Abstract
AIM: To investigate the role of the -347G→GA polymorphism in the progression of colorectal cancer (CRC).
METHODS: We designed a case-control study based on a population of CRC patients in China and normal healthy controls with no history of tumors or inherited diseases. Polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) analyses were used to genotype the variants, and immunohistochemical staining was performed to measure the expression of E-cadherin in different allele cases among the CRC patients and normal controls.
RESULTS: The GA-allele (G/GA heterozygous and GA/GA homozygous) did not increase the risk of CRC compared with the G-allele (OR = 1.232, 95% CI = 0.898-1.691). However, the frequencies of the GA-allele were higher in poorly differentiated (P = 0.002) and proximal (P = 0.019) CRC patients than in normal controls. We also observed that E-cadherin expression was lower in poorly differentiated CRC patients than in well differentiated CRC patients (P = 0.001). Furthermore, E-cadherin expression was lower for the GA-allele than for the G-allele (G/G heterozygous) in CRC patients (P = 0.018). In contrast, there was no significant difference in E-cadherin expression for the G-allele and GA-allele in normal controls (P = 0.292).
CONCLUSION: The -347G→GA promoter polymorphism in E-cadherin gene is associated with specific CRC features, and may be a prognostic factor rather than a susceptibility factor during the progression of CRC.
Keywords: Allele, Colorectal cancer, E-cadherin, Polymorphism, Prognosis
INTRODUCTION
Colorectal cancer (CRC) is one of the most common cancers in Western countries[1], and is becoming more prevalent in Asian countries, especially China[2]. The etiopathogenesis of CRC is considered to be multifactorial, and to include high red meat intake[3], high alcohol intake[4] and smoking[5]. However, no single environmental or lifestyle factor has consistently been associated with the risk of CRC. Recently, more and more views support genetic predisposition as the basis of many diseases, especially cancers[6]. However, CRC is divided into hereditary and sporadic cases, which show distinct genetic alterations and exhibit different key events leading to neoplastic growth.
E-cadherin is one of the major constituents of cell-adhesion complexes in epithelial cells[7,8]. It is a 97-kDa transmembrane glycoprotein encoded by the E-cadherin gene (CDH1) located on chromosome 16q22.1. It plays important roles in the establishment of adherent-type junctions by mediating calcium-dependent cellular interactions, and is thought to be a tumor suppressor protein[7]. Partial or total loss of E-cadherin expression occurs in the majority of human carcinomas[9]. Besides its role in physical cell-cell adhesions, E-cadherin is also thought to be involved in intracellular signaling in normal epithelial cells, since downregulation of this molecule in epithelial cells is frequently associated with tumor formation and differentiation[10].
It is not yet understood how the expression of E-cadherin is regulated, and this may occur via loss of heterozygosity, gene mutations or methylation of the coding region. Recently, the promoter region of CDH1 was reported to be highly polymorphic[11]. One of the polymorphisms is the -347G→GA (rs5030625) single nucleotide polymorphism (SNP) upstream from the transcriptional start site[12]. Just as nucleotide variations in the coding region of a gene can alter protein expression[12,13], the -347G→GA polymorphism within the promoter region may change the transcriptional efficiency of CDH1. For example, the GA-allele has a weak transcriptional factor-binding strength and transcriptional activity compared with the G-allele[12], suggesting that the GA-allele may be associated with tumor formation or differentiation.
In the present study, we carried out a hospital-based case-control study to explore the association of the CDH1 -347G→GA polymorphism with sporadic CRC in China. In addition, we measured the expression of E-cadherin in different allele cases including CRC patients and normal controls by immunohistochemical staining to check the function of the -347G→GA polymorphism in vitro.
MATERIALS AND METHODS
Subjects
The study included 290 sporadic CRC patients and 335 normal healthy controls (Table 1) enrolled from The Affiliated Drum Tower Hospital of Nanjing University Medical School between 2004 and 2008. Most of the patients had recently received a final diagnosis of CRC and were scheduled for surgery if no clinical metastases were detected, or would receive chemotherapy. A small number of the CRC patients had previously received surgery or chemotherapy. None of the subjects were blood-related. Patients affected by CRC were considered eligible if they had a histological diagnosis and were free from any known diseases with a genetic predisposition. Controls were selected from trauma patients or puerperal women in the same hospital during this time period. None of the controls had a history of malignancy. All the subjects were interviewed by a trained interviewer using a pretested questionnaire to obtain information on their sociodemographic characteristics, dietary habits, smoking and drinking status, and their individual and family history of cancer. Sporadic CRC cases and controls were matched for age, sex, smoking and drinking history.
