Abstract
AIM: To study the outcome and prognostic factors in a series of patients with extrahepatic cholangiocarcinoma and determine the impact of comorbidity on survival.
METHODS: A retrospective analysis of 68 patients with extrahepatic cholangiocarcinoma (perihilar, n = 37; distal, n = 31) seen at a single tertiary-care institution during the period 1999-2003 was performed. Data on presentation, management, and outcome were assessed by chart review. Pathologic confirmation was obtained in 37 cases (54.4%). Comorbidity was evaluated by using the Charlson comorbidity index (CCI).
RESULTS: Mean age at diagnosis was 73.4 ± 11.5 years. Jaundice was the most common symptom presented (86.8%). Median CCI score was 1 (range, 0 to 4). Nineteen patients (27.9%) underwent tumor resection. Palliative biliary drainage was performed in 39 patients (57.4%), and 6 patients (8.8%) received only best supportive care. Tumor-free margin status (R0) was achieved in 15 cases (78.9% of resection group). Baseline serum carbohydrate antigen 19-9 (CA 19-9) level was revealed to be an independent predictor of surgical treatment (P = 0.026). Overall median survival was 3.1 ± 0.9 mo, with 1- and 2-year survival rates of 21% and 7%, respectively. In the univariate analysis, tumor resection, CCI score, and serum CA 19-9 levels correlated significantly with outcome. In the multivariate analysis, only resection (HR 0.10; 95% CI, 0.02-0.51, P = 0.005) and a CCI score ≥ 2 (HR 3.36; 95% CI, 1.0-10.9, P = 0.045) were found to independently predict survival.
CONCLUSION: Tumor resection and comorbidity emerged as significant prognostic variables in extrahepatic cholangiocarcinoma. Comorbidity evaluation instruments should be applied in the clinical management of such patients.
Keywords: Charlson index, Cholangiocarcinoma, Comorbidity, Prognosis, Survival
INTRODUCTION
Cholangiocarcinoma is a relatively uncommon malignant tumor arising in the intrahepatic or extrahepatic biliary ducts. It accounts for about 3% of all gastrointestinal cancers globally and constitutes the second most common primary hepatic malignant disease, with approximately 5000 new cases being diagnosed annually in the United States[1]. Although the entire biliary tree is potentially at risk, the perihilar region is the most frequently involved site, accounting for about 60% of all tumors[1]. However, it has been suggested that the incidence of intrahepatic forms of the disease is currently increasing in the US and the United Kingdom[2]. Cholangiocarcinoma has been characterized as a slow-growing and late metastasizing tumor, tending to spread longitudinally along the bile ducts with neural, perineural, and subepithelial extensions. Because of the late presentation of symptoms, tumors are usually diagnosed in their later stages, and thus most therapeutic approaches are not curative[1]. The prognosis for patients with unresectable disease is dismal, with the reported median survival time less than 1 year from diagnosis[3].
In developed countries, the peak age at diagnosis of cholangiocarcinoma has shifted from the sixth decade of life during the 1970s, toward the seventh decade and older nowadays. According to Patel, the median age at the time of death in individuals with intrahepatic cholangiocarcinoma - based on US vital-statistics data referring to the period between 1973 and 1997 - was 71 years for males and 74 years for females[2]. Recent works from various European institutions have reported a median age at diagnosis of 64 to 75 years[4-7]. Due to the increased ageing of the Western population, there is an emerging need to develop a means to characterize the “functional age” of older patients in order to optimize therapeutic strategies for cancer and design new multi-disciplinary approaches. Multiple studies have demonstrated that the amount of comorbidity (defined as the presence of diseases or disorders which exist before cancer diagnosis and are not treatment-related adverse effects) significantly impacts on various prognostic outcomes in oncologic patients, such as functional status[8], healthcare resources use[9], therapeutic decision-making[10], and overall survival[11]. The Charlson comorbidity index (CCI) was originally developed by Mary Charlson and colleagues in 1987 from the study of 1-year all-cause mortality in a cohort of more than 500 patients admitted to a medical unit of a teaching hospital[12]. This index has been validated in predicting mortality risk associated with a wide range of medical conditions, and constitutes one of the most commonly used comorbidity indices to date. Correlations between the severity of comorbid conditions, assessed by means of the CCI, and diverse outcomes have been observed in patients with colorectal[13], head and neck[14], non-small cell lung[15], bladder[10], clear cell renal[16], and ovarian cancer[17]. Some reports indicate that the impact of comorbidity on survival may vary among populations with different cancers. To the best of our knowledge, no previous studies have focused on the role of age and comorbidity, quantified using the CCI, on treatment decisions and clinical outcomes for patients with biliary tract malignancies. Thus, we conducted a single-center analysis of consecutive Spanish patients with extrahepatic cholangiocarcinoma to investigate the impact of these variables on the choice of therapeutic approach and patients’ overall survival.
MATERIALS AND METHODS
The tumor registry of the University Hospital “12 de Octubre” compiles data on all new cancer cases in Health Area 11 of the Community of Madrid (Central Spain), with 593 931 inhabitants in 2007. We carried out a retrospective analysis of all the patients with extrahepatic cholangiocarcinoma (code C24.0 according to the 2nd edition of the International Classification of Diseases for Oncology) consecutively diagnosed at our institution between January 1, 1999 and December 31, 2003. Full clinical documentation was available for 68 subjects; they were then included in the study. Diagnosis of cholangiocarcinoma was based on clinical, imaging, cytologic or histopathologic findings. All patients underwent ultrasound examination of the liver and gallbladder as the first diagnostic imaging approach. Further procedures included triple phase helical computed tomography in 60 patients (88.2%), cholangio-magnetic resonance imaging in 14 patients (20.6%), and cholangiography by means of either the percutaneous transhepatic (PTC) or endoscopic retrograde approach (ERCP) in 39 (57.4%) and 32 (47.1%) patients, respectively. Pathologic confirmation was established in 37 cases (54.4%), a proportion in accordance with previously published series[4,6,18,19], and was based on either histologic or cytologic samples (24 and 13 cases, respectively).
