind to LuxR-type proteins, and destabilize them (19). Primary and immortalized human epithelial cell lines inactivate a P. aeruginosa AHL autoinducer, suggesting that humans may have evolved quorum-sensing interference strategies for resisting pathogens (20). These natural quorum-sensing interference strategies have been exploited in a number of systems to inhibit bacteria that depend on quorum sensing for virulence. Analogous mechanisms for enhancing quorum sensing–controlled behaviors probably also exist and may play out in niches in which such behaviors benefit the organisms cohabitating with quorum-sensing bacteria.
Intracellular Small-Molecule Signaling
How are population-wide responses to small molecules related to the responses of individual bacteria? Decoding extracellular information requires signal transduction across the bacterial cell membrane. For quorum-sensing signals, this is mediated either by diffusion of auto-inducers across the membrane or by phosphor-relay from membrane-bound receptors feeding into cytoplasmic second-messenger cascades. Second-messenger systems can integrate many sensory inputs and offer flexibility of recognition and response.
Adenosine 3′,5′ monophosphate (cAMP) and guanosine-3,5-bis(pyrophosphate) (ppGpp) are common second messengers in bacteria. cAMP is synthesized from ATP by one or more adenylate cyclases, and it allosterically activates a transcription factor, catabolite regulation protein (CRP), to regulate catabolic operons for use of alternative carbon sources and other cellular processes (21). ppGpp is produced from guanosine 5′-triphosphate (GTP) by a ribosome-associated protein in response to low levels of charged tRNAs. ppGpp binds to RNA polymerase and alters its activity to repress genes encoding ribosomal RNA and tRNA (22), whereas genes specifying amino acid synthesis and transport are activated (23). Guanosine 3′,5′-monophosphate (cGMP), an important second messenger in eukaryotes, appears to be rarely used in bacteria. Rather, new evidence suggests that the cyclic dinucleotide 3′,5′-cyclic diguanylic acid (cdiGMP) (Fig. 1) is widely used by bacteria. We concentrate on this molecule because recent research indicates that it serves as the focal point for several extracellular sensory inputs, and because of its role in regulating complex cellular processes that are also regulated by quorum sensing.
cdiGMP was first identified as an allosteric activator of cellulose synthase in Gluconacetobacter (formerly Acetobacter) xylinum (24), a bacterium associated with grapes. Diguanylate cyclases (DGCs) and phosphodiesterases A (PDEAs) are responsible for synthesis and breakdown of cdiGMP, respectively (25–27). The best studied DGC is PleD, one of several signaling proteins required for cellular differentiation in the aquatic bacterium Caulobacter crescentus. Analysis of the crystal structure of PleD complexed with cdiGMP led to a model in which two PleD monomers dimerize and catalyze cdiGMP synthesis from two molecules of GTP (28). Phosphorylation of PleD regulates its DGC activity, presumably by promoting dimerization (25). Regulation of DGC activity by protein phosphorylation also occurs in Borrelia burgdorferi, the causative agent of Lyme disease (26). Thus, phosphorelays can influence second-messenger cdiGMP pathways. It is not known if other signaling systems, such as those involving cAMP or ppGpp, intersect with cdiGMP pathways.
DGC and PDEA domain proteins are found in most bacterial phyla but are absent from Archaea and Eukarya (30). In some genera, such as Vibrio and Pseudomonas, these modules exist in many dozens of proteins. Their occurrence in transmembrane or membrane-associated proteins that contain sensory domains led to the prediction that DGC and PDEA domains were important in relaying external sensory information into the cytoplasm. Environmental stimuli, such as molecular oxygen, amino acids, electrons, and photons, are believed to regulate the activity of DGC or PDEA proteins (29, 30), and it seems that cdiGMP is the common second messenger for many external signals.
It is clear that one regulatory function mediated by cdiGMP is the control of gene expression. For example, early during cholera disease, the internal cdiGMP concentration in Vibrio cholerae is reduced, leading to activation of virulence genes and repression of biofilm-formation genes (31, 32). Similarly, reduction of cdiGMP in Salmonella enterica serovar Typhimurium activates the expression of virulence genes required for survival within host cells (33). Despite rapid advances in our understanding of cdiGMP as a signaling molecule, we have yet to discover the molecular mechanism underlying its regulatory effects. It may influence DNA binding proteins and thus directly affect gene expression, and/or act on structural proteins and enzymes and direct cell physiology through posttranscriptional mechanisms.
