Abstract
AIM: To evaluate gastrointestinal (GI) symptoms and breath hydrogen responses to oral fructose-sorbitol (F-S) and glucose challenges in eating disorder (ED) patients.
METHODS: GI symptoms and hydrogen breath concentration were monitored in 26 female ED inpatients for 3 h, following ingestion of 50 g glucose on one day, and 25 g fructose/5 g sorbitol on the next day, after an overnight fast on each occasion. Responses to F-S were compared to those of 20 asymptomatic healthy females.
RESULTS: F-S provoked GI symptoms in 15 ED patients and one healthy control (P < 0.05 ED vs control). Only one ED patient displayed symptom provocation to glucose (P < 0.01 vs F-S response). A greater symptom response was observed in ED patients with a body mass index (BMI) ≤ 17.5 kg/m2 compared to those with a BMI > 17.5 kg/m2 (P < 0.01). There were no differences in psychological scores, prevalence of functional GI disorders or breath hydrogen responses between patients with and without an F-S response.
CONCLUSION: F-S, but not glucose, provokes GI symptoms in ED patients, predominantly those with low BMI. These findings are important in the dietary management of ED patients.
Keywords: Fructose, Sorbitol, Malabsorption syndromes, Functional gastrointestinal disorders, Eating disorders, Underweight
INTRODUCTION
A high prevalence of gastrointestinal (GI) symptoms, fulfilling criteria for functional GI disorders (FGID), is present in patients with eating disorders (ED), with more than half of a large sample of ED inpatients meeting the symptom criteria for irritable bowel syndrome (IBS)[1]. In addition to the distress caused by these chronic or recurrent GI symptoms, such symptoms may potentially interfere with the nutritional rehabilitation of ED patients.
Ingestion of fructose-sorbitol (F-S) is an established means of GI symptom provocation in IBS patients[2,3]. It remains controversial whether such symptom provocation is related to hydrogen production in the colon as a result of incomplete small bowel absorption. Because both fruit and sorbitol-containing “diet” products are frequently consumed by ED patients[4], we hypothesized that ingestion of both these substances together may be an important factor in the genesis of the GI symptoms in ED patients. Furthermore, we hypothesized that certain characteristics associated with ED, such as body weight, and behavioral and psychological features, could influence the responses to F-S symptom provocation.
The specific aims of this study were therefore: (1) to determine the prevalence of F-S symptom provocation in female ED patients when compared to healthy female subjects; (2) to determine the specificity of any positive symptom response to F-S by evaluating whether, in ED patients, symptom provocation is greater after F-S ingestion than after glucose ingestion, a substance not recognized to provoke GI symptoms; (3) to examine the relationships between body mass index (BMI), menstrual status, behavioral and psychological characteristics, type of ED and the F-S symptom responses; (4) to determine if symptom provocation is related to the presence of symptoms compatible with IBS, or to the number of FGIDs present in an ED patient; and (5) to determine whether F-S symptom provocation in ED patients, if present, is related to incomplete small bowel absorption of F-S detected by breath hydrogen testing.
MATERIALS AND METHODS
Subjects
Twenty-six consecutive female ED inpatients (23 ± 7 years, BMI 18.6 ± 3.6 kg/m2) from the Eating Disorder Unit at the Northside Clinic, Sydney, Australia, participated in the study. Inclusion criteria were eating disorder diagnosis[5] confirmed by a specialist psychiatrist and a specialist psychologist, minimum age 16 years, and no known organic GI or other systemic disease. The study was conducted 2 wk after admission to hospital. ED assessment included current BMI, menstrual status, and ED behaviors (self-induced vomiting, laxative abuse, binge eating). Ten patients had a diagnosis of anorexia nervosa, 5 bulimia nervosa, and 11 eating disorder not otherwise specified (EDNOS: 6 purging type and 5 restricting type). Twenty asymptomatic, normal weight, healthy women (31 ± 9 years) who underwent F-S provocation breath testing in our laboratory using the same protocol as the ED patients formed a control group. Informed consent was obtained from all subjects, and the study was approved by the Ethics Committee of the Northside Clinic.