Demographic data, predisposing factors, clinical manifestations at admission, laboratory and imaging findings, pathology reports, therapeutic approaches, and all-cause mortality were assessed by review of medical records. Additional variables such as length of hospital stay or presence of perioperative complications were specifically recorded in patients who underwent surgical resection as the first-line treatment. Extrahepatic cholangiocarcinomas were classified as perihilar (those involving or requiring resection of the hepatic duct bifurcation) or distal types (those involving the distal extrahepatic, or intrapancreatic portion of the bile duct and potentially amenable to pancreatoduodenectomy). The American Joint Committee on Cancer (AJCC) 2003 criteria were used for TNM (Tumor, Node, Metastases) staging of the tumor[20]. Perihilar tumors were classified according to the modified Bismuth-Corlette classification[21]. CCI scores were calculated by the method previously reported by Charlson et al[12], in which each specific comorbid condition is weighted and scored (Table 1). These scores were determined by one of the authors (Fernández-Ruiz M) who was blinded to survival status.
Table 1.
The Charlson comorbidity index (CCI)[12]
| Weight1 | Comorbid condition |
| 1 | Myocardial infarction |
| Congestive heart failure | |
| Peripheral vascular disease | |
| Cerebrovascular disease (except hemiplegia) | |
| Dementia | |
| Chronic obstructive pulmonary disease | |
| Connective tissue disease | |
| Peptic ulcer disease | |
| Mild liver disease | |
| Diabetes (without complications) | |
| 2 | Hemiplegia |
| Moderate or severe renal disease | |
| Diabetes with end-organ damage (retinopathy, neuropathy, etc) | |
| Any second solid tumor (nonmetastatic), leukemia or lymphoma | |
| 3 | Moderate or severe liver disease |
| 6 | Metastatic solid tumor |
| AIDS |
Optionally, the age index leads to adding 1 point for each decade over 40 years.
The primary endpoint of the study was overall survival, defined as the interval (in months) between tumor diagnosis and death or completion of the follow-up period (December 31, 2004). Patients lost during this period were recorded as of the last known contact in our institution. Quantitative data are shown as mean ± SD, or median ± range or 95% confidence interval (95% CI), as appropriate. Qualitative variables are expressed as absolute and relative frequencies. Nominal variables were compared by the χ2 test or Fisher’s exact test. Two-tailed Student’s t test (or U Mann-Whitney test when the assumption of normality did not hold) were applied for continuous variables. We used logistic regression analysis in order to identify factors predictive of tumor resectability. Survival curves were estimated by the Kaplan-Meier product-limit method, and differences between groups were compared with the log-rank test (univariate analysis). Multivariate analysis was based on the stepwise forward Cox proportional hazards model, using survival as the dependent variable and those factors demonstrating statistical significance in the univariate analysis as covariates. To assess the role of CCI as a predictor of mortality, survival analysis was carried out with the cohort divided into 2 groups based on its median value (CCI score equal or lower than 1, or greater than 1). We also dichotomized other continuous variables by using their mean or median values, except for total bilirubin (cut-off value at 10 mg/dL) and hemoglobin (cut-off value at 12 g/dL). Differences were considered significant at P < 0.05. All statistical analysis was performed using the software package SPSS, version 12.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Patients’ characteristics
A total of 68 consecutive patients diagnosed with extrahepatic cholangiocarcinoma during the study period were analyzed. Their baseline characteristics stratified by primary tumor location are summarized in Table 2. There were 34 males and 34 females, with a mean age at diagnosis of 73.4 ± 11.5 years (range, 42 to 96 years). Forty-seven patients were older than 70 (69.1%) years. Regarding the risk factors for the development of cholangiocarcinoma, only 1 patient from the cohort had a previous diagnosis of primary sclerosing cholangitis (PSC). A history of underlying chronic liver disease was recognized in 5 cases (7.4%): hepatitis B and C infection (2 patients each), and chronic alcoholism (1 patient). Five patients (7.4%) had previously undergone cholecystectomy. No cases of Caroli’s disease, choledochal cyst, hepatolithiasis, or exposure to chemical agents were found. A family history of malignancy was reported in 5 patients (7.4%). Major clinical symptoms at admission were jaundice (86.8%), abdominal pain (36.7%), and weight loss (27.9%). Age and sex distribution, predisposing factors, clinical manifestations, and duration of symptoms were similar between patients with perihilar and distal lesions. The serum lactate dehydrogenase level in patients with distal cholangiocarcinoma (169 ± 54 IU/L) was lower than that in patients with perihilar tumors (269 ± 180 IU/L, P = 0.005), while there was a nearly significant difference in serum carbohydrate antigen 19-9 (CA 19-9) levels at diagnosis (87.6 IU/L vs 989 IU/L, respectively, P = 0.057). Other hematologic and liver function tests were similar in both groups.
Table 2.