Table 1.
Clinical characteristics of the CRC patients and normal controls
| CRC patients (%) | Normal controls (%) | P | |
| Sex | |||
| Male | 188 (64.8) | 212 (63.3) | |
| Female | 102 (35.2) | 123 (36.7) | 0.6881 |
| Age (yr) | |||
| Range | 27-85 | 15-81 | |
| Mean | 63.51 ± 13.24 | 58.62 ± 17.45 | 0.4072 |
| Smoking history | |||
| Yes | 117 (40.3) | 122 (36.4) | |
| No | 173 (59.7) | 213 (63.6) | 0.3141 |
| Drinking history | |||
| Yes | 128 (44.1) | 135 (40.3) | |
| No | 162 (55.9) | 200 (59.7) | 0.3321 |
| Stage | |||
| Dukes A | 12 | ||
| Dukes B | 89 | ||
| Dukes C | 102 | ||
| Dukes D | 87 | ||
| Location | |||
| Ascending, transverse | 138 | ||
| Descending, sigmoid | 61 | ||
| Rectum | 91 | ||
| Total | 290 | 335 |
Two-tailed χ2-test;
Two-tailed t-test; CRC: Colorectal cancer.
The study was approved by the Institutional Review Boards of the Drum Tower Hospital. All the participants provided written informed consent at the time of recruitment and agreed to blood collection.
DNA extraction
Peripheral venous blood (5 mL) was drawn from each subject before they received surgery or chemotherapy, and was placed in tubes containing EDTA and stored at -70°C until analysis. Total genomic DNA was extracted using a purification kit (Promega, Madison, WI, USA) according to the manufacturer’s instructions.
Genotyping analysis
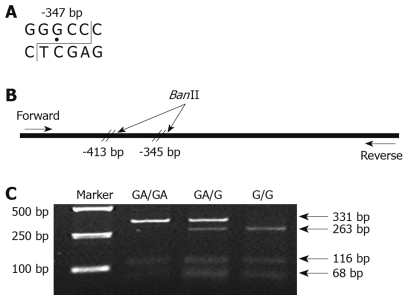
The CDH1 -347G→GA polymorphism was genotyped by the PCR-RFLP method. A 447-bp fragment containing the -347G→GA polymorphism in the CDH1 promoter was amplified with the following primers: forward, 5'-GCCCCGACTTGTCTCTCTAC-3'; reverse, 5'-GGCCACAGCCAATCAGCA-3'. PCR amplification was carried out in a volume of 25 μL containing 20 ng of genomic DNA, 1 μL of primers, 20 μmol/L each of the forward and reverse primers, 2.5 μL of 10 × PCR buffer (Mg2+-free), 2 μL of dNTP, 1.5 μL of MgCl2 and 0.5 U of Taq polymerase (TaKaRa Biotechnology, Dalian, China). The amplification was performed in a programmable thermal cycler (MWG Biotech AG, Ebersberg, Germany) as follows: 1 cycle of 95°C for 2 min; 35 cycles of 94°C for 1 min, 61°C for 1 min and 72°C for 1 min; and a final cycle of 72°C for 10 min. The PCR product was digested with BanII (TaKaRa Biotechnology) at 37°C overnight (Figure 1A and B). After digestion, the products were separated by 3% agarose gel electrophoresis and stained with ethidium bromide (Figure 1C). GA/GA homozygous cases were represented by DNA bands of 332 and 116 bp. G/G homozygous cases were represented by DNA bands of 263, 116 and 68 bp. GA/G heterozygous cases displayed a combination of both alleles (332, 263, 116 and 68 bp).
Figure 1.