GI symptom, psychological and behavioral assessment
The following self-report questionnaires were administered to the ED patients: the Rome II modular questionnaire[6], the Beck Depression Inventory (BDI)[7], the Eating Attitudes Test (EAT)[8], the Eysenck Personality Questionnaire - neuroticism (EPQ)[9], the Spielberger State-Trait Anxiety Inventory (STAI)[10], the Brief Symptom Inventory - somatization (BSI)[11], and the Quality of Life Eating Disorder Scale (QOL ED)[12] of the computerized Eating and Exercise Examination[13].
Experimental protocol
On the evening prior to the test day, subjects ate a carbohydrate-free dinner, and then fasted from midnight until the end of testing the following day. Immediately prior to testing the subjects brushed their teeth, and subjects were not allowed to eat, drink, or smoke cigarettes during the testing[3].
In the ED patients, substrate testing took place on 2 consecutive days. On the first day patients underwent a challenge with a glucose solution and on the second day with an F-S solution. Three baseline breath hydrogen samples were obtained on each day. Patients then ingested 50 g glucose dissolved in 250 mL water (2200 mOsm/L), or an F-S mixture of 25 g fructose and 5 g sorbitol dissolved in 250 mL water (1680 mOsm/L)[3,14]. Breath samples were obtained at 10 min intervals for a 3 h period, and the hydrogen concentration (ppm) of each sample was determined[3] on a portable instrument (Gastrolyser II, Bedfont Pty Ltd., UK). The instrument was calibrated with research grade hydrogen, as per the manufacturer’s recommendations. Patients were blind to the order of substrate challenge, and were not aware of the hydrogen concentrations obtained.
Symptom assessment during provocative testing
A standard proforma assessment of GI symptoms was completed before, and at 1, 2, and 3 h following ingestion of the test solutions[3]. The symptoms rated were: abdominal pain, abdominal discomfort, abdominal bloating, abdominal distension, belching, nausea, loose or increased frequency of bowel motions, sensation of fullness, borborygmi, and flatulence. These symptoms were each rated on a score of 0 to 3, as follows: absent (score 0); mild (score 1); moderate (score 2); or severe (score 3).
Statistical analysis
An hourly symptom score was determined by summing the individual symptom scores (corrected for baseline) for each symptom at hour 1, hour 2 and hour 3. A total symptom score was obtained by summing the 3-hourly symptom scores. A symptom response was defined as a total symptom score of 5 or greater; this measure was used to relate symptom provocation to BMI, behavioral characteristics and psychological measures, type of ED and presence of FGIDs. A BMI ≤ 17.5 kg/m2 was used to determine 2 categories of ED patients[5]. The total number of FGIDs was determined for each ED patient. A breath hydrogen level ≥ 20 ppm above baseline was used as a cut-off value to categorize each subject as either a “malabsorber”, or an “absorber” (i.e. no breath hydrogen response)[3]. Mouth-to-cecum transit time was defined as the time between ingestion of the solution and an increase in breath hydrogen of 10 ppm in 3 consecutive samples[15]. Peak hydrogen level was taken as the maximum hydrogen value recorded during the 3-h test period. To test for differences between groups, the Chi square with Fisher’s Exact test for low cell numbers, ANOVA, and the Student’s t-test were used where appropriate. Unless otherwise noted, results are presented as frequencies or mean ± SD, and P values < 0.05 are considered significant.
RESULTS
F-S symptom provocation in ED patients and controls
Fifteen (58%) ED patients compared to one (5%) control subject reported one or more individual symptoms at 3 h after F-S provocation (P < 0.05).