Demographic, clinical, and laboratory data of patients at baseline (mean ± SD) n (%)
| Variable | Perihilar (n = 37) | Distal (n = 31) | Total (n = 68) |
| Age (yr) | 74.2 ± 10.5 | 72.4 ± 12.6 | 73.4 ± 11.5 |
| Sex (M/F) | 18/19 | 16/15 | 34/34 |
| Smoking | 13 (35.1) | 8 (25.8) | 21 (30.9) |
| Predisposing factor | 5 (13.5) | 1 (3.2) | 6 (8.8) |
| Family history of malignancy | 1 (2.7) | 4 (12.9) | 5 (7.4) |
| Clinical manifestations | |||
| Jaundice | 33 (89.2) | 26 (83.9) | 59 (86.8) |
| Abdominal pain | 16 (43.2) | 9 (29.0) | 25 (36.7) |
| Weight loss | 11 (29.7) | 8 (25.8) | 19 (27.9) |
| Fever | 0 (0) | 3 (9.7) | 3 (4.4) |
| Casual diagnosis | 0 (0) | 1 (3.2) | 1 (1.5) |
| Symptoms duration (mo) [median (range)] | 0.3 (0.01-12) | 0.5 (0.05-3.5) | 0.5 (0.01-12) |
| AST (IU/L) | 146 ± 104 | 129 ± 121 | 138 ± 111 |
| ALT (IU/L) | 211 ± 166 | 172 ± 139 | 193 ± 144 |
| γ-GT (IU/L) | 629 ± 377 | 811 ± 660 | 712 ± 529 |
| LDH (IU/L)a | 269 ± 180 | 169 ± 54 | 223 ± 174 |
| Albumin (g/dL) | 3.3 ± 0.4 | 3.2 ± 0.7 | 3.2 ± 0.6 |
| Bilirubin (mg/dL) | 15.6 ± 9.1 | 12.6 ± 9.5 | 14.2 ± 9.3 |
| Hemoglobin (g/dL) | 13.4 ± 1.4 | 12.7 ± 2.3 | 13.1 ± 1.9 |
| Platelets (× 1000/μL) | 257 ± 99 | 294 ± 103 | 274 ± 102 |
| Creatinine (mg/dL) | 0.8 ± 0.4 | 0.8 ± 0.3 | 0.8 ± 0.3 |
| CA 19-9 (IU/L) [median (range)]1 | 989 (2.9-65 920) | 87.6 (9-11 641) | 269 (2.9-65 920) |
Data available for 29 patients;
P = 0.005; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; CA 19-9: Carbohydrate antigen 19-9; γ-GT: γ-glutamyltranspeptidase; LDH: Lactate dehydrogenase.
The median CCI score was 1 (range, 0 to 4). Thirty-one patients (45.6%) had no comorbidities (CCI score of 0), 18 (26.5%) had a modest comorbidity level (CCI score of 1), and 19 (27.9%) had a high comorbidity level (CCI score ≥ 2). The most common comorbid conditions encountered were hypertension (44%), diabetes mellitus (17.6%), chronic obstructive pulmonary disease (16%), coronary heart disease (11.7%), and cerebrovascular disease (8.8%). A history of previous malignancy was identified in 4 patients (5.8%).
Treatment approaches
After initial assessment, 23 patients (33.8%) were considered to have potentially resectable disease and underwent laparotomy with curative intent. At exploration, 4 patients had findings (locally advanced tumor) that precluded resection. Surgical therapy in the remaining 19 patients (27.9%) consisted of partial duodenopancreatectomy with radical lymphadenectomy in 13 cases, excision of the extrahepatic biliary tree in 4 cases, and extrahepatic duct resection associated with left hemihepatectomy in 2 cases. Median hospital stay was 30 d (range, 15 to 66 d). Major postoperative complications occurred in 14 patients (60.8% of the surgical group) and included sepsis (4 cases), surgical wound infection (3 cases), digestive tract bleeding (3 cases), and death within 30 d after procedure (3 cases). Primary tumor location, age, and baseline serum levels of carcinoembrionary antigen (CEA) and CA 19-9 were identified as predictive variables for resectable disease, whereas CCI scores did not differ significantly between patients who had surgery and those who did not (Table 3). In logistic regression multivariate analysis, only serum CA 19-9 levels ≥ 270 IU/L predicted unresectability (OR, 0.07; 95% CI, 0.0-0.7; P = 0.026).
Table 3.
Analysis of predictive variables for resectable tumor
| Variable |
Univariate |
Multivariate |
||||
| OR | 95% CI | P | OR | 95% CI | P | |
| Age ≥ 73 yr | 0.24 | 0.0-0.7 | 0.012 | - | ||
| Distal location | 5.27 | 1.6-17.1 | 0.004 | - | ||
| CEA ≥ 5 IU/L | 0.09 | 0.0-0.6 | 0.013 | - | ||
| CA 19-9 ≥ 270 IU/L | 0.05 | 0.0-0.5 | 0.003 | 0.07 | 0.0-0.7 | 0.026 |
CEA: Carcinoembrionary antigen; OR: Odds ratio.
Palliative biliary drainage was performed in 39 subjects (57.4%) with non-resectable tumors. Thirteen patients (19.1%) underwent endoscopic biliary stenting by ERCP, whereas percutaneous approach by PTC was required in 26 patients (38.2%), associated with stent placement in 14 cases. Chemo- or brachy-radiotherapy were employed as adjuvant treatment in 3 and 7 subjects, respectively. Finally, 6 patients (8.8%) received only best supportive care.
Macroscopic and microscopic appearance
TNM staging distribution by primary location is presented in Table 4. The majority of tumors were pathologically classified as T3 (36.7%) and metastatic spread was identified in 13.2% of patients. According to the Bismuth-Corlette classification of perihilar cholangiocarcinomas, 6 out of the 37 patients with such tumors were diagnosed as stage I, 8 as stage II, 5 as stage IIIa, 7 as stage IIIb, and 7 as stage IV. Four patients remained unclassified. Of the 19 patients who underwent resection, 15 (78.9%) had negative histologic margins (R0 resection), whereas in 3 cases (15.8%) the margins were microscopically involved with the tumor (R1 resection).