One model for cdiGMP signaling is that it rapidly diffuses throughout the cytoplasm to act as a common allosteric regulator of proteins that control various processes. A contrasting model is that cdiGMP is spatially restricted to microdomains near the cytoplasmic membrane, where fluxes in its concentration mediate allosteric regulation of nearby membrane-bound or membrane-associated proteins. This would allow the cell to have distinct cdiGMP-regulated responses to different stimuli. Consistent with this second model, in G. xylinus, virtually all DGC and PDEA proteins, 90% of the total cellular cdiGMP, and the only known target of cdiGMP regulation (cellulose synthase) are located in the membrane fraction (27, 34). Additionally, PleD becomes localized to one cell pole in C. crescentus following phosphorylation and activation by its cognate sensor kinases, suggesting that a localized flux of cdiGMP may be important for subsequent developmental changes in this organism (26). A somewhat analogous situation exists in eukaryotic cells where diffusion of cAMP appears, in some cases, to be restricted to cellular microdomains by the organization of cAMP-specific phosphodies-terases into a boundary (35, 36).
The preponderance of proteins containing DGC and PDEA domains in some species of bacteria (e.g., 61 in V. cholerae) lends credence to the concept that spatial restriction of cdiGMP provides high-fidelity signaling. However, recent data suggest that the enzymatic activities of most DGC and PDEA proteins are tightly regulated by phosphorylation or other modifications (25). Thus, the background activities of DGC and PDEA enzymes could be minimal under most conditions, so spatial restriction of cdiGMP may not be a general requirement. Identifying the subcellular locations of and protein-protein interactions between bacterial DGC, PDEA, and cdiGMP-regulated proteins will help to distinguish whether one or the other model, or both models, operate.
Quorum sensing and cdiGMP signaling regulate some of the same complex processes, namely biofilm formation, multicellularity, and virulence, so it stands to reason that these two signaling pathways may be linked. Although a direct connection, such as an autoinducer activating a membrane-bound DGC or PDEA, has yet to be reported, the evidence for indirect interplay is strong. In V. cholerae, the quorum sensing–regulated transcription factor AphA influences expression of genes encoding DGCs and PDEAs in addition to those encoding virulence factors (37). Also, formation of a symbiotic biofilm community between the hyperthermophiles Thermotoga maritima and Methanococcus jannaschii is mediated by quorum sensing and, very likely, cdiGMP signaling, because genes for DGCs and PDEAs were up-regulated and down-regulated, respectively, during formation of the biofilm (38).
Conclusions and Prospects
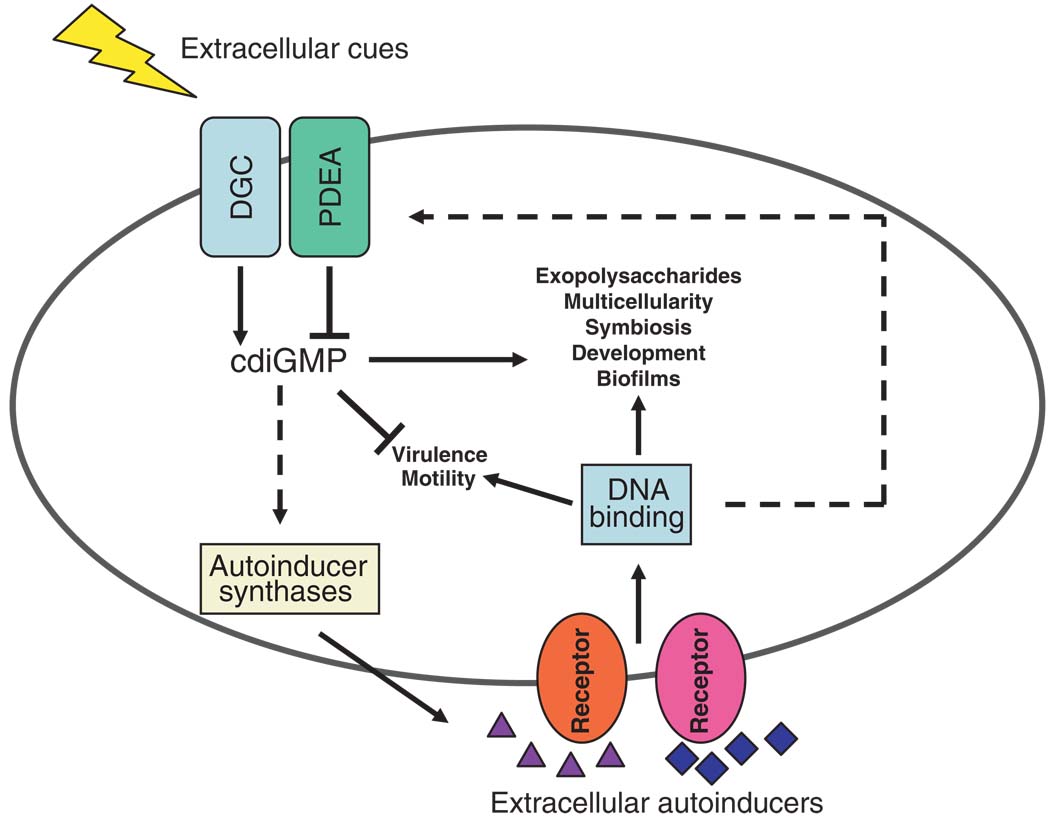