Genotyping analysis of the CDH1 -347G→GA polymorphism by PCR-RFLP analysis. A: Structural diagram of the restriction enzyme analysis for BanII; B: Schematic overview of the E-cadherin gene promoter PCR fragment and the locations of the BanII restriction enzyme sites; C: RFLP analysis of the -347G→GA polymorphism using BanII digestion. GA/GA homozygote: 332 and 116 bp; GA/G heterozygote: 332, 263, 116 and 68 bp; G/G homozygote: 263, 116 and 68 bp.
Immunohistochemistry
CRC tissue samples were collected from CRC patients who underwent surgery. Normal colon tissue samples were collected from outpatients via colonic biopsies during colonoscopy examination.
Immunohistochemistry was carried out using the avidin-biotin-peroxidase complex method. The central region of CRC tissue samples and normal colon tissue samples were cut into 4-μm sections, mounted on glass slides, deparaffinized and rehydrated by xylene and graded ethanol solutions. Endogenous peroxidase activity was blocked by incubation with 3% hydrogen peroxide for 20 min at room temperature. For antigen retrieval, the sections were heated in a pressure cooker for 5 min. The sections then were sequentially incubated with primary mouse antibodies against E-cadherin (Santa Cruz Biotechnology, Santa Cruz, CA, USA; dilution, 1:150; monoclonal antibody) overnight at 4°C, a biotinylated goat anti-mouse secondary antibody (Santa Cruz Biotechnology) for 30 min, and peroxidase-conjugated streptavidin for 10 min. Samples were colored with diaminobenzidine (Boster, Wuhan, China) and counterstained with hematoxylin. Images were captured under an Olympus-BX50F4 microscope (Olympus Corporation, Tokyo, Japan) and quantitatively analyzed using the Image-Pro Plus 6.0 software (Media Cybernetics, Bethesda, USA).
Sections of normal colorectal tissue were used as positive controls for E-cadherin staining. Negative controls were prepared by replacing the primary antibody with nonimmune IgG.
Statistical analysis
The distributions of the clinical characteristics of the CRC patients and normal controls were analyzed by an unpaired two-tailed t-test. This test was also used to compare E-cadherin expression between the G-allele and GA-allele. The χ2-test was used to test the differences in the allele frequencies between normal controls and CRC patients. The genotype data were further stratified by age, sex, smoking, alcohol intake, tumor location, pathologic grouping and clinical stage of CRC. The odds ratio (OR) and 95% confidence interval (CI) were calculated using an unconditional logistic regression model to evaluate the risk of the CDH1 -347G→GA polymorphism for CRC. We performed all analyses with the SPSS 15.0 software package (SPSS Inc, Chicago, USA). Values of P < 0.05 were considered statistically significant.
RESULTS
All the recruited subjects were successfully genotyped. The study included 290 CRC patients and 335 normal controls with available data. The clinical characteristics of the study subjects are summarized in Table 1. No significant differences were noted in the distribution frequencies for sex, smoking and drinking.
As shown in Table 2, the G-allele and GA-allele frequencies were 52.8% and 47.2%, respectively, in the CRC patients, and 57.9% and 42.1%, respectively, in the controls. There was no significant difference between the patients and the controls (χ2 = 1.671, P = 0.198). A logistic regression analysis revealed that the GA-allele did not increase the risk of CRC (OR = 1.232, 95% CI = 0.898-1.691). However, when the CRC patients were stratified by age, sex, smoking, drinking, tumor location, pathologic grouping and clinical stage, the GA-allele frequency was higher in poorly differentiated CRC patients than in normal controls (χ2-test, P = 0.002). In addition, the GA-allele frequency was higher in proximal CRC patients than in normal controls (χ2-test, P = 0.019).
Table 2.
