Abstract
Background
China's National Free Antiretroviral Treatment Program began in 2002 and, by August 2008, included over 52,000 patients.
Objective
To report five year outcomes on adult mortality and immunological treatment failure rates and risk factors.
Design
Open cohort analysis of prospectively collected observational database.
Patients
All patients in national treatment database from June 2002-August 2008. Patients excluded if not started on triple therapy or had missing treatment regimen information.
Intervention
Antiretroviral therapy per Chinese national treatment guidelines.
Measurements
Mortality rate and immunologic treatment failure rate using World Health Organization criteria.
Results
Of 52,191 total patients, 48,785 were included. Median age was 38 years, 58% were male, 53% were infected through plasma/blood, and median baseline CD4 cell count was 118/μL. Mortality was greatest during the first three months of treatment (22.6/100 person-years) but declined to a steady rate of 4-5/100 person-years after six months and maintained over the subsequent 4½ years. Baseline CD4 cell count <50/μL (adjusted hazard ratio [HR] 3.3, 95% confidence interval [CI] 2.9-3.8, compared to ≥200/μL) and having 4-5 baseline symptom categories (adjusted HR 3.4, 95% CI 2.9-4.0, compared to no baseline symptoms) were the strongest mortality risk factors. Treatment failure was determined among 31,070 with ≥1 follow-up CD4 cell count. Overall, 25% (12.0/100 person-years) failed treatment with the cumulative treatment failure rate increasing to 50% at five years.
Limitation
Immunologic treatment failure does not necessarily correlate well with virologic treatment failure.
Conclusions
The National Free Antiretroviral Treatment Program reduced mortality among adult AIDS patients in China to rates comparable to other low or middle-income countries. A cumulative immunological treatment failure rate of 50% after five years, with limited availability of second-line regimens, is of great concern.
Introduction
In China, an estimated 700,000 people are infected with the human immunodeficiency virus (HIV), of whom about 85,000 have developed the acquired immunodeficiency syndrome (AIDS) (1). Of these, a cumulative 223,501 and 62,838, respectively, had been identified as of October 2007 (2). Before 2002, when China initiated its National Free Antiretroviral Treatment Program as a pilot project among former plasma donors (3, 4), antiretroviral therapy was not readily available. Treatment was rapidly scaled up and, by August 2008, over 52,000 people had received first-line highly active antiretroviral therapy (HAART). A few nongovernmental organizations also provide treatment in China and some patients self-pay but an estimated 97% of patients in China receive free treatment through the national program. Currently, all HIV-infected individuals who meet the national treatment criteria are eligible for treatment and patients have been treated in all 31 provinces, autonomous regions, and municipalities in China.
The feasibility of implementing HAART in developing countries has been demonstrated in multiple studies, with one year outcomes often comparable to those in developed countries (5-10). Longer term data of the sustainability of such outcomes have also been reported but have either been in relatively small numbers or for only slightly longer durations (11-22). We report the five year outcomes on mortality and immunological treatment failure rates and risk factors of all previously treatment-naïve adult patients enrolled in the China National Free Antiretroviral Treatment Program.
Methods
Study Design and Setting
The National Free Antiretroviral Treatment Program and its observational database has been previously described (3, 4, 23). Briefly, all HIV positive patients in China who meet the national treatment guidelines of CD4 cell count <200/μL, total lymphocyte count <1200/mL, or World Health Organization (WHO) stage III or IV disease are eligible to receive HAART (24). The first line treatment regimen is composed of zidovudine or stavudine with nevirapine, all Chinese generically produced. Didanosine (generic) was used as the third drug until 2005, when lamivudine (branded) became available. Following treatment initiation, visits are scheduled at 2 weeks, 1 month, 2 months, 3 months, and then every three months thereafter. Local healthcare providers from the program complete visit-specific forms at each visit.
Patient Selection
All patients in the database from June 2002 through 30-Aug-2008 were eligible. Patients were excluded if they did not receive treatment through the national program, were not previously ART-naïve, were <18 years old at treatment initiation, were not started on appropriate triple therapy, or had missing treatment dates. Thirty-five CD4 cell counts >3000/μL were considered inaccurate and excluded as well. Patients without a treatment termination date were considered active if their most recent follow-up visit was within six months of 30-Aug-2008 and late if not. Henan Province did not participate in the national treatment database until July 2006 so baseline CD4 cell counts for Henan patients before July 2006 were collected instead from the national HIV epidemiology database, independently maintained at the China CDC.
Variables and Data Collection
Case report forms from each visit were forwarded to the Chinese Center for Disease Control and Prevention (China CDC) via DataFax (Clinical DataFax Systems Inc., Hamilton, Ontario, Canada). Data collected included demographics, current symptoms, laboratory results, treatment regimen start/stop dates and reason for change, and treatment termination reasons (24). Each data field of each form was manually compared to the faxed digital image by two separate reviewers to ensure accurate electronic transcription of data. Quality control queries were sent to each site to resolve missing or discrepant data. Patients with a prefectural/city level address were considered urban and those with a district/county or below level address were considered rural. Survival was calculated from the date of antiretroviral treatment initiation until death or date of last follow-up. For the analysis of immunological treatment failure and CD4 cell count response, only patients with ≥1 follow-up CD4 cell count were included. Immunological treatment failure was defined using WHO criteria: 1) after six months on treatment, CD4 cell count <100/μL; 2) after six months on treatment, CD4 cell count return to or below pre-treatment level; or 3) CD4 cell count <50% of peak on-treatment level (25). Patients meeting any of these criteria were considered treatment failures. Time to failure was defined as treatment initiation date to first CD4 cell count date meeting one of the three criteria. Data for other patients were censored on the patients' last follow-up visit date. A sensitivity analysis was also performed using one year as the treatment failure cutpoint instead of six months. For the analysis of CD4 cell count response, a three month window (six weeks before and after) around each time point was used to define the CD4 cell count for that interval. The initial six weeks after treatment initiation was included with the three month time point. If more than one CD4 cell count was performed during any interval, the one closest to each three month time point was used. The last pre-treatment CD4 cell count was defined as the baseline.
Because of the difficulty in making definitive diagnoses of opportunistic infections in rural settings, easily diagnosed signs and symptoms were used as a proxy. These were collected as part of a general review of systems and physical exam during the baseline patient visit and were categorized as: 1) fever, 2) pulmonary (cough, dyspnea, chest pain, night sweats, or lymphadenopathy), 3) gastrointestinal (nausea, vomiting, or diarrhea), 4) skin or mucosal (rash, thrush, or oral hairy leukoplakia), and 5) central nervous system (headache or visual changes).