F-S vs glucose symptom provocation in ED patients
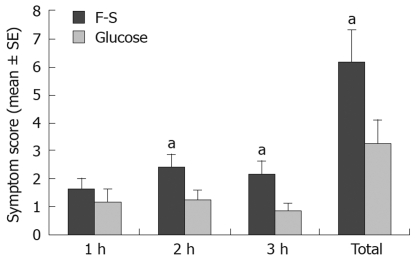
Hourly and total symptom scores following F-S and glucose ingestion are shown in Figure 1. There was a significantly greater symptom score following F-S than there was following glucose at both 2 and 3 h. Fourteen (55%) patients exhibited a symptom response to F-S, while only one (4%) patient exhibited a symptom response to glucose (P < 0.01).
Figure 1.
Hourly and total symptom scores in eating disorder patients following ingestion of 25 g fructose-5 g sorbitol (F-S) and 50 g glucose (aP < 0.05 vs glucose).
F-S symptom provocation and ED characteristics
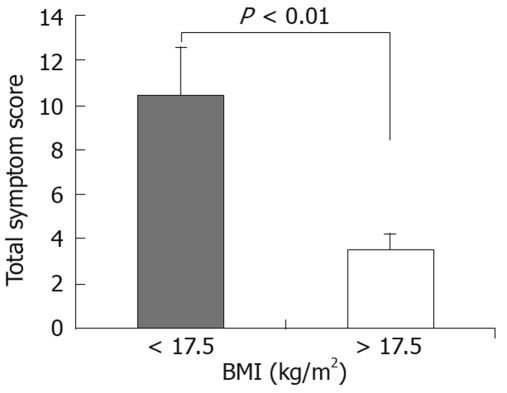
Total symptom score for patients with a BMI ≤ 17.5 kg/m2 was significantly higher than that of patients with a BMI >17.5 (Figure 2). Excluding 7 patients who were taking oral contraception, 7 (64%) of the 11 patients with a symptom response were suffering from oligo- or secondary amenorrhoea, compared with 2 (25%) of the 8 patients with no symptom response; this difference did not reach statistical significance.
Figure 2.
Total symptom scores (mean ± SE) in eating disorder patients following ingestion of 25 g fructose-5 g sorbitol, grouped according to body mass index (BMI).
There was no significant difference in type of ED diagnosis in patients with and without an F-S symptom response (Table 1), although 80% of anorexia nervosa patients showed a symptom response. There was a significant difference in binge eating behavior between patients with and without an F-S symptom response (Table 1). There were no differences in psychological or QOL ED scores between ED patients with and without an F-S symptom response (Table 2).
Table 1.
Type of eating disorder and ED behaviors in patients according to symptom response to fructose-sorbitol
| No symptom response (n = 12) | Symptom response (n = 14) | |
| Type of eating disorder | f (%) | f (%) |
| Anorexia nervosa | 2 (17) | 8 (57) |
| Bulimia nervosa | 4 (33) | 1 (7) |
| EDNOS | 6 (50) | 5 (36) |
| Eating disorder behaviors | ||
| Self-induced vomiting | 7 (58) | 6 (43) |
| Laxative abuse | 1 (8) | 1 (7) |
| Binge eating | 7 (58) | 2 (14)1 |
ED: Eating disorder; EDNOS: Eating disorder not otherwise specified; f: Frequency;
χ2 > 5.54, df = 1, P < 0.05 (Fisher’s exact test).
Table 2.