Distribution of the CDH1 -347G→GA polymorphism between CRC patients and normal controls
| G-allele (%)1 | GA-allele (%)2 | OR (95% CI)3 | χ2 | P | |
| Controls | 194 (57.9) | 141 (42.1) | |||
| Patients | 153 (52.8) | 137 (47.2) | 1.232 (0.898-1.691) | 1.671 | 0.198 |
| Sex | |||||
| Male | 98 (52.1) | 90 (47.9) | 1.264 (0.882-1.809) | 1.633 | 0.233 |
| Female | 55 (53.9) | 47 (46.1) | 1.176 (0.753-1.836) | 0.508 | 0.495 |
| Age (yr) | |||||
| > 60 | 95 (55.2) | 77 (44.8) | 1.115 (0.770-1.616) | 0.333 | 0.571 |
| ≤ 60 | 58 (49.2) | 60 (50.8) | 1.423 (0.934-2.169) | 2.712 | 0.107 |
| Smoking | |||||
| Yes | 57 (48.7) | 60 (51.3) | 1.448 (0.949-2.210) | 2.967 | 0.105 |
| No | 96 (55.5) | 77 (44.5) | 1.104 (0.762-1.598) | 0.273 | 0.637 |
| Drinking | |||||
| Yes | 66 (51.6) | 62 (48.4) | 1.292 (0.859-1.945) | 1.516 | 0.249 |
| No | 87 (53.7) | 75 (46.3) | 1.186 (0.813-1.730) | 0.786 | 0.386 |
| Location | |||||
| Proximal4 | 63 (45.7) | 75 (54.3) | 1.638 (1.099-2.441) | 5.919 | 0.019 |
| Distal5 | 90 (59.2) | 62 (40.8) | 0.948 (0.642-1.399) | 0.073 | 0.843 |
| Differentiation | |||||
| Well | 116 (56.3) | 90 (43.7) | 1.067 (0.752-1.516) | 0.133 | 0.721 |
| Poorly | 16 (33.3) | 32 (66.7) | 2.752 (1.454-5.209) | 10.240 | 0.002 |
| Other | 21 (58.3) | 15 (41.7) | 0.983 (0.489-1.974) | 0.002 | 1.000 |
| Stage | |||||
| Dukes A/B | 58 (57.4) | 43 (42.6) | 1.020 (0.650-1.600) | 0.007 | 1.000 |
| Dukes C/D | 95 (50.3) | 94 (49.7) | 1.361 (0.951-1.948) | 2.856 | 0.100 |
G/G homozygous;
GA/GA homozygous; GA/G heterozygous;
95% confidence interval;
Ascending colon, transverse colon;
Descending colon, sigmoid colon, rectum; CDH1: E-cadherin gene.
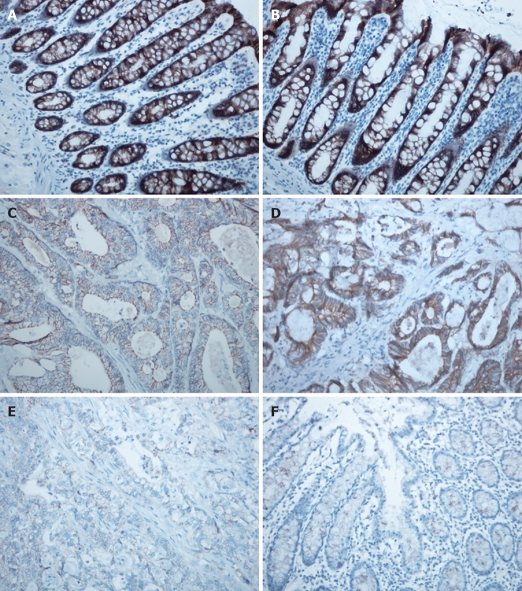
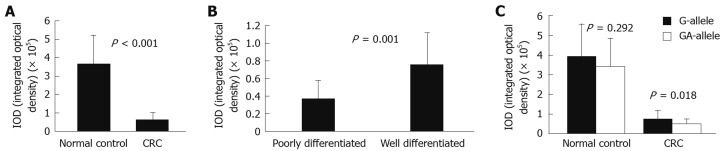
Immunohistochemical staining for E-cadherin was evaluated in 42 CRC patients (G-allele vs GA-allele: 20 vs 22) and 37 normal controls (G-allele vs GA-allele: 19 vs 18) (Figure 2). E-cadherin expression levels with the G-allele and GA-allele were compared between CRC patients and normal controls, as well as between poorly differentiated and well differentiated CRC patients (Figure 3). E-cadherin expression was significantly higher in normal controls, well differentiated CRC patients and the G-allele CRC patients than in CRC patients (t-test, P < 0.001), poorly differentiated CRC patients (t-test, P = 0.001) and the GA-allele CRC patients (t-test, P = 0.018), however, there was no significant difference in E-cadherin expression between the G-allele and GA-allele in normal controls (t-test, P = 0.292).