Analysis
Baseline characteristics between cohorts were compared using the Mann-Whitney test for continuous variables because none fulfilled the Kolmogorov-Smirnov test for normality. Pearson χ2 statistic was used for dichotomous and categorical variables. Cox proportional hazard modeling was used to assess hazard ratios (HR) between the outcome (mortality or treatment failure) and potential risk factors. Covariates predetermined to be clinically significant were entered into full multivariable Cox models. Survival curves were calculated by life tables with statistical significance between groups assessed by the log-rank test because the assumption of proportionality was fulfilled. CD4 cell counts over time were modeled using the mixed linear model with maximum likelihood estimation. Data were analyzed using SPSS version 13.0 (SPSS Inc., Chicago, IL) and SAS version 9.13 (SAS Institute Inc., Cary, NC). All hypothesis testing was 2-sided with α=0.05. This analysis was approved by the institutional review board of the China CDC.
Funding Source
This study was funded by the applied research program on AIDS prevention and treatment of the China Ministry of Health, the U.S. National Institutes of Health, and a cooperative agreement from the U.S. Centers for Disease Control and Prevention Global AIDS Program to the China CDC. The U.S. sponsors, through co-authors MB and RYC, were involved in the study design, data analysis and interpretation, manuscript writing, and decision to submit the paper for publication.
Results
Of 52,191 AIDS patients in the National Free Antiretroviral Treatment Program database through 30-Aug-2008, 3406 were excluded (Figure 1). Of the 48,785 patients included, 70% were active, 10% were late, and 13% died (90% AIDS-related). Seven percent terminated treatment for medication adverse effects (46%), patient request (35%), poor adherence (9%), and other (10%). Patients from Henan Province comprised 47%, Yunnan 15%, Guangxi 11%, Anhui 5%, and Xinjiang, Hubei, and Guangdong 3% each, with the remaining 13% distributed among the other 24 provinces, autonomous regions, and municipalities. Median follow-up time was 17 months (interquartile range [IQR] 5-37) with a median of 7 follow-up visits (IQR 4-9). Median age was 38 years, 58% were male, 75% were married, and 53% were infected through plasma or blood (Table 1). Eighty-two percent were classified as rural, with 91% of former plasma donors being rural and 72% of non-former plasma donors being rural (P<0.001 for difference). Baseline median CD4 cell count was 118/μL (among those with a baseline CD4 cell count) and 81% had at least one baseline symptom category. Treatment regimens consisted of zidovudine or stavudine and nevirapine as two of the three drugs, with zidovudine and stavudine usage rates similar. Didanosine was the third drug in 46% and lamivudine in 43%. These four regimens comprised 89% of all initial regimens. A small amount of efavirenz (9%) was used for those diagnosed with HIV-tuberculosis co-infection.
Figure 1.
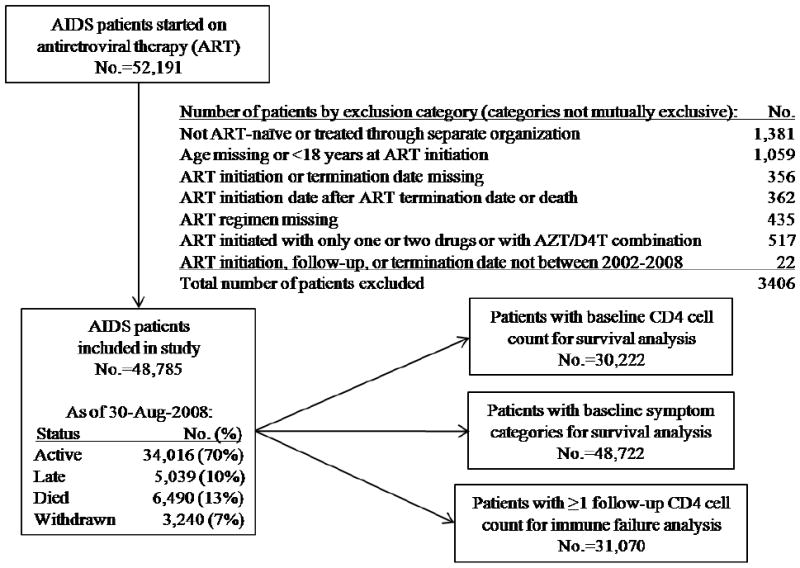
Schematic diagram of the 52,191 total patients in the National Free Antiretroviral Treatment Program, China, June 2002 – August 2008.
Note: Active patients are those without a treatment termination date recorded who were last seen within six months of August 30, 2008. Late patients are those without a treatment termination date recorded but had not been seen within six months of August 30, 2008. Withdrawn patients are patients with a treatment termination date recorded for any reason.
Table 1.
Baseline characteristics of the 48,785 patients included in the analysis, stratified by mortality and treatment failure, from the National Free Antiretroviral Treatment Program, China, June 2002 – August 2008.