In our opinion, future research will reveal an explicit connection between extracellular quorum-sensing signaling and intracellular cdiGMP signaling. That quorum sensing and cdiGMP play critical roles in biofilm formation, as well as in other related processes, is a clue that the two signaling processes converge. Many non–mutually exclusive possibilities exist for interconnections between quorum sensing and cdiGMP signaling in different bacteria (Fig. 3). As in T. maritima, quorum sensing could regulate the expression of genes encoding proteins with DGC and PDEA activities, and thus, quorum sensing controls the cellular level of cdiGMP. Alternatively or additionally, cdiGMP could impinge on the expression or the activity of autoinducer synthases and thus affect production of quorum-sensing signals. Feedback regulation between the two kinds of signaling processes might also exist. Ultimately, if a molecular link is discovered between quorum sensing and cdiGMP signaling, it will confirm that bacteria can convert extracellular population-density information into intracellular second-messenger signals that change gene expression, cellular physiology, and group behavior. It is very likely that similar molecular signaling mechanisms underpin collective behaviors of cells in higher organisms.
Fig. 3.
Possible convergence of quorum sensing and cdiGMP signaling in the regulation of diverse bacterial behaviors. Cell density–dependent extracellular autoinducers activate membrane-bound (shown) or cytoplasmic sensory receptor proteins (not shown) which, through DNA binding proteins, regulate cellular processes including those listed. A variety of extracellular signals activate membrane-bound (shown) or cytoplasmic DGC and PDEA domain proteins (not shown), which synthesize and hydrolyze cdiGMP, respectively. cdiGMP functions as an activator or repressor of many of the same cellular processes regulated by quorum signaling (listed). Hypothetical connections between quorum-sensing and cdiGMP signaling are denoted by dashed lines.
References and Notes
- 1.Waters CM, Bassler BL. Annu. Rev. Cell Dev. Biol. 2005;21:319. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 2.Fuqua C, Parsek MR, Greenberg EP. Annu. Rev. Genet. 2001;35:439. doi: 10.1146/annurev.genet.35.102401.090913. [DOI] [PubMed] [Google Scholar]
- 3.Kleerebezem M, Quadri LE, Kuipers OP, de Vos WM. Mol. Microbiol. 1997;24:895. doi: 10.1046/j.1365-2958.1997.4251782.x. [DOI] [PubMed] [Google Scholar]
- 4.More MI, et al. Science. 1996;272:1655. doi: 10.1126/science.272.5268.1655. [DOI] [PubMed] [Google Scholar]
- 5.Zhang RG, et al. Nature. 2002;417:971. doi: 10.1038/nature00833. [DOI] [PubMed] [Google Scholar]
- 6.Novick RP. Mol. Microbiol. 2003;48:1429. doi: 10.1046/j.1365-2958.2003.03526.x. [DOI] [PubMed] [Google Scholar]
- 7.Pesci EC, et al. Proc. Natl. Acad. Sci. U.S.A. 1999;96:11229. doi: 10.1073/pnas.96.20.11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deziel E, et al. Proc. Natl. Acad. Sci. U.S.A. 2004;101:1339. doi: 10.1073/pnas.0307694100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh PK, et al. Nature. 2000;407:762. doi: 10.1038/35037627. [DOI] [PubMed] [Google Scholar]
- 10.Schuster M, Lostroh CP, Ogi T, Greenberg EP. J. Bacteriol. 2003;185:2066. doi: 10.1128/JB.185.7.2066-2079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lazdunski AM, Ventre I, Sturgis JN. Nat. Rev. Microbiol. 2004;2:581. doi: 10.1038/nrmicro924. [DOI] [PubMed] [Google Scholar]
- 12.Mashburn LM, Whiteley M. Nature. 2005;437:422. doi: 10.1038/nature03925. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, et al. Nature. 2002;415:545. doi: 10.1038/415545a. [DOI] [PubMed] [Google Scholar]