Figure 2.
Immunohistochemical staining for E-cadherin. A,B: E-cadherin expression in normal colorectal tissue with the G-allele (A) and GA-allele (B) (each, × 200); C,D: E-cadherin expression in well differentiated CRC with the GA-allele (C) and G-allele (D) (each, × 200); E: E-cadherin expression in poorly differentiated CRC with the GA-allele (× 200); F: Negative control (× 200).
Figure 3.
E-cadherin expression in colon tissues of different types. A: E-cadherin expression is significantly higher in normal controls than in CRC patients (t-test, P < 0.001); B: E-cadherin expression is significantly higher in well differentiated CRC patients than in poorly differentiated CRC patients (t-test, P = 0.001); C: There is no significant difference in E-cadherin expression between the G-allele and GA-allele in normal controls (t-test, P = 0.292). However, E-cadherin expression is significantly higher for the G-allele than for the GA-allele in CRC patients (t-test, P = 0.018).
DISCUSSION
Several molecular epidemiological studies have confirmed an association between the CDH1 -347G→GA polymorphism and the risk of cancers, including gastric, colorectal and esophageal cancers[14,15]. The authors of these studies proposed that the CDH1 -347G→GA polymorphism may be functional, and that the GA-allele could lead to transcriptional downregulation of CDH1 and low expression of E-cadherin compared with the G-allele, thereby increasing the risk of cancer. However, recent studies have indicated that some functional polymorphisms may play more important roles in the prognosis of cancer than in its formation[16,17]. To further investigate the association between the functional CDH1 -347G→GA polymorphism and sporadic CRC, we conducted the present case-control study in a Chinese population.
We found that the GA-allele did not increase the risk of CRC compared with the G-allele in this Chinese population. This finding is not consistent with the study by Shin et al[18], who reported that the GA-allele was associated with a significantly increased risk of CRC in Korea. Furthermore, their GA-allele frequency in normal controls (41/147, 27.9%) was obviously lower than the frequency observed in the present study (141/335, 42.1%). These discrepancies may be caused by racial differences. At the same time, we found that there was no significant difference in E-cadherin expression between the G-allele and GA-allele in normal controls, as evaluated by immunohistochemical staining. In other words, the GA-allele did not increase the risk of CRC or influence the expression of E-cadherin in normal controls.
However, the frequency of the GA-allele was significantly higher in our poorly differentiated and proximal CRC patients than in the normal controls. In addition, E-cadherin expression was lower in the poorly differentiated CRC patients than in the well differentiated CRC patients, and the E-cadherin expression was lower for the GA-allele than for the G-allele in CRC patients. Considering the above-described findings, the CDH1 -347G→GA polymorphism did not appear to influence E-cadherin expression in normal controls. However, it may play a role in the expression of E-cadherin after CRC formation, and may also be associated with the cell differentiation of CRC. These findings are reminiscent of the study of Cano et al[19], who found that the Snail gene family members were expressed in human carcinoma cells, especially poorly differentiated carcinoma cells, and could repress the transcriptional activity of CDH1 through binding to a specified structure or zone in CDH1. We speculate that this specific structure or zone may be associated with certain functional polymorphisms.
Although the idea that E-cadherin is associated with tumors has been accepted by most researchers, the molecular mechanisms for the involvement of E-cadherin in tumor formation remain controversial. For example, it remains unknown whether E-cadherin acts as a susceptibility factor or a prognostic factor. Supporters of the former idea believe that loss of E-cadherin during tumor formation leads to accumulation of β-catenin in the cytoplasm, which could stimulate the β-catenin pathway[20]. However, several studies have shown that low expression of E-cadherin can occur after the formation of a tumor in response to alterations in the internal environment, such as hypoxic conditions, growth factor expression, tumor suppressor protein actions etc, all of which can modulate the expression of E-cadherin through various signaling pathways[21]. Therefore, it is possible that the CDH1 -347G→GA polymorphism may change the transcriptional activity of CDH1 after tumor formation by combining with other factors, and thereby influencing the differentiation of tumor cells.