Table 4.
AJCC-TNM staging distribution
| Perihilar (n = 37) | Distal (n = 31) | Total (n = 68) | |
| Tumor status | |||
| T1 | 3 | 3 | 6 |
| T2 | 8 | 5 | 13 |
| T3 | 16 | 9 | 25 |
| T4 | 3 | 6 | 9 |
| Unknown | 7 | 8 | 15 |
| Lymph node status | |||
| N0 | 27 | 19 | 46 |
| N1 | 10 | 10 | 20 |
| Unknown | 1 | 1 | 2 |
| Metastases status | |||
| M0 | 31 | 28 | 59 |
| M1 | 6 | 3 | 9 |
AJCC: American Joint Committee on Cancer; TNM: Tumor, Node, Metastases.
Survival analysis
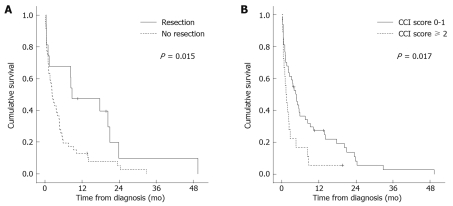
Median follow-up time for the entire cohort was 2.7 mo (range, 0.07 to 49.4 mo). Five patients (7.4%) were lost to follow-up. At the end of observation, 60 of 68 patients (88.2%) had died, with an overall median survival of 3.1 mo (95% CI, 1.4-4.8). Survival rates at 1, 2, and 3 years were 21%, 7%, and 2% respectively. Tumor progression (27.9%), infection (13.2%), liver failure (8.8%), and bleeding (4.4%) were the most frequently recorded causes of death. The clinical, tumor-related, and treatment-related variables evaluated by univariate and multivariate analysis to determine their impact on outcome are presented in Table 5. For the univariate log-rank analysis, surgical resection, lower comorbidity index (CCI score < 2), and lower serum CA 19-9 levels (< 270 IU/L) correlated significantly with better survival. Patients who underwent resection had a longer median survival than those who did not undergo such treatment (8.7 ± 5.7 mo vs 2.3 ± 0.4 mo, P = 0.015) (Figure 1A). Regarding the presence and number of comorbid conditions, the median survival in patients with a CCI score of 0 or 1 was longer than that in patients with a higher score (4.7 ± 0.8 mo vs 1.4 ± 0.5 mo, P = 0.017) (Figure 1B). This difference remained significant in the subgroup of patients who did not undergo surgical resection (3.6 ± 1.0 mo vs 1.1 ± 0.5 mo, P = 0.001). Within the resection group, median survival in patients with a CCI score of 0 or 1 (17.7 ± 7.9 mo) was also longer than that in patients with a score ≥ 2 (8.3 ± 7.5 mo), although the difference did not achieve statistical significance (P = 0.25). On Cox multivariate analysis, the performance of surgical resection [Hazard Ratio (HR) 0.10; 95% CI, 0.02-0.51, P = 0.005] and the number of comorbid conditions (HR 3.36; 95% CI, 1.0-10.9, P = 0.045) emerged as independent predictors of survival (Table 5).
Table 5.
Univariate and multivariate analysis for prognostic survival variables
| Variable (n) | Median survival (mo) | 95% CI | P (univariate) | P (multivariate) | HR | 95% CI |
| Age at diagnosis | ||||||
| < 73 yr (30) | 4.7 | 0.7-8.7 | 0.054 | - | ||
| ≥ 73 yr (38) | 2.2 | 0.7-3.8 | ||||
| History of weight loss | ||||||
| No (49) | 3.6 | 0.9-6.2 | 0.085 | - | ||
| Yes (19) | 2.1 | 0.5-3.6 | ||||
| CCI score | ||||||
| 0-1 (49) | 4.7 | 3.1-6.3 | 0.017 | 0.045 | 3.36 | 1.0-10.9 |
| ≥ 2 (19) | 1.4 | 0.4-2.3 | ||||
| Total bilirubin | ||||||
| < 10 mg/dL (23) | 3.6 | 0.7-6.6 | 0.583 | - | ||
| ≥ 10 mg/dL (45) | 2.3 | 0-0-4.6 | ||||
| Hemoglobin | ||||||
| ≥ 12 g/dL (49) | 3.6 | 1.4-5.7 | 0.097 | - | ||
| < 12 g/dL (19) | 2.2 | 0.1-4.4 | ||||
| CA 19-91 | ||||||
| < 270 IU/L (15) | 8.4 | 0.0-18.3 | 0.012 | 0.089 | - | - |
| ≥ 270 IU/L (14) | 2.7 | 0.2-5.2 | ||||
| Primary location | ||||||
| Perihilar (37) | 3.1 | 1.1-5.2 | 0.994 | - | ||
| Distal (31) | 2.5 | 0.0-6.3 | ||||
| Lymph node status2 | ||||||
| No (46) | 2.3 | 0.0-5.0 | 0.286 | - | ||
| Yes (20) | 3.6 | 1.5-5.8 | ||||
| Metastases | ||||||
| No (59) | 3.6 | 1.3-6.1 | 0.068 | - | ||
| Yes (9) | 0.7 | 0.3-1.1 | ||||
| Tumor resection | ||||||
| No (49) | 2.3 | 1.4-3.2 | 0.015 | 0.005 | 0.1 | 0.02-0.51 |
| Yes (19) | 8.7 | 0.0-19.8 | ||||
| Adjuvant therapy3 | ||||||
| No (41) | 2.1 | 1.0-3.1 | 0.129 | - | ||
| Yes (8) | 5.1 | 2.5-3.2 | ||||
Data available for 29 patients;
Data available for 66 patients;
Denotes chemotherapy or brachy-radiotherapy in patients not receiving surgical resection; HR: Hazard ratio; CCI: Charlson comorbidity index.