- 14.Miller ST, et al. Mol. Cell. 2004;15:677. doi: 10.1016/j.molcel.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 15.Taga ME, Semmelhack JL, Bassler BL. Mol. Microbiol. 2001;42:777. doi: 10.1046/j.1365-2958.2001.02669.x. [DOI] [PubMed] [Google Scholar]
- 16.Xavier KB, Bassler BL. Nature. 2005;437:750. doi: 10.1038/nature03960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji G, Beavis R, Novick RP. Science. 1997;276:2027. doi: 10.1126/science.276.5321.2027. [DOI] [PubMed] [Google Scholar]
- 18.Dong YH, et al. Nature. 2001;411:813. doi: 10.1038/35081101. [DOI] [PubMed] [Google Scholar]
- 19.Manefield M, et al. Microbiology. 2002;148:1119. doi: 10.1099/00221287-148-4-1119. [DOI] [PubMed] [Google Scholar]
- 20.Chun CK, Ozer EA, Welsh MJ, Zabner J, Greenberg EP. Proc. Natl. Acad. Sci. U.S.A. 2004;101:3587. doi: 10.1073/pnas.0308750101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harman JG. Biochim. Biophys. Acta. 2001;1547:1. doi: 10.1016/s0167-4838(01)00187-x. [DOI] [PubMed] [Google Scholar]
- 22.Reiness G, Yang HL, Zubay G, Cashel M. Proc. Natl. Acad. Sci. U.S.A. 1975;72:2881. doi: 10.1073/pnas.72.8.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paul BJ, Berkmen MB, Gourse RL. Proc. Natl. Acad. Sci. U.S.A. 2005;102:7823. doi: 10.1073/pnas.0501170102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ross P, et al. Nature. 1987;325:279. doi: 10.1038/325279a0. [DOI] [PubMed] [Google Scholar]
- 25.Paul R, et al. Genes Dev. 2004;18:715. doi: 10.1101/gad.289504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryjenkov DA, Tarutina M, Moskvin OV, Gomelsky M. J. Bacteriol. 2005;187:1792. doi: 10.1128/JB.187.5.1792-1798.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tal R, et al. J. Bacteriol. 1998;180:4416. doi: 10.1128/jb.180.17.4416-4425.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan C, et al. Proc. Natl. Acad. Sci. U.S.A. 2004;101:17084. doi: 10.1073/pnas.0406134101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romling U, Gomelsky M, Galperin MY. Mol. Microbiol. 2005;57:629. doi: 10.1111/j.1365-2958.2005.04697.x. [DOI] [PubMed] [Google Scholar]
- 30.Jenal U. Curr. Opin. Microbiol. 2004;7:185. doi: 10.1016/j.mib.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 31.Tischler AD, Camilli A. Mol. Microbiol. 2004;53:857. doi: 10.1111/j.1365-2958.2004.04155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tischler AD, Camilli A. Infect. Immun. 2005;73:5873. doi: 10.1128/IAI.73.9.5873-5882.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hisert KB, et al. Mol. Microbiol. 2005;56:1234. doi: 10.1111/j.1365-2958.2005.04632.x. [DOI] [PubMed] [Google Scholar]
- 34.Weinhouse H, et al. FEBS Lett. 1997;416:207. doi: 10.1016/s0014-5793(97)01202-7. [DOI] [PubMed] [Google Scholar]
- 35.Perry SJ, et al. Science. 2002;298:834. doi: 10.1126/science.1074683. [DOI] [PubMed] [Google Scholar]
- 36.Cui X, et al. Eur. J. Pharmacol. 2002;451:295. doi: 10.1016/s0014-2999(02)02294-x. [DOI] [PubMed] [Google Scholar]
- 37.Kovacikova G, Lin W, Skorupski K. Mol. Microbiol. 2005;57:420. doi: 10.1111/j.1365-2958.2005.04700.x. [DOI] [PubMed] [Google Scholar]
- 38.Johnson MR, et al. Mol. Microbiol. 2005;55:664. doi: 10.1111/j.1365-2958.2004.04419.x. [DOI] [PubMed] [Google Scholar]
- 39.This work was funded by the Howard Hughes Medical Institute and the NIH.