| Total (No.=48785) |
Alive (No.=42295) |
Dead (No.=6490) |
Total (No.=31070) |
Treatment Success (No.=23373) |
Treatment Failure (No.=7697) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Value | No. | Value | No. | Value | P Value |
No. | Value | No. | Value | No. | Value | P Value |
|
| Age, median (IQR), years | 48785 | 38 (33-46) | 42295 | 38 (33-45) | 6490 | 40 (34-49) | <0.001 | 31070 | 38 (33-46) | 23373 | 38 (33-45) | 7697 | 39 (34-47) | <0.001 |
| Female, No. (%) | 48761 | 20441 (42) | 42273 | 18087 (43) | 6488 | 2354 (36) | <0.001 | 31061 | 13526 (44) | 23366 | 10345 (44) | 7695 | 3181 (41) | <0.001 |
| Married, No. (%) | 48728 | 36302 (75) | 42246 | 31441 (74) | 6482 | 4861 (75) | 0.33 | 31049 | 23590 (76) | 23356 | 17683 (76) | 7693 | 5907 (77) | 0.056 |
| Infection route, Former plasma donors/blood transfusion, No. (%) | 48785 | 25778 (53) | 42295 | 21283 (50) | 6490 | 4495 (69) | <0.001 | 31070 | 17222 (55) | 23373 | 12106 (52) | 7697 | 5116 (67) | <0.001 |
| Weight, median (IQR), kg | ||||||||||||||
| Female | 20381 | 53 (48-59) | 18037 | 53 (48-60) | 2344 | 50 (45-55) | <0.001 | 13499 | 53 (48-60) | 10325 | 53 (48-60) | 3174 | 53 (48-60) | 0.11 |
| Male | 28229 | 59 (52-64) | 24113 | 59 (53-65) | 4116 | 55 (50-61) | <0.001 | 17481 | 60 (54-65) | 12983 | 60 (53-65) | 4498 | 60 (54-65) | 0.69 |
| Baseline CD4 cell count (/μL), median (IQR) | 30222 | 118 (37-203) | 27319 | 127 (42-208) | 2903 | 47 (15-121) | <0.001 | 19738 | 122 (41-203) | 14845 | 117 (42-192) | 4893 | 146 (36-251) | 0.001 |
| CD4 <50/μL, No. (%) | 9037 (30) | 7543 (28) | 1494 (52) | <0.001 | 5621 (29) | 4170 (28) | 1451 (30) | 0.035 | ||||||
| Hemoglobin, median (IQR), g/dL | 42333 | 12 (11-14) | 37041 | 12 (11-14) | 5292 | 11 (10-13) | <0.001 | 27149 | 12 (11-14) | 20598 | 12 (11-14) | 6551 | 12 (11-14) | 0.27 |
| Hemoglobin <8 g/dL, No. (%) | 2070 (5) | 1554 (4) | 516 (10) | <0.001 | 1100 (4) | 807 (4) | 293 (5) | 0.047 | ||||||
| ALT (SGPT), median (IQR), U/L | 39720 | 28 (19-42) | 34977 | 28 (19-42) | 4743 | 30 (19-44) | 0.001 | 25466 | 28 (19-42) | 19525 | 28 (19-42) | 5941 | 28 (19-42) | 0.39 |
| ALT >100 U/L, No. (%) | 1370 (3.4) | 1187 (3) | 183 (4) | 0.10 | 834 (3) | 646 (3) | 188 (3) | 0.59 | ||||||
| Number of baseline symptom categories | 48722 | 42244 | 6478 | 31042 | 23350 | 7692 | ||||||||
| 0 | 9102 (19) | 8647 (21) | 455 (7) | <0.001 | 5916 (19) | 4736 (20) | 1180 (15) | |||||||
| 1 | 7533 (16) | 6898 (16) | 635 (10) | 4932 (16) | 3793 (16) | 1139 (15) | ||||||||
| 2-3 | 19636 (40) | 16747 (40) | 2889 (45) | 12324 (40) | 9187 (39) | 3137 (41) | ||||||||
| 4-5 | 12451 (26) | 9952 (24) | 2499 (39) | 7870 (25) | 5634 (24) | 2236 (29) | <0.001 | |||||||
Note: IQR=interquartile range; SD=standard deviation; ALT=alanine aminotransferase; SGPT=serum glutamic pyruvic transaminase
Five year outcomes were measured by mortality and immunological treatment failure rates. Overall, 6,490 people died across the five years, of whom 69% were former plasma donors (Table 1). Mortality was greatest during the first three months of treatment (22.6 deaths/100 person-years) but declined rapidly to 4-5 deaths/100 person-years after six months and maintained over the subsequent 4½ years (Figure 2a). Overall, 90% were alive at one year and 76% at five years. In an adjusted Cox regression analysis, the strongest risk factors for death were having a low CD4 cell count and multiple baseline symptom categories at treatment initiation. Those with CD4 cell counts <50/μL had an adjusted HR 3.3 (95% confidence interval [CI] 2.9-3.8) compared to those with CD4 cell counts ≥200/μL (Table 2). Patients with 4-5 baseline symptom categories had an adjusted HR 3.4 (95% CI 2.9-4.0) compared to those with none. This effect was even more pronounced for those dying within the first six months after treatment initiation (CD4 <50/μL: adjusted HR 4.9, 95% CI 4.1-6.0; 4-5 baseline symptom categories: adjusted HR 4.7, 95% CI 3.8-5.8). The increased risk for mortality by baseline CD4 cell count and number of baseline symptom categories persisted across the five years of this analysis (Figure 2b).
Figure 2.
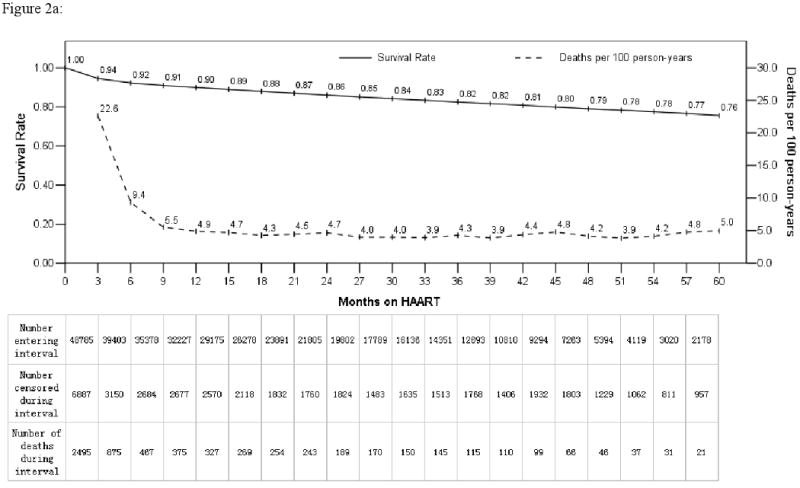
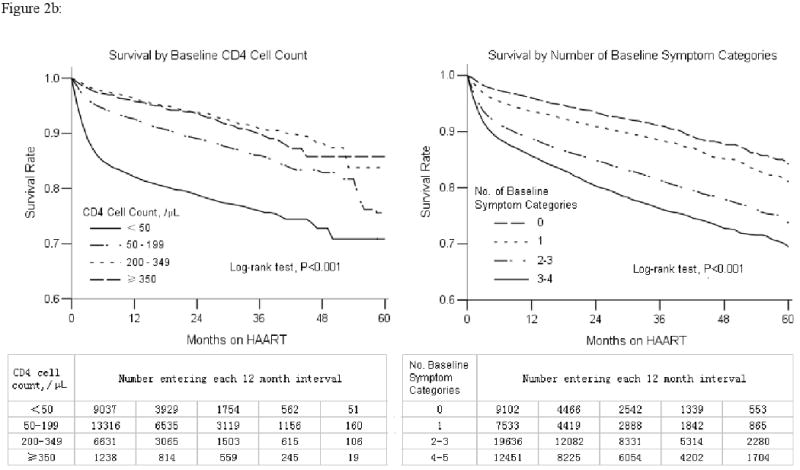
Overall change over time by mortality and life table survival rate (2a) and life table survival stratified by baseline CD4 cell count and number of baseline symptom categories (2b) following treatment initiation for previously antiretroviral therapy naïve adult AIDS patients in the National Free Antiretroviral Treatment Program, China, June 2002 – August 2008. Mortality rate shown reflects each three month interval.