Figure 1.
Kaplan-Meier survival curves stratified by treatment approach (log-rank test) (A) and Charlson comorbidity index (CCI) score (log-rank test) (B).
DISCUSSION
Our study is a retrospective analysis of the clinical and evolutive characteristics of a consecutive series of Spanish patients diagnosed with extrahepatic cholangiocarcinoma. The results of the study are in addition to the limited works published to date in our country regarding this condition[22-24]. We have demonstrated that the presence and number of comorbid conditions, as assessed by the CCI, act as independent factors of unfavorable prognosis. Patients with higher associated comorbidity (CCI score ≥ 2) had significantly shorter median survival than those with a lower burden of comorbidity (CCI score < 2). This difference was upheld specifically in patients not subject to surgery. To the best of our knowledge, our work is the very first study to demonstrate the impact of comorbidity - as assessed by the CCI criteria - on the survival of patients with malignancies of the biliary tract, and is in accordance with previous studies focused on other solid-organ malignancies[10,13-17].
Extrahepatic cholangiocarcinoma is a rare condition in the Western world. In a study performed in Spain between 1994 and 1996, Mena et al[22] estimated its incidence as 3.23 new cases per 100 000 inhabitants per year. A more recent work, based on data from the nationwide Danish Cancer Registry, revealed a reduced progression of incidence from 1978 (1.05 cases per 100 000 inhabitants per year) up to 2002 (0.74 cases per 100 000 inhabitants per year)[25]. In contrast, the incidence and mortality of intrahepatic forms of the disease seem to have experienced a sustained increase in the last few decades[1,2]. The cause of this rise is unknown and does not appear to be explained simply by improvements in diagnosis or changes in coding practice. PSC remains the most common predisposing condition in the development of cholangiocarcinoma in Western countries[1]. Cirrhosis of any cause and, more specifically, hepatitis B and C virus infection, have recently been linked to this type of cancer[2]. In our study, only a reduced percentage of patients were associated with some of these risk factors, including 1 case of PSC. These circumstances are common in the literature[5-7,18] and appears to suggest the concurrence of other etiopathogenic mechanisms yet to be clarified. Our experience confirms the poor prognosis associated with extrahepatic cholangiocarcinoma, with a median survival of 3.1 mo and a 3-year survival probability of 2%, slightly lower than that described in previous studies with similar clinical and epidemiologic characteristics[6,7,24]. The mean age at diagnosis of the patients analyzed in this study (73.4 years) was higher than that reported by other authors (64 years in the study by Figueras et al[23], 67 years in the study by Weber et al[5]) and may have conditioned the reduced rate of resectability obtained in our series (27.9%). Distal tumor location was associated with a higher probability of receiving surgical resection in the univariate analysis, a finding previously reported in the literature[19,26,27]. Determination at diagnosis of serum CA 19-9 levels ≥ 270 IU/L emerged in the logistic regression multivariate analysis as the only factor independently predictive of unresectability (OR, 0.07; 95% CI, 0.0-0.7). Gerhardt et al[6] revealed that initial levels of this tumor marker in patients with perihilar cholangiocarcinoma subject to resection were significantly lower in comparison to those affected by unresectable disease. Kau et al[28] reported similar findings in subjects with periampular carcinoma. As suggested by these authors, the presence of high serum levels of CA 19-9 probably reflects a greater tumor mass. Consequently, the univariate analysis of survival in our study showed worse prognosis in patients with higher serum levels of CA 19-9 at the time of diagnosis (≥ 270 IU/L), while this difference did not remain significant in the Cox multivariate model.
As early as 1964, Feinstein highlighted the role of comorbidity when explaining the difference between estimated survival according to the TNM system in patients with lung cancer and that observed in clinical practice[29]. Since then, various comorbidity scales have been designed; the CCI, the Kaplan-Feinstein Index (KFI), the Cumulative Illness Rating Scale (CIRS), and the Index of Co-Existent Disease (ICED) feature among the most widely used, although no single index has yet emerged as clearly superior to the others[11]. The majority of these scales were not designed specifically for subjects with neoplastic disease; as commented, the CCI was developed to analyze 1-year mortality on the basis of data from an internal medicine inpatient department[12]. The KFI was created by these authors in 1974 from a cohort of diabetic subjects[11]. Only in the last few years have some specific instruments been developed and validated for oncologic patients. The National Institute on Aging (NIA) and National Cancer Institute (NCI) Comorbidity Index[30] and the Adult Comorbidity Evaluation-27 (ACE-27)[31] are probably the most notable. The latter was developed by Piccirillo et al[31] and modifications as well as additions of comorbid ailments have been carried out on the KFI, which is currently available online (http://cancercomorbidity.wustl.edu). This index has demonstrated its usefulness when analyzing the influence of comorbidity on the prognosis of adult patients with carcinoma of unknown primary site (CUP)[32], resected colon cancer[33], or recently diagnosed head and neck cancer[34]. However, in spite of the progress made in the last few decades on the design and perfection of new scales, the CCI remains one of the most popular and extensively validated comorbidity instruments. Since its original formulation by Charlson et al[12] the CCI has exhibited good prognostic value for predicting cancer patient survival in numerous retrospective studies[10,13-17]. Reviews of the CCI suggest it has good reliability, excellent correlation with mortality and progression-free survival outcomes, and is easily modified, particularly to account for the effect of age[11]. Versions of the index adapted to databases via the International Classification of Diseases (ICD)-9 or based on the evaluation of self-reported comorbidities have been also developed. One of its limitations when applied to oncologic patients is characterized by the exclusion of certain comorbidities, such as nonmalignant hematopoietic disorders (i.e. anemia) or polyneuropathy[10,11].