Psychological characteristics and QOL ED in ED patients according to symptom response to fructose-sorbitol (mean ± SD)
| No symptom response (n = 12) | Symptom response (n = 14) | |
| Eating attitudes test | 62 ± 26 | 67 ± 19 |
| BSI somatization | 14.0 ± 8.1 | 13.6 ± 7.1 |
| BDI depression | 30.4 ± 12.6 | 30.8 ± 7.0 |
| STAI trait anxiety | 63.6 ± 9.7 | 61.0 ± 7.2 |
| STAI state anxiety | 60.1 ± 9.4 | 63.3 ± 10.2 |
| EPQ neuroticism | 19.3 ± 2.5 | 20.6 ± 2.5 |
| QOL ED global | 17.0 ± 3.2 | 14.7 ± 2.4 |
| QOL ED psychological | 3.3 ± 1.1 | 3.2 ± 1.3 |
BSI: Brief Symptom Inventory; BDI: Beck Depression Inventory; STAI: Spielberger State-Trait Anxiety Inventory; EPQ: Eysenck Personality Questionnaire; QOL ED: Quality of Life Eating Disorder Scale.
F-S symptom provocation and FGID
Sixteen patients fulfilled the criteria for IBS, while 8 patients had 3 or more FGID diagnoses. There was no significant difference in the prevalence of IBS or in the proportion of patients with 3 or more FGIDs, in ED patients with (73%, 36% respectively) and without (80%, 40%) an F-S symptom response.
F-S breath hydrogen response in ED patients and controls
Thirteen (50%) ED patients and 14 (70%) control subjects malabsorbed F-S; this difference was not significant. There were no significant differences in the peak hydrogen levels between ED “malabsorbers” and control subject “malabsorbers” (71 ± 31 ppm vs 48 ± 30 ppm). In ED patients, there were no significant differences in total symptom scores between F-S “malabsorbers” (8 ± 7) and “absorbers” (5 ± 3). When the ED “malabsorbers” were compared with the ED “absorbers” there were no significant differences in the prevalence of IBS (85% vs 63%), or the number of ED patients with ≥ 3 FGIDs (46% vs 25%). The mouth-to-cecum transit time for ED “malabsorbers” was significantly longer (106 ± 35 min) than for control subject “malabsorbers” (54 ± 25 min, P < 0.001).
DISCUSSION
This study is the first to examine the GI symptom responses to F-S ingestion in patients with eating disorders. The key findings are that F-S provoked symptoms in more than half of female ED patients. This is a significantly greater proportion than that found in healthy individuals. Moreover, the response was specific for F-S ingestion. Additionally, there was a greater symptom response in patients at lower BMI values; consistent with this finding, symptom provocation was more common in anorexia nervosa patients. Symptom provocation was not related to the patients’ psychological characteristics, to the presence of chronic digestive tract symptoms such as IBS, or to the presence of a positive breath hydrogen response to F-S.
These findings are clinically relevant to the day-to-day management of ED patients. Eating disorder patients preferentially select low energy foods, including fruit and diet drinks[4]. Fructose, a monosaccharide, is found naturally in fruit, including apples, grapes and stone fruit[16]. Sorbitol, a sugar alcohol, is present in diet drinks, chewing gum, artificial sweeteners and in some fruits such as apples. These 2 substances are therefore likely to be commonly ingested by ED patients, representing a potential source of GI distress that would impact negatively on their nutritional management. In this context, F-S provocative testing could prove valuable in identifying those patients with symptom sensitivity to these substances. Such testing may be of particular importance in very low weight ED patients. Breath testing in association with the F-S challenge, however, does not appear to provide additional clinically useful information. Thus an F-S challenge without breath testing would be sufficient. This approach would also obviate the need for dietary restriction prior to the challenge, a restriction that is contraindicated in the treatment of ED patients in whom “normal” eating behavior is being established.
We chose a dose of F-S (25 g fructose and 5 g sorbitol) that had been previously evaluated in IBS patients[2,3], and shown to produce a greater symptom response than a dose of 20 g fructose and 5 g sorbitol[3]. A recent study has supported a dose of 25 g fructose as the optimal challenge for testing for fructose malabsorption[17]. The dose of glucose was chosen based on that recommended for the evaluation of small bowel bacterial overgrowth[18].