Table 2.
Rates and risk factors for death or treatment failure by Cox proportional hazards regression among 48,785 patients included in the analysis (death) or the 31,070 with at least one follow-up CD4 cell count recorded (treatment failure) in the National Free Antiretroviral Treatment Program, China, June 2002 – August 2008.
| Death | Treatment Failure | Death or Treatment Failure† | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate (No.=48785) | Multivariable (No.=24526) |
Univariate (No.=31070) | Multivariable (No.=15869) |
Univariate (No.=48785) | Multivariable (No.=24526) |
||||||
| No. | Rate per 100 patient- years |
HR (95% CI) |
HR (95% CI) | No. | Rate per 100 patient- years |
HR (95% CI) |
HR (95% CI) | Rate per 100 patient- years |
HR (95% CI) | HR (95% CI) | |
| Age, years | |||||||||||
| 18-44 | 35247 | 7.1 | 1.0 | 1.0 | 22404 | 12.1 | 1.0 | 1.0 | 15.0 | 1.0 | 1.0 |
| 45-59 | 11642 | 7.5 | 1.2 (1.1-1.2) | 1.3 (1.1-1.4) | 7592 | 12.0 | 1.0 (0.9-1.0) | 1.2 (1.1-1.3) | 15.3 | 1.0 (0.9-1.0) | 1.2 (1.1-1.3) |
| ≥60 | 1896 | 14.0 | 1.8 (1.6-2.0) | 1.9 (1.5-2.2) | 1074 | 13.8 | 1.2 (1.0-1.3) | 1.1 (0.9-1.3) | 22.7 | 1.6 (1.5-1.7) | 1.5 (1.3-1.7) |
| Sex | |||||||||||
| Female | 20441 | 5.7 | 1.0 | 1.0 | 13526 | 10.5 | 1.0 | 1.0 | 12.7 | 1.0 | 1.0 |
| Male | 28320 | 9.0 | 1.5 (1.4-1.5) | 1.4 (1.3-1.5) | 17535 | 13.6 | 1.3 (1.3-1.4) | 1.2 (1.1-1.3) | 17.7 | 1.4 (1.4-1.5) | 1.2 (1.2-1.3) |
| Marriage | |||||||||||
| Married/Live Together | 36302 | 7.0 | 1.0 | 1.0 | 23590 | 11.7 | 1.0 | 1.0 | 14.6 | 1.0 | 1.0 |
| Single/Divorced/Widowed | 12426 | 9.2 | 1.2 (1.1-1.3) | 1.2 (1.1-1.3) | 7459 | 13.9 | 1.2 (1.2-1.3) | 1.1 (1.0-1.1) | 18.2 | 1.3 (1.2-1.3) | 1.1 (1.1-1.2) |
| Infection route | |||||||||||
| Sexual transmission | 13923 | 8.0 | 1.0 | 1.0 | 8715 | 14.0 | 1.0 | 1.0 | 18.2 | 1.0 | 1.0 |
| Injection drug users | 6700 | 10.4 | 1.2 (1.1-1.3) | 1.3 (1.1-1.5) | 3623 | 16.0 | 1.2 (1.1-1.3) | 1.1 (0.9-1.2) | 21.1 | 1.2 (1.1-1.3) | 1.2 (1.1-1.3) |
| Former plasma donors/blood transfusion | 25778 | 7.0 | 1.2 (1.1-1.3) | 1.4 (1.3-1.5) | 17222 | 11.2 | 0.7 (0.6-0.7) | 1.4 (1.3-1.5) | 14.0 | 0.6 (0.6-0.7) | 1.0 (0.9-1.0) |
| Baseline CD4 cell count, cells/μL | |||||||||||
| ≥200 | 7869 | 3.6 | 1.0 | 1.0 | 5124 | 26.0 | 1.0 | 1.0 | 20.6 | 1.0 | 1.0 |
| 50-199 | 13316 | 6.7 | 1.8 (1.6-2.0) | 1.7 (1.5-2.0) | 8993 | 13.2 | 0.5 (0.5-0.6) | 0.5 (0.5-0.6) | 16.3 | 0.8 (0.7-0.9) | 0.8 (0.7-0.9) |
| <50 | 9037 | 15.9 | 4.1 (3.6-4.6) | 3.3 (2.9-3.8) | 5621 | 20.9 | 0.8 (0.8-0.9) | 0.9 (0.8-0.9) | 29.6 | 1.6 (1.5-1.7) | 1.4 (1.3-1.5) |
| Hemoglobin, g/dL | |||||||||||
| ≥8 | 40263 | 6.8 | 1.0 | 1.0 | 26049 | 12.0 | 1.0 | 1.0 | 14.8 | 1.0 | 1.0 |
| <8 | 2070 | 19.4 | 2.6 (2.4-2.8) | 2.1 (1.9-2.4) | 1100 | 16.1 | 1.4 (1.2-1.5) | 1.1 (1.0-1.3) | 28.6 | 2.0 (1.9-2.2) | 1.5 (1.4-1.7) |
| ALT (SGPT), U/L | |||||||||||
| <100 | 38350 | 7.0 | 1.0 | 1.0 | 24632 | 11.9 | 1.0 | 1.0 | 15.0 | 1.0 | 1.0 |
| ≥100 | 1370 | 11.1 | 1.4 (1.2-1.6) | 1.2 (0.9-1.4) | 834 | 15.1 | 1.3 (1.1-1.5) | 1.1 (0.9-1.3) | 21.1 | 1.5 (1.4-1.7) | 1.1 (1.0-1.3) |
| Number of baseline symptom categories | |||||||||||
| 0 | 9102 | 3.7 | 1.0 | 1.0 | 5916 | 12.2 | 1.0 | 1.0 | 12.5 | 1.0 | 1.0 |
| 1 | 7533 | 4.9 | 1.5 (1.3-1.6) | 1.6 (1.3-2.0) | 4932 | 11.8 | 0.9 (0.9-1.0) | 1.0 (0.9-1.2) | 12.7 | 1.0 (0.9-1.0) | 1.1 (1.0-1.3) |
| 2-3 | 19636 | 8.0 | 2.5 (2.2-2.7) | 2.4 (2.0-2.8) | 12324 | 12.2 | 1.0 (0.9-1.0) | 1.1 (1.0-1.2) | 15.7 | 1.2 (1.1-1.3) | 1.3 (1.2-1.4) |
| 4-5 | 12451 | 9.9 | 3.1 (2.8-3.5) | 3.4 (2.9-4.0) | 7870 | 12.2 | 1.0 (0.9-1.0) | 1.1 (1.0-1.2) | 17.5 | 1.3 (1.3-1.4) | 1.5 (1.4-1.6) |
Numbers for Death or Treatment Failure column are the same as numbers for Death column.