Not surprisingly, comorbidity has a greater impact on biologically indolent cancers (e.g. prostate or breast), rather than aggressive tumors[31]. Chronic conditions, such as those included in the CCI, exert their influence on survival at mid-term and long-term follow-up, losing part of its relevance in the presence of aggressive entities. Although malignancies of the biliary tract are supposed to be relatively slow-growing[4], the late diagnosis at advanced stages of the disease determines its poor prognosis as we reproduced in our series (median survival of 3.1 mo). Consequently, it would be reasonable to suppose that the burden of comorbidity should exert a minor influence on prognosis in patients with extrahepatic cholangiocarcinoma. In support of this hypothesis, tumor progression was the most frequently recorded cause of death in our study (27.9%), ahead of others more directly related to associated comorbidities (e.g. infection or liver failure). Nonetheless, our findings clearly reveal that patients with a higher comorbidity level (CCI score ≥ 2) presented significantly lower survival in comparison to the remainder of the cohort, and that this effect was independent of other prognostic variables. The literature contains some equivalent examples in relation to other tumors with aggressive biological behaviour and poor outcome. Seve et al[32] demonstrated in a cohort of 389 patients with CUP and a median survival of just 12 wk, that the number of comorbid conditions (as assessed by the ACE-27) entailed a worse prognosis, specifically in subjects with impaired functional class. Firat et al[35] found that comorbidity (evaluated by the CIRS and CCI) was an important prognostic factor in stage III non-small cell lung cancer and concluded that comorbidity should be taken into account even in advanced-stage disease. Similar findings have been reported in ovarian cancer patients with regional spread (FIGO stage II and III) or distant metastases (FIGO stage IV)[17]. It has been hypothesized that the type of comorbidities and cancer may interact on a physiologic level resulting in increased aggressiveness and metastatic potential[36]. Another plausible explanation may be related to the nature of therapeutic management. Older and sicker patients are often excluded from prospective clinical trials and are not usually eligible for aggressive cancer therapies, such as duodenopancreatectomy or resection of the biliary tree[10]. However, we have not been able to demonstrate the presence of differences in the CCI scores between surgical and nonsurgical groups. The impact of comorbidity on patient survival in our series is also upheld after specific analysis according to the type of treatment, while that difference did not attain statistical significance in subjects who underwent surgical resection (17.7 mo vs 8.3 mo in patients with CCI score < 2 or ≥ 2, respectively; P = 0.25).
There are a number of limitations in our single-center study to be considered. The retrospective design hinders identification and control of variables with a potential influence on survival, such as performance status or conditions not included in the CCI. We had no control over the quality of the medical records reviewed. The reduced size of the sample analysed, in particular among subjects undergoing surgical resection (n = 19), could have hindered demonstration of significant differences in the survival of this subgroup according to their level of comorbidity. Therefore, we cannot generalize the validity of our results on other cohorts of younger patients subject to a higher proportion of surgical procedures. As we previously pointed out, the CCI presents some drawbacks in the evaluation and quantification of comorbidity in individuals with malignant diseases, in comparison with other more recent and specific indices[30,31]. Nonetheless, its broad dissemination and simple application guarantee the usefulness of the CCI both in clinical practice and in the context of retrospective studies. As highlighted by Extermann, this index exhibits excellent test-retest reliability, that ranged from 0.86 among a cohort of elderly cancer subjects to 0.92 in surgical patients; its inter-rater reliability is also acceptable, attaining 0.945 in some series[11]. A recent study which compared its capacity for prognostic prediction in patients undergoing resection of colorectal carcinoma with that of other recently developed instruments (ACE-27 and NIA/NCI) has concluded the existence of close similarities between them[33].
In conclusion, we have demonstrated that comorbidity exerts an adverse impact on survival in patients diagnosed with extrahepatic cholangiocarcinoma, even after multivariate adjustment for other well-known prognostic factors such as age at diagnosis, primary tumor location, lymph node status, metastatic spread, or nature of the therapeutic approach. With this aim in mind, the CCI constitutes an easy and intuitive instrument in daily clinical practice. In our experience, patients with a CCI score ≥ 2 had higher all-cause mortality compared to patients with a CCI score < 2. These data suggest that comorbidity evaluation should be added to the decision-making process in patients with extrahepatic cholangiocarcinoma in order to improve therapy selection and prognostic estimation. However, further prospective studies should be conducted to specifically examine the relationship between comorbidity and treatment outcomes such as mid-term efficacy and incidence of postoperative complications.
COMMENTS
Background
Cholangiocarcinoma is a relatively uncommon malignant tumor that is associated with a poor prognosis. Many studies have reported that the presence of comorbidity influences various prognostic outcomes in cancer patients, including treatment decision-making and survival.
Research frontiers
A correlation between the severity of comorbid conditions, as assessed by the Charlson comorbidity index (CCI), and overall survival has been described in patients with various solid-organ malignancies, such as colorectal, head and neck, bladder, clear cell renal, or ovarian cancer.
Innovations and breakthroughs
The number of comorbid conditions has an adverse impact on survival in patients diagnosed with extrahepatic cholangiocarcinoma, even after multivariate adjustment for other established prognostic factors such as age, tumor location, or type of treatment. Patients with a CCI score ≥ 2 had higher all-cause mortality compared to patients with a CCI score < 2. No previous studies have focused on the role of comorbidity on treatment decisions and clinical outcomes for patients with this disease.
Applications
The study results suggest that comorbidity evaluation should be routinely added to the decision-making process in subjects with extrahepatic cholangiocarcinoma with the aim of improving their treatment selection criteria and survival estimation.