The mechanisms involved in the symptom response to F-S are not clear. The response was not related to the presence of F-S malabsorption or to the psychological characteristics of the patients. An osmotic effect is unlikely, given that the glucose challenge - of similar osmolality to the F-S solution - did not provoke symptoms. The mechanism, however, does appear to be a genuine physiologic phenomenon. A factor associated with low body weight, and presumably a significant negative energy balance, appears to be involved. A role of negative energy balance is also indicated by the observed increased menstrual disturbance among the symptom responders. Underlying disordered gut physiology in the ED patients, as evidenced by a prolongation of mouth-cecum transit time, is another factor of potential relevance. Despite this, there was no evidence for the presence of small bowel bacterial overgrowth in our patients, based on the glucose breath hydrogen testing and using accepted definitions[14,18].
In conclusion, given the novel findings observed in this study, further research should be undertaken to enable the formulation of more precise guidelines regarding F-S sensitivity and the dietary management of ED patients. Future studies should address the role of different F-S doses in relation to body weight and the extent to which various foods containing F-S provoke symptoms.
COMMENTS
Background
Eating disorder (ED) patients display a high prevalence of gastrointestinal symptoms and functional gastrointestinal disorders such as irritable bowel syndrome (IBS). These symptoms may interfere with their nutritional management. Ingestion of fructose-sorbitol (F-S) is an established means of gastrointestinal symptom provocation in irritable bowel syndrome patients. Surprisingly, although ED patients are known to consume “diet” products containing fructose and sorbitol, their gastrointestinal symptom responses to F-S provocation have not been studied.
Research frontiers
It remains controversial whether ingestion of F-S (25 g fructose and 5 g sorbitol) provokes symptoms in IBS by hydrogen production in the colon as a result of incomplete small bowel absorption. Because both fruit and sorbitol-containing “diet” products are frequently consumed by ED patients, ingestion of both these substances together may be an important factor in the genesis of gastrointestinal symptoms in ED patients. Furthermore, certain characteristics associated with ED, such as body weight, and behavioral and psychological features, could influence the responses to F-S symptom provocation.
Innovations and breakthroughs
The key findings of this study are that F-S provoked gastrointestinal symptoms in more than half of the female ED patients, a significantly greater proportion than that found in healthy individuals; the response was specific for F-S ingestion; and there was a greater symptom response in patients at lower BMI values. Consistent with this last finding, symptom provocation was more common in anorexia nervosa patients. Hence negative energy balance appears to play a role in F-S sensitivity in these patients.
Applications
As fructose and sorbitol are likely to be commonly ingested by ED patients, representing a potential source of gastrointestinal distress that would impact negatively on their nutritional management, F-S provocative testing could prove valuable in identifying those patients with symptom sensitivity to these substances. Further studies could address the role of different F-S doses in relation to body weight and the extent to which various foods containing F-S provoke symptoms.
Terminology
Fructose: A monosaccharide, found naturally in fruit, including apples, grapes and stone fruit; Sorbitol: A sugar alcohol, present in diet drinks, chewing gum, artificial sweeteners and in some fruits such as apples.
Peer review
This study documents the provocation of symptoms in patients with eating disorders who have been challenged with a fructose-sorbitol dose.
Acknowledgments
The assistance of the staff and patients of the Eating Disorders Unit, the Northside Clinic, Sydney, Australia is gratefully acknowledged.