Note: HR=hazard ratio; CI=confidence interval; ALT=alanine aminotransferase; SGPT=serum glutamic pyruvic transaminase
To understand treatment outcomes further, a subgroup of patients with at least one follow-up CD4 cell count were identified, on which immunologic treatment failure rates and CD4 cell count responses could be analyzed. Among the 48,785 patients, 31,070 (64%) had at least one follow-up CD4 cell count (median 3, IQR 2-5) performed during a median follow-up time of 25 months (IQR 12-42), with a median of 8 follow-up visits (IQR 5-10). In comparing baseline characteristics between the 31,070 with follow-up CD4 cell counts and the 17,715 without, significantly fewer of those with follow-up CD4 cell counts had baseline CD4 cell counts <50/μL (28.5% vs. 32.6% among those with baseline CD4 cell counts, P<0.001) but a similar proportion had baseline symptom categories (81.9% vs. 82.0%). Overall, 7697/31,070 patients (25%) met the definition for immunological treatment failure across the five years, 67% of whom were former plasma donors (Table 1). The cumulative proportion of treatment failure patients increased over time, from 12% (11.6/100 person-years) at one year to 50% (12.0/100 person-years overall) at five years (Figure 3). If the treatment failure cutpoint were changed from six months to one year, the cumulative failure rate would be much lower at one year (6%) but only slightly lower at five years (47%). The mean CD4 cell count response over time demonstrates the contrast between the treatment success and failure cohorts, with the treatment success group continuing to rise across the five years while the treatment failure group peaked under 250/μL at 30 months, then declined thereafter (Figure 3).
Figure 3.
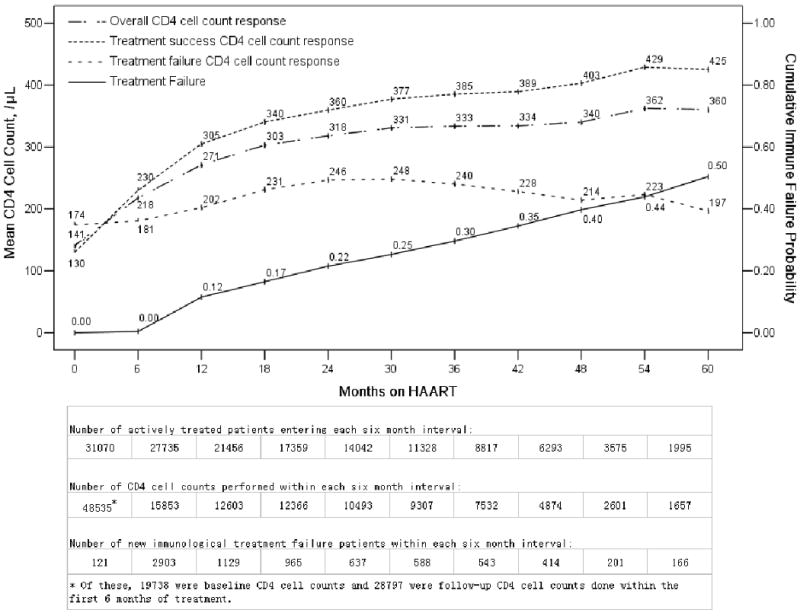
Cumulative immunological treatment failure rate and CD4 cell count response stratified by treatment success and failure over time among the 31,070 patients with at least one follow-up CD4 cell count in the National Free Antiretroviral Treatment Program, China, June 2002 – August 2008. CD4 cell counts over time were modeled using the mixed linear model with maximum likelihood estimation.
In the Cox regression analysis of treatment failure, low baseline CD4 cell counts were protective (Table 2), in contrast to the survival analysis (Figure 2b). The reason for this relates to the difference in number of CD4 cell counts done between those categorized as treatment failure versus success. The treatment failure group had median 4 (IQR 3-6) follow-up CD4 cell counts whereas the treatment success group had median 3 (IQR 2-5, P<0.001). Thus, with significantly fewer follow-up CD4 cell counts, some of those categorized as treatment successes were likely categorized as such simply because they had less opportunity to be identified as treatment failures. To confirm that this explains the seemingly protective effect of low CD4 cell counts on treatment failure, a univariate analysis of the number of follow-up CD4 cell counts was done. Each additional CD4 cell count was associated with a slightly increased risk of treatment failure (HR 1.08, 95% CI 1.07-1.09). A second univariate analysis of treatment failure patients only was done. The results were consistent with the mortality analysis, with those with the lowest CD4 cell counts at the highest risk of failure (CD4 <50/μL=HR 1.5, 95% CI 1.4-1.6; CD4 50-199/μL=HR 1.1, 95% CI 1.0-1.1; CD4 ≥200/μL=HR 1.0).
Being a former plasma donor was protective in the univariate model for treatment failure (HR 0.7, 95% CI 0.7-0.7) but at higher risk in the multivariable model (adjusted HR 1.4, 95% CI 1.3-1.5, Table 2). This change in point estimate was due to the large number of missing baseline CD4 cell counts among former plasma donors because this cohort was treated the earliest in China. Among the 31,070 patients evaluated for treatment failure, 61% of former plasma donors, 8% of sexual transmissions, and 2% of injection drug users were missing baseline CD4 cell counts. As such, only a minority of former plasma donors entered the multivariable model, biasing the final results. When CD4 cell counts were excluded from the multivariable model, the former plasma donor point estimate (adjusted HR 0.5, 95% CI 0.4-0.6) was in the same direction as the univariate result.
Discussion
China's National Free Antiretroviral Treatment Program faces severe resource limitations similar to those in other low or middle income countries because those infected are predominantly poor and live in rural, severely under-resourced areas of the country. Significant effort has been made to develop an infrastructure capable of treating large numbers of people across a wide geographic area, including training of rural healthcare workers, limited CD4 cell count monitoring, and using mostly Chinese-produced generic HIV drugs with almost no second line treatment options (3, 4). Thus, the rapid scaling up of China's national treatment program from 2002-2008 to treat 48,785 previously ART-naïve adult AIDS patients has two most notable outcomes. The first is its success in decreasing mortality from 22.6/100 person-years at three months to about 4-5/100 person-years after six months on HAART. This reduction in mortality, to levels similar to other resource-limited countries (5, 8, 18, 21), was then sustained across the subsequent 4½ years. The second notable outcome is that immunological treatment failure rates increased over time to about 50% at five years in the absence of readily available second-line treatment options.