In conclusion, we found that the CDH1 -347G→GA polymorphism was associated with specific CRC features. Furthermore, the GA-allele was a repressive factor for the transcriptional activity of CDH1, but may begin to function after the formation of CRC and play a role in the differentiation of tumor cells. This also implies that the CDH1 -347G→GA polymorphism may be a prognostic factor rather than a susceptibility factor during the progression of CRC.
COMMENTS
Background
The progression of colorectal cancer (CRC) is a major cause of cancer death in Western populations, and is becoming more prevalent in Asian countries such as China. Although it is well known that high red meat intake, high alcohol intake and smoking are associated with the risk of CRC, host genetic factors may be one of the critical factors in carcinogenesis.
Research frontiers
E-cadherin is thought to be involved in intracellular signaling in epithelial cells, since downregulation of this molecule in epithelial cells is frequently associated with tumor formation and differentiation. The -347G→GA polymorphism within the promoter region is considered to be functional, which may change the transcriptional efficiency of E-cadherin gene (CDH1).
Innovations and breakthroughs
Most previous studies concentrated on the association of polymorphisms with the formation of carcinomas. This is probably the first report on the relationship between CDH1 -347G→GA polymorphism and the prognosis of CRC, and we found that the -347G→GA polymorphism may be a prognostic factor rather than a susceptive factor during the progression of CRC.
Applications
These findings may help doctors to choose an appropriate treatment for different CRC patients.
Terminology
E-cadherin: E-cadherin is a 97-kDa transmembrane glycoprotein, which is one of the major constituents of cell-adhesion complexes in epithelial cells. CDH1: CDH1 is the gene which encodes E-cadherin, and is located on chromosome 16q22.1.
Peer review
It is a well-written and well-designed study, with large observed samples, and with important scientific merit.
Footnotes
Peer reviewers: Inti Zlobec, PhD, Institute for Pathology, University Hospital Basel, Schoenbeinstrasse 40, Basel, CH-4031, Switzerland; Ferenc Sipos, MD, PhD, Cell Analysis Laboratory, 2nd Department of Internal Medicine, Semmelweis University, Szentkirályi u. 46, Budapest 1088, Hungary
S- Editor Tian L L- Editor Webster JR E- Editor Lin YP
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Fengju S, Guanglin W, Kexin C. Incidence of colon cancer in Tianjin, China, 1981-2000. Asia Pac J Public Health. 2005;17:22–25. doi: 10.1177/101053950501700106. [DOI] [PubMed] [Google Scholar]
- 3.Chao A, Thun MJ, Connell CJ, McCullough ML, Jacobs EJ, Flanders WD, Rodriguez C, Sinha R, Calle EE. Meat consumption and risk of colorectal cancer. JAMA. 2005;293:172–182. doi: 10.1001/jama.293.2.172. [DOI] [PubMed] [Google Scholar]
- 4.Cho E, Smith-Warner SA, Ritz J, van den Brandt PA, Colditz GA, Folsom AR, Freudenheim JL, Giovannucci E, Goldbohm RA, Graham S, et al. Alcohol intake and colorectal cancer: a pooled analysis of 8 cohort studies. Ann Intern Med. 2004;140:603–613. doi: 10.7326/0003-4819-140-8-200404200-00007. [DOI] [PubMed] [Google Scholar]
- 5.Giovannucci E. An updated review of the epidemiological evidence that cigarette smoking increases risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2001;10:725–731. [PubMed] [Google Scholar]