Terminology
Comorbidity in cancer patients may be defined as the presence of diseases or disorders which exist before cancer diagnosis and are not treatment-related adverse effects. The CCI, in which each specific comorbid condition is weighted and scored, was originally developed from the study of 1-year all-cause mortality in a cohort of patients admitted to the medical unit of a teaching hospital.
Peer review
This paper is a retrospective research about the impact of comorbidity on extrahepatic cholangiocarcinoma. We are sure that this kind of research is fairly important in the clinical treatment of biliary tract malignancies. Although the data was accurate and stated in detail, this research included a relatively small size of sample, so it can not get more significant results and weakened the conclusion. Anyway, this paper gave us some new information about the prognostic of biliary tract malignancies.
Acknowledgments
The authors thank Mrs. Monserrat Pilas (Hospital Tumor Registry, University Hospital “12 de Octubre”) for her valuable assistance.
Footnotes
Peer reviewer: Wei Tang, MD, Assistant Professor, H-B-P Surgery Division, Artificial Organ and Transplantation Division, Department of surgery, Graduate School of Medicine, The University of Tokyo, Tokyo 113-8655, Japan
S- Editor Wang JL L- Editor Webster JR E- Editor Lin YP
References
- 1.Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet. 2005;366:1303–1314. doi: 10.1016/S0140-6736(05)67530-7. [DOI] [PubMed] [Google Scholar]
- 2.Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353–1357. doi: 10.1053/jhep.2001.25087. [DOI] [PubMed] [Google Scholar]
- 3.Jarnagin WR, Fong Y, DeMatteo RP, Gonen M, Burke EC, Bodniewicz BS J, Youssef BA M, Klimstra D, Blumgart LH. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507–517; discussion 517-519. doi: 10.1097/00000658-200110000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mansfield SD, Barakat O, Charnley RM, Jaques BC, O'Suilleabhain CB, Atherton PJ, Manas D. Management of hilar cholangiocarcinoma in the North of England: pathology, treatment, and outcome. World J Gastroenterol. 2005;11:7625–7630. doi: 10.3748/wjg.v11.i48.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber A, Landrock S, Schneider J, Stangl M, Neu B, Born P, Classen M, Rösch T, Schmid RM, Prinz C. Long-term outcome and prognostic factors of patients with hilar cholangiocarcinoma. World J Gastroenterol. 2007;13:1422–1426. doi: 10.3748/wjg.v13.i9.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerhardt T, Milz S, Schepke M, Feldmann G, Wolff M, Sauerbruch T, Dumoulin FL. C-reactive protein is a prognostic indicator in patients with perihilar cholangiocarcinoma. World J Gastroenterol. 2006;12:5495–5500. doi: 10.3748/wjg.v12.i34.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexopoulou A, Soultati A, Dourakis SP, Vasilieva L, Archimandritis AJ. Cholangiocarcinoma: a 7-year experience at a single center in Greece. World J Gastroenterol. 2008;14:6213–6217. doi: 10.3748/wjg.14.6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garman KS, Pieper CF, Seo P, Cohen HJ. Function in elderly cancer survivors depends on comorbidities. J Gerontol A Biol Sci Med Sci. 2003;58:M1119–M1124. doi: 10.1093/gerona/58.12.m1119. [DOI] [PubMed] [Google Scholar]
- 9.Seo PH, Pieper CF, Cohen HJ. Effects of cancer history and comorbid conditions on mortality and healthcare use among older cancer survivors. Cancer. 2004;101:2276–2284. doi: 10.1002/cncr.20606. [DOI] [PubMed] [Google Scholar]
- 10.Koppie TM, Serio AM, Vickers AJ, Vora K, Dalbagni G, Donat SM, Herr HW, Bochner BH. Age-adjusted Charlson comorbidity score is associated with treatment decisions and clinical outcomes for patients undergoing radical cystectomy for bladder cancer. Cancer. 2008;112:2384–2392. doi: 10.1002/cncr.23462. [DOI] [PubMed] [Google Scholar]
- 11.Extermann M. Measuring comorbidity in older cancer patients. Eur J Cancer. 2000;36:453–471. doi: 10.1016/s0959-8049(99)00319-6. [DOI] [PubMed] [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 13.Ouellette JR, Small DG, Termuhlen PM. Evaluation of Charlson-Age Comorbidity Index as predictor of morbidity and mortality in patients with colorectal carcinoma. J Gastrointest Surg. 2004;8:1061–1067. doi: 10.1016/j.gassur.2004.09.045. [DOI] [PubMed] [Google Scholar]
- 14.Singh B, Bhaya M, Stern J, Roland JT, Zimbler M, Rosenfeld RM, Har-El G, Lucente FE. Validation of the Charlson comorbidity index in patients with head and neck cancer: a multi-institutional study. Laryngoscope. 1997;107:1469–1475. doi: 10.1097/00005537-199711000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Birim O, Kappetein AP, Bogers AJ. Charlson comorbidity index as a predictor of long-term outcome after surgery for nonsmall cell lung cancer. Eur J Cardiothorac Surg. 2005;28:759–762. doi: 10.1016/j.ejcts.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 16.Santos Arrontes D, Fernández Aceñero MJ, García González JI, Martín Muñoz M, Paniagua Andrés P. Survival analysis of clear cell renal carcinoma according to the Charlson comorbidity index. J Urol. 2008;179:857–861. doi: 10.1016/j.juro.2007.10.048. [DOI] [PubMed] [Google Scholar]