In this study, AIDS mortality among the 48,785 patients was highest during the first six months of treatment, similar to other studies identified through an English-language MEDLINE search (5-8, 11, 26-30). Our initially high mortality, however, was likely not due primarily to treatment related factors, such as severe immune reconstitution inflammatory syndrome. In a previous analysis of 4,093 former plasma donor AIDS patients, we showed that mortality declined from 27.3/100 person-years pre-treatment to 4.6/100 person-years post-treatment (31). This previously reported decline in mortality is consistent with the results of our current, much larger analysis. Thus, although immune reconstitution inflammatory syndrome may play a contributing role, the 22.6 deaths/100 person-years over the first three months of treatment in this study likely reflects primarily the natural history of untreated AIDS, followed by a dramatic decline in mortality over the subsequent three months of HAART due to a reconstituting immune system. Our analysis of risk factors associated with mortality supports this conclusion, with those most immunosuppressed at baseline at the greatest risk of death, particularly in the first six months of treatment. Of note, this increased mortality still persisted across five years when compared to those starting treatment at less immunosuppressed levels (Figure 2b), similar to other studies (32, 33). Furthermore, our use of easily diagnosed baseline symptom categories predicted mortality as well as CD4 cell counts (Table 2) and, if validated by other studies, could be useful in other resource-limited settings.
Our analysis of immunological treatment outcomes demonstrates increasing failure over time when second-line treatment options are not widely available. Most studies of treatment outcomes only report change in CD4 cell count over time (9), not treatment failure rates. One year immunological treatment failure rates reported from Zambia (13/100 person-years) (5), Haiti (9%) (6), and Asia (6.7/100 person-years) (34) are similar to our results but use differing definitions of failure, making direct comparisons difficult. To maintain uniformity and allow comparability between cohorts, we used WHO criteria to define immunologic treatment failure. There are limitations with this approach because immunologic criteria for treatment failure are less sensitive and specific than virologic criteria (35-39), causing our failure rates to be overestimates. Conversely, with a median of only three CD4 cell counts per patient during follow-up, our failure rate at any individual time point is likely an underestimate because if more CD4 cell counts were done, more patients would be found to be in treatment failure. Our rates, therefore, should be viewed as general estimates rather than precise values. Finally, the decreased number of patients included in the multivariable model, primarily due to missing baseline CD4 cell counts, limits the generalizability of our results. These limitations, however, do not alter the very concerning overall trend of significantly increasing immunological treatment failure rates to around 50% at five years when almost no second line treatment options are available.
There are limitations to our study in addition to those discussed above. First, observational databases have potential inherent biases. Data quality is dependent on healthcare providers across the country completing data forms accurately. Missing data due to lack of patient follow-up or lab tests are problematic. However, the data collected are a reflection of real-world treatment realities in a resource-limited setting. With 48,785 patients, and results consistent with other developing country cohorts, it is unlikely that any single bias affected the results dramatically. Second, 10% of our patients were late. If mortality was significantly higher in this group, our overall mortality rate would be underestimated (40). We reviewed the independently maintained national HIV epidemiology database and found that among 3589 (71% of our late patients) with follow-up information, 321 (9%) had died. This is consistent with our overall mortality rate of 13%. Thus, it is not likely that these late patients have significantly different mortality outcomes. Third, we unfortunately did not have a reliable measure of medication adherence, which has been correlated with treatment failure (41, 42). The current case report form does have an adherence question but the way the question is phrased is so vague that the data are difficult to interpret. The form is being revised and the next version will ask a much more specific question about the number of missed doses in the previous seven days. Finally, a recent analysis demonstrated low sensitivity of the WHO immunological treatment failure criteria compared with virologic criteria (39). While the immediate, temporal correlation of immunologic and virologic treatment failure may be low, our analysis shows long-term outcome differences using the immunologic criteria with a sharp contrast between the CD4 cell count responses of the immunologic treatment failure and success cohorts (Figure 3). How these long-term immunologic outcomes correspond to virologic outcomes remain to be seen.
In summary, our analysis of the National Free Antiretroviral Treatment Program highlights both areas of success and areas needing improvement. First is not only the success of reducing mortality among AIDS patients to levels reported by other low or middle-income countries but that this low mortality rate was maintained across five years using primarily Chinese-produced generic drugs. However, the unacceptably high initial mortality following treatment initiation emphasizes the need to begin treatment sooner due to the significant associations of low baseline CD4 cell count and baseline symptoms with mortality. The national treatment program needs to encourage earlier HIV treatment through increased screening and work to reduce stigma and discrimination, which deter people from being screened and accessing care. The national treatment program is also changing its treatment initiation criterion from CD4 cell count below 200/μL to below 350/μL. Second is that successful first-line regimens will not last and that second-line regimens are essential. There is an urgent need to develop more precise definitions of treatment failure and to evaluate critically the usefulness and cost-effectiveness of monitoring tools to support long-term ART (43-46). A comprehensive approach to HIV/AIDS incorporating both efforts to initiate treatment earlier and provide second-line therapy is necessary if the short-term gains achieved from first-line therapy are to be sustained and improved. With longer-term data on the longevity of first-line antiretroviral treatment regimens in resource-limited settings, such as in our analysis, policy makers and funders of healthcare can develop more concrete estimates in planning for the amount and cost of second-line treatment regimens required in the coming years. In China, second-line therapy is now being introduced to the national treatment program and the challenge will be how to scale up access in a way which does not merely postpone treatment failure and the need for third-line treatment. Finally, as treatment improves and patients live longer, the National Free Antiretroviral Treatment Program will need to deal with co-morbidities such as long-term treatment side effects, hepatitis B and C virus co-infections, and heart disease (47).
Acknowledgments
We acknowledge the tireless labor of the many HIV healthcare providers across the country for their dedication in providing conscientious treatment and care to their patients and in completing the countless numbers of data forms that made this work possible. We also acknowledge the many international institutions and organizations which have provided technical support to the National Free Antiretroviral Treatment Program over the past several years, including the World Health Organization, UNAIDS, Global Fund to Fight AIDS, Tuberculosis and Malaria, U.S. Centers for Disease Control and Prevention Global AIDS Program, National Institute of Allergy and Infectious Diseases of the U.S. National Institutes of Health, Clinton Foundation, Pangaea Global AIDS Foundation, University of North Carolina at Chapel Hill, University of Maryland Institute of Human Virology, and Médecins Sans Frontières.