Footnotes
Peer reviewer: Dr. Philip Abraham, Professor, Consultant Gastroenterologist & Hepatologist, P. D. Hinduja National Hospital & Medical Research Centre, Veer Savarkar Marg, Mahim, Mumbai 400 016, India
S- Editor Tian L L- Editor Cant MR E- Editor Lin YP
References
- 1.Boyd C, Abraham S, Kellow J. Psychological features are important predictors of functional gastrointestinal disorders in patients with eating disorders. Scand J Gastroenterol. 2005;40:929–935. doi: 10.1080/00365520510015836. [DOI] [PubMed] [Google Scholar]
- 2.Rumessen JJ, Gudmand-Høyer E. Functional bowel disease: malabsorption and abdominal distress after ingestion of fructose, sorbitol, and fructose-sorbitol mixtures. Gastroenterology. 1988;95:694–700. doi: 10.1016/s0016-5085(88)80016-7. [DOI] [PubMed] [Google Scholar]
- 3.Symons P, Jones MP, Kellow JE. Symptom provocation in irritable bowel syndrome. Effects of differing doses of fructose-sorbitol. Scand J Gastroenterol. 1992;27:940–944. doi: 10.3109/00365529209000167. [DOI] [PubMed] [Google Scholar]
- 4.Baş M, Kiziltan G. Relations among weight control behaviors and eating attitudes, social physique anxiety, and fruit and vegetable consumption in Turkish adolescents. Adolescence. 2007;42:167–178. [PubMed] [Google Scholar]
- 5.Diagnostic and statistical manual of mental disorders. 4th edition (DSM-IV) Vol. 42. Washington DC: American Psychiatric Association; 1994. [Google Scholar]
- 6.Drossman DA, Corraziari EC, Talley NJ, Thompson WG, Whitehead WE. Rome II integrative questionnaire. In: Drossman DA, Corraziari EC, Talley NJ, Thompson WG, Whitehead WE, et al., editors. Rome II: the functional gastrointestinal disorders. McLean VA: Degnon Associates; 2000. pp. 691–710. [Google Scholar]
- 7.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 8.Garner DM, Garfinkel PE. The Eating Attitudes Test: an index of the symptoms of anorexia nervosa. Psychol Med. 1979;9:273–279. doi: 10.1017/s0033291700030762. [DOI] [PubMed] [Google Scholar]
- 9.Eysenck HJ, Eysenck SBG. Manual of the Eysenck Personality Questionnaire (Junior and Adult) Vol. 9. Kent: Seven Oaks; 1975. [Google Scholar]
- 10.Spielberger CD, Gorsuch RL, Lushene RE. STAI Manual for the State-Trait Anxiety Inventory. Vol. 9. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- 11.Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med. 1983;13:595–605. [PubMed] [Google Scholar]
- 12.Abraham SF, Brown T, Boyd C, Luscombe G, Russell J. Quality of life: eating disorders. Aust N Z J Psychiatry. 2006;40:150–155. doi: 10.1080/j.1440-1614.2006.01762.x. [DOI] [PubMed] [Google Scholar]
- 13.Abraham S, Lovell , N . Eating and exercise examination—computerized (EEE-C). Version 4.0 Manual. Vol. 40. Sydney: Ashwood Medical; 1999. [Google Scholar]
- 14.Simrén M, Stotzer PO. Use and abuse of hydrogen breath tests. Gut. 2006;55:297–303. doi: 10.1136/gut.2005.075127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kellow JE, Borody TJ, Phillips SF, Haddad AC, Brown ML. Sulfapyridine appearance in plasma after salicylazosulfapyridine. Another simple measure of intestinal transit. Gastroenterology. 1986;91:396–400. doi: 10.1016/0016-5085(86)90574-3. [DOI] [PubMed] [Google Scholar]
- 16.Rumessen JJ. Fructose and related food carbohydrates. Sources, intake, absorption, and clinical implications. Scand J Gastroenterol. 1992;27:819–828. doi: 10.3109/00365529209000148. [DOI] [PubMed] [Google Scholar]
- 17.Rao SS, Attaluri A, Anderson L, Stumbo P. Ability of the normal human small intestine to absorb fructose: evaluation by breath testing. Clin Gastroenterol Hepatol. 2007;5:959–963. doi: 10.1016/j.cgh.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh VV, Toskes PP. Small bowel bacterial overgrowth: presentation, diagnosis, and treatment. Curr Gastroenterol Rep. 2003;5:365–372. doi: 10.1007/s11894-003-0048-0. [DOI] [PubMed] [Google Scholar]