- 17.Tetsche MS, Dethlefsen C, Pedersen L, Sorensen HT, Norgaard M. The impact of comorbidity and stage on ovarian cancer mortality: a nationwide Danish cohort study. BMC Cancer. 2008;8:31. doi: 10.1186/1471-2407-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farhat MH, Shamseddine AI, Tawil AN, Berjawi G, Sidani C, Shamseddeen W, Barada KA. Prognostic factors in patients with advanced cholangiocarcinoma: role of surgery, chemotherapy and body mass index. World J Gastroenterol. 2008;14:3224–3230. doi: 10.3748/wjg.14.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu XF, Zhou XT, Zou SQ. An analysis of 680 cases of cholangiocarcinoma from 8 hospitals. Hepatobiliary Pancreat Dis Int. 2005;4:585–588. [PubMed] [Google Scholar]
- 20.American Joint Committee on Cancer. AJCC Cancer Staging Manual. 6th ed. New York, NY: Springer; 2002. pp. 145–150. [Google Scholar]
- 21.Bismuth H, Nakache R, Diamond T. Management strategies in resection for hilar cholangiocarcinoma. Ann Surg. 1992;215:31–38. doi: 10.1097/00000658-199201000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mena FJ, Velicia R, Valbuena MC, González JM, Caro-Patón A, Pérez-Miranda M, Bellido J. Carcinoma of the extrahepatic biliary tree: analysis of 15 cases. Rev Esp Enferm Dig. 1999;91:297–304. [PubMed] [Google Scholar]
- 23.Figueras J, Llado L, Valls C, Serrano T, Ramos E, Fabregat J, Rafecas A, Torras J, Jaurrieta E. Changing strategies in diagnosis and management of hilar cholangiocarcinoma. Liver Transpl. 2000;6:786–794. doi: 10.1053/jlts.2000.18507. [DOI] [PubMed] [Google Scholar]
- 24.Margarit C, Escartín A, Bellmunt J, Allende E, Bilbao I. [Peripheral cholangiocarcinoma: results of surgical treatment] Gastroenterol Hepatol. 2006;29:215–223. doi: 10.1157/13085990. [DOI] [PubMed] [Google Scholar]
- 25.Jepsen P, Vilstrup H, Tarone RE, Friis S, Sørensen HT. Incidence rates of intra- and extrahepatic cholangiocarcinomas in Denmark from 1978 through 2002. J Natl Cancer Inst. 2007;99:895–897. doi: 10.1093/jnci/djk201. [DOI] [PubMed] [Google Scholar]
- 26.Nakeeb A, Pitt HA, Sohn TA, Coleman J, Abrams RA, Piantadosi S, Hruban RH, Lillemoe KD, Yeo CJ, Cameron JL. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224:463–473; discussion 473-475. doi: 10.1097/00000658-199610000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forsmo HM, Horn A, Viste A, Hoem D, Ovrebo K. Survival and an overview of decision-making in patients with cholangiocarcinoma. Hepatobiliary Pancreat Dis Int. 2008;7:412–417. [PubMed] [Google Scholar]
- 28.Kau SY, Shyr YM, Su CH, Wu CW, Lui WY. Diagnostic and prognostic values of CA 19-9 and CEA in periampullary cancers. J Am Coll Surg. 1999;188:415–420. doi: 10.1016/s1072-7515(98)00326-3. [DOI] [PubMed] [Google Scholar]
- 29.Feinstein AR. Symptomatic patterns, biologic behavior, and prognosis in cancer of the lung. Practical application of Boolean Algebra and clinical taxonomy. Ann Intern Med. 1964;61:27–43. doi: 10.7326/0003-4819-61-1-27. [DOI] [PubMed] [Google Scholar]
- 30.Havlik RJ, Yancik R, Long S, Ries L, Edwards B. The National Institute on Aging and the National Cancer Institute SEER collaborative study on comorbidity and early diagnosis of cancer in the elderly. Cancer. 1994;74:2101–2106. doi: 10.1002/1097-0142(19941001)74:7+<2101::aid-cncr2820741718>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 31.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL Jr. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291:2441–2447. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 32.Seve P, Sawyer M, Hanson J, Broussolle C, Dumontet C, Mackey JR. The influence of comorbidities, age, and performance status on the prognosis and treatment of patients with metastatic carcinomas of unknown primary site: a population-based study. Cancer. 2006;106:2058–2066. doi: 10.1002/cncr.21833. [DOI] [PubMed] [Google Scholar]
- 33.Hines RB, Chatla C, Bumpers HL, Waterbor JW, McGwin G Jr, Funkhouser E, Coffey CS, Posey J, Manne U. Predictive capacity of three comorbidity indices in estimating mortality after surgery for colon cancer. J Clin Oncol. 2009;27:4339–4345. doi: 10.1200/JCO.2009.22.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yung KC, Piccirillo JF. The incidence and impact of comorbidity diagnosed after the onset of head and neck cancer. Arch Otolaryngol Head Neck Surg. 2008;134:1045–1049. doi: 10.1001/archotol.134.10.1045. [DOI] [PubMed] [Google Scholar]
- 35.Firat S, Byhardt RW, Gore E. Comorbidity and Karnofksy performance score are independent prognostic factors in stage III non-small-cell lung cancer: an institutional analysis of patients treated on four RTOG studies. Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 2002;54:357–364. doi: 10.1016/s0360-3016(02)02939-5. [DOI] [PubMed] [Google Scholar]
- 36.Newschaffer CJ, Bush TL, Penberthy LE, Bellantoni M, Helzlsour K, Diener-West M. Does comorbid disease interact with cancer? An epidemiologic analysis of mortality in a cohort of elderly breast cancer patients. J Gerontol A Biol Sci Med Sci. 1998;53:M372–M378. doi: 10.1093/gerona/53a.5.m372. [DOI] [PubMed] [Google Scholar]