- 6.Garinis GA, Menounos PG, Spanakis NE, Papadopoulos K, Karavitis G, Parassi I, Christeli E, Patrinos GP, Manolis EN, Peros G. Hypermethylation-associated transcriptional silencing of E-cadherin in primary sporadic colorectal carcinomas. J Pathol. 2002;198:442–449. doi: 10.1002/path.1237. [DOI] [PubMed] [Google Scholar]
- 7.Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- 8.Koriyama C, Akiba S, Itoh T, Sueyoshi K, Minakami Y, Corvalan A, Yonezawa S, Eizuru Y. E-cadherin and beta-catenin expression in Epstein-Barr virus-associated gastric carcinoma and their prognostic significance. World J Gastroenterol. 2007;13:3925–3931. doi: 10.3748/wjg.v13.i29.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen HC, Chu RY, Hsu PN, Hsu PI, Lu JY, Lai KH, Tseng HH, Chou NH, Huang MS, Tseng CJ, et al. Loss of E-cadherin expression correlates with poor differentiation and invasion into adjacent organs in gastric adenocarcinomas. Cancer Lett. 2003;201:97–106. doi: 10.1016/j.canlet.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer. 2004;4:118–132. doi: 10.1038/nrc1276. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura A, Shimazaki T, Kaneko K, Shibata M, Matsumura T, Nagai M, Makino R, Mitamura K. Characterization of DNA polymorphisms in the E-cadherin gene (CDH1) promoter region. Mutat Res. 2002;502:19–24. doi: 10.1016/s0027-5107(02)00024-6. [DOI] [PubMed] [Google Scholar]
- 12.Shin Y, Kim IJ, Kang HC, Park JH, Park HR, Park HW, Park MA, Lee JS, Yoon KA, Ku JL, et al. The E-cadherin -347G->GA promoter polymorphism and its effect on transcriptional regulation. Carcinogenesis. 2004;25:895–899. doi: 10.1093/carcin/bgh073. [DOI] [PubMed] [Google Scholar]
- 13.Li LC, Chui RM, Sasaki M, Nakajima K, Perinchery G, Au HC, Nojima D, Carroll P, Dahiya R. A single nucleotide polymorphism in the E-cadherin gene promoter alters transcriptional activities. Cancer Res. 2000;60:873–876. [PubMed] [Google Scholar]
- 14.Zhang XF, Wang YM, Wang R, Wei LZ, Li Y, Guo W, Wang N, Zhang JH. [Correlation of E-cadherin polymorphisms to esophageal squamous cell carcinoma and gastric cardiac adenocarcinoma] Ai Zheng. 2005;24:513–519. [PubMed] [Google Scholar]
- 15.Zhang B, Pan K, Liu Z, Zhou J, Gu L, Ji J, Ma J, You WC, Deng D. Genetic polymorphisms of the E-cadherin promoter and risk of sporadic gastric carcinoma in Chinese populations. Cancer Epidemiol Biomarkers Prev. 2008;17:2402–2408. doi: 10.1158/1055-9965.EPI-08-0315. [DOI] [PubMed] [Google Scholar]
- 16.Huang CS, Kuo SH, Lien HC, Yang SY, You SL, Shen CY, Lin CH, Lu YS, Chang KJ. The CYP19 TTTA repeat polymorphism is related to the prognosis of premenopausal stage I-II and operable stage III breast cancers. Oncologist. 2008;13:751–760. doi: 10.1634/theoncologist.2007-0246. [DOI] [PubMed] [Google Scholar]
- 17.Kim JG, Chae YS, Sohn SK, Cho YY, Moon JH, Park JY, Jeon SW, Lee IT, Choi GS, Jun SH. Vascular endothelial growth factor gene polymorphisms associated with prognosis for patients with colorectal cancer. Clin Cancer Res. 2008;14:62–66. doi: 10.1158/1078-0432.CCR-07-1537. [DOI] [PubMed] [Google Scholar]
- 18.Shin Y, Kim IJ, Kang HC, Park JH, Park HW, Jang SG, Lee MR, Jeong SY, Chang HJ, Ku JL, et al. A functional polymorphism (-347 G-->GA) in the E-cadherin gene is associated with colorectal cancer. Carcinogenesis. 2004;25:2173–2176. doi: 10.1093/carcin/bgh223. [DOI] [PubMed] [Google Scholar]
- 19.Cano A, Pérez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 20.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baranwal S, Alahari SK. Molecular mechanisms controlling E-cadherin expression in breast cancer. Biochem Biophys Res Commun. 2009;384:6–11. doi: 10.1016/j.bbrc.2009.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]