Primary Funding Sources: This study was supported by the applied research program on AIDS prevention and treatment of the China Ministry of Health (WA-2006-03), the U.S. National Institutes of Health (U2R TW006918 and R03 TW008203), and a cooperative agreement from the U.S. Centers for Disease Control and Prevention Global AIDS Program to the China CDC (1U2GPS001188-01).
Footnotes
Conflict of Interest Disclosure: All authors report no financial disclosures.
Reproducible Research Statement: Study protocol, statistical code, and data set: Not available.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the U.S. Department of Health and Human Services.
References
- 1.Wang L, Wang N, Wang L, et al. The 2007 Estimates for People at Risk for and Living With HIV in China: Progress and Challenges. J Acquir Immune Defic Syndr. 2009;50:414–418. doi: 10.1097/QAI.0b013e3181958530. [DOI] [PubMed] [Google Scholar]
- 2.State Council AIDS Working Committee Office, UN Theme Group on AIDS in China. A Joint Assessment of HIV/AIDS Prevention, Treatment and Care in China (2007) 2007 December 1; [Google Scholar]
- 3.Zhang FJ, Pan J, Yu L, Wen Y, Zhao Y. Current progress of China's free ART program. Cell Res. 2005;15:877–82. doi: 10.1038/sj.cr.7290362. [DOI] [PubMed] [Google Scholar]
- 4.Zhang F, Haberer JE, Wang Y, et al. The Chinese free antiretroviral treatment program: challenges and responses. AIDS. 2007;21 8:S143–8. doi: 10.1097/01.aids.0000304710.10036.2b. [DOI] [PubMed] [Google Scholar]
- 5.Stringer JS, Zulu I, Levy J, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006;296:782–93. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 6.Severe P, Leger P, Charles M, et al. Antiretroviral therapy in a thousand patients with AIDS in Haiti. N Engl J Med. 2005;353:2325–34. doi: 10.1056/NEJMoa051908. [DOI] [PubMed] [Google Scholar]
- 7.Ferradini L, Jeannin A, Pinoges L, et al. Scaling up of highly active antiretroviral therapy in a rural district of Malawi: an effectiveness assessment. Lancet. 2006;367:1335–42. doi: 10.1016/S0140-6736(06)68580-2. [DOI] [PubMed] [Google Scholar]
- 8.Braitstein P, Brinkhof MW, Dabis F, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–24. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 9.Ivers LC, Kendrick D, Doucette K. Efficacy of antiretroviral therapy programs in resource-poor settings: a meta-analysis of the published literature. Clin Infect Dis. 2005;41:217–24. doi: 10.1086/431199. [DOI] [PubMed] [Google Scholar]
- 10.Jahn A, Floyd S, Crampin AC, et al. Population-level effect of HIV on adult mortality and early evidence of reversal after introduction of antiretroviral therapy in Malawi. Lancet. 2008;371:1603–11. doi: 10.1016/S0140-6736(08)60693-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coetzee D, Hildebrand K, Boulle A, et al. Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. AIDS. 2004;18:887–95. doi: 10.1097/00002030-200404090-00006. [DOI] [PubMed] [Google Scholar]
- 12.Corey DM, Kim HW, Salazar R, et al. Effectiveness of combination antiretroviral therapy on survival and opportunistic infections in a developing world setting: an observational cohort study. J Acquir Immune Defic Syndr. 2007;44:451–5. doi: 10.1097/QAI.0b013e31802f8512. [DOI] [PubMed] [Google Scholar]
- 13.Madec Y, Laureillard D, Pinoges L, et al. Response to highly active antiretroviral therapy among severely immuno-compromised HIV-infected patients in Cambodia. AIDS. 2007;21:351–9. doi: 10.1097/QAD.0b013e328012c54f. [DOI] [PubMed] [Google Scholar]
- 14.Laurent C, Ngom Gueye NF, Ndour CT, et al. Long-term benefits of highly active antiretroviral therapy in Senegalese HIV-1-infected adults. J Acquir Immune Defic Syndr. 2005;38:14–7. doi: 10.1097/00126334-200501010-00003. [DOI] [PubMed] [Google Scholar]
- 15.Fairall LR, Bachmann MO, Louwagie GM, et al. Effectiveness of antiretroviral treatment in a South African program: a cohort study. Arch Intern Med. 2008;168:86–93. doi: 10.1001/archinternmed.2007.10. [DOI] [PubMed] [Google Scholar]
- 16.Ferradini L, Laureillard D, Prak N, et al. Positive outcomes of HAART at 24 months in HIV-infected patients in Cambodia. AIDS. 2007;21:2293–301. doi: 10.1097/QAD.0b013e32828cc8b7. [DOI] [PubMed] [Google Scholar]
- 17.Grinsztejn B, Veloso VG, Pilotto JH, Campos DP, Keruly JC, Moore RD. Comparison of clinical response to initial highly active antiretroviral therapy in the patients in clinical care in the United States and Brazil. J Acquir Immune Defic Syndr. 2007;45:515–20. doi: 10.1097/QAI.0b013e3180decb6a. [DOI] [PubMed] [Google Scholar]
- 18.Mermin J, Were W, Ekwaru JP, et al. Mortality in HIV-infected Ugandan adults receiving antiretroviral treatment and survival of their HIV-uninfected children: a prospective cohort study. Lancet. 2008;371:752–9. doi: 10.1016/S0140-6736(08)60345-1. [DOI] [PubMed] [Google Scholar]
- 19.Toure S, Kouadio B, Seyler C, et al. Rapid scaling-up of antiretroviral therapy in 10,000 adults in Cote d'Ivoire: 2-year outcomes and determinants. AIDS. 2008;22:873–82. doi: 10.1097/QAD.0b013e3282f768f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sherer R, Gui X, Zhan F, Teter C, Ping DL, Wykoff RF. Rapid antiretroviral therapy scale-up in Hubei Province, China. Health Aff (Millwood) 2008;27:1140–7. doi: 10.1377/hlthaff.27.4.1140. [DOI] [PubMed] [Google Scholar]
- 21.Van der Borght SF, Clevenbergh P, Rijckborst H, et al. Mortality and morbidity among HIV type-1-infected patients during the first 5 years of a multicountry HIV workplace programme in Africa. Antivir Ther. 2009;14:63–74. [PubMed] [Google Scholar]
- 22.Bussmann H, Wester CW, Ndwapi N, et al. Five-year outcomes of initial patients treated in Botswana's National Antiretroviral Treatment Program. AIDS. 2008;22(17):2303–11. doi: 10.1097/QAD.0b013e3283129db0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang FJ, editor. China Free ART Manual. Beijing: Chinese Center for Disease Control and Prevention; Jan, 2005. [Google Scholar]
- 24.Chinese Center for Disease Control and Prevention. China Free ART Manual. 2005 January; [Google Scholar]
- 25.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents: Recommendation for a public health approach. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 26.Marazzi MC, Liotta G, Germano P, et al. Excessive early mortality in the first year of treatment in HIV type 1-infected patients initiating antiretroviral therapy in resource-limited settings. AIDS Res Hum Retroviruses. 2008;24:555–560. doi: 10.1089/aid.2007.0217. [DOI] [PubMed] [Google Scholar]
- 27.Kamya MR, Mayanja-Kizza H, Kambugu A, et al. Predictors of long-term viral failure among Ugandan children and adults treated with antiretroviral therapy. J Acquir Immune Defic Syndr. 2007;46:187–93. doi: 10.1097/QAI.0b013e31814278c0. [DOI] [PubMed] [Google Scholar]
- 28.Johannessen A, Naman E, Ngowi BJ, et al. Predictors of mortality in HIV-infected patients starting antiretroviral therapy in a rural hospital in Tanzania. BMC Infect Dis. 2008;8:52. doi: 10.1186/1471-2334-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barth RE, van der Meer JT, Hoepelman AI, et al. Effectiveness of highly active antiretroviral therapy administered by general practitioners in rural South Africa. Eur J Clin Microbiol Infect Dis. 2008;27:977–84. doi: 10.1007/s10096-008-0534-2. [DOI] [PubMed] [Google Scholar]
- 30.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. Aids. 2008;22:1897–908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang F, Dou Z, Yu L, et al. The effect of highly active antiretroviral therapy on mortality among HIV-infected former plasma donors in China. Clin Infect Dis. 2008;47:825–33. doi: 10.1086/590945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hogg RS, Yip B, Chan KJ, et al. Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. JAMA. 2001;286:2568–77. doi: 10.1001/jama.286.20.2568. [DOI] [PubMed] [Google Scholar]
- 33.Antiretroviral Therapy Cohort Collaboration. Importance of baseline prognostic factors with increasing time since initiation of highly active antiretroviral therapy: collaborative analysis of cohorts of HIV-1-infected patients. J Acquir Immune Defic Syndr. 2007;46:607–15. doi: 10.1097/QAI.0b013e31815b7dba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou J, Paton NI, Ditangco R, et al. Experience with the use of a first-line regimen of stavudine, lamivudine and nevirapine in patients in the TREAT Asia HIV Observational Database. HIV Med. 2007;8:8–16. doi: 10.1111/j.1468-1293.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hosseinipour M, vanOosterhout J, Weigel R, et al. Validating clinical and immunological definitions of antiretroviral treatment failure in Malawi (abstract WEAB101). International AIDS Society Conference; Sydney, Australia. July 2007. [Google Scholar]
- 36.Basenero A, Castelnuovo B, Birabwa E, et al. Inadequacy of clinical and immunological criteria in identifying virologic failure of 1st line ART: the Ugandan experience (abstract WEAB102); International AIDS Society Conference; Sydney, Australia. July 2007. [Google Scholar]
- 37.Moore DM, Mermin J, Awor A, Yip B, Hogg RS, Montaner JS. Performance of immunologic responses in predicting viral load suppression: implications for monitoring patients in resource-limited settings. J Acquir Immune Defic Syndr. 2006;43:436–9. doi: 10.1097/01.qai.0000243105.80393.42. [DOI] [PubMed] [Google Scholar]
- 38.Bisson GP, Gross R, Strom JB, et al. Diagnostic accuracy of CD4 cell count increase for virologic response after initiating highly active antiretroviral therapy. AIDS. 2006;20:1613–9. doi: 10.1097/01.aids.0000238407.00874.dc. [DOI] [PubMed] [Google Scholar]
- 39.Mee P, Fielding KL, Charalambous S, Churchyard GJ, Grant AD. Evaluation of the WHO criteria for antiretroviral treatment failure among adults in South Africa. AIDS. 2008;22:1971–7. doi: 10.1097/QAD.0b013e32830e4cd8. [DOI] [PubMed] [Google Scholar]
- 40.Bisson GP, Gaolathe T, Gross R, et al. Overestimates of survival after HAART: implications for global scale-up efforts. PLoS ONE. 2008;3:e1725. doi: 10.1371/journal.pone.0001725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bisson GP, Gross R, Bellamy S, et al. Pharmacy refill adherence compared with CD4 count changes for monitoring HIV-infected adults on antiretroviral therapy. PLoS Med. 2008;5:e109. doi: 10.1371/journal.pmed.0050109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lima VD, Harrigan R, Bangsberg DR, et al. The Combined Effect of Modern Highly Active Antiretroviral Therapy Regimens and Adherence on Mortality Over Time. J Acquir Immune Defic Syndr. 2009;50:529–536. doi: 10.1097/QAI.0b013e31819675e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moore DM, Mermin J. Monitoring antiretroviral failure in resource-poor settings. Lancet. 2008;371:1396–7. doi: 10.1016/S0140-6736(08)60607-8. [DOI] [PubMed] [Google Scholar]
- 44.Phillips AN, Pillay D, Miners AH, Bennett DE, Gilks CF, Lundgren JD. Outcomes from monitoring of patients on antiretroviral therapy in resource-limited settings with viral load, CD4 cell count, or clinical observation alone: a computer simulation model. Lancet. 2008;371:1443–51. doi: 10.1016/S0140-6736(08)60624-8. [DOI] [PubMed] [Google Scholar]
- 45.Mills EJ, Nachega JB, Buchan I, et al. Adherence to antiretroviral therapy in sub-Saharan Africa and North America: a meta-analysis. JAMA. 2006;296:679–90. doi: 10.1001/jama.296.6.679. [DOI] [PubMed] [Google Scholar]
- 46.The ART-LINC Collaboration of the International Databases to Evaluate AIDS. Antiretroviral therapy in resource-limited settings 1996 to 2006: patient characteristics, treatment regimens and monitoring in sub-Saharan Africa, Asia and Latin America. Trop Med Int Health. 2008;13:1–10. doi: 10.1111/j.1365-3156.2008.02078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bartlett JG. Significant challenges facing HIV practitioners. J Infect Dis. 2008;197:S250–1. doi: 10.1086/533417. [DOI] [PubMed] [Google Scholar]


