Abstract
AIM: To evaluate the impact of splenectomy on long-term survival, postoperative morbidity and mortality of patients with gastric cancer by performing a meta-analysis.
METHODS: A search of electronic databases to identify randomized controlled trials in The Cochrane Library trials register, Medline, CBMdisc (Chinese Biomedical Database) and J-STAGE, etc was performed. Data was extracted from the studies by 2 independent reviewers. Outcome measures were survival, postoperative morbidity and mortality and operation-related events. The meta-analyses were performed by RevMan 4.3.
RESULTS: Three studies comprising 466 patients were available for analysis, with 231 patients treated by gastrectomy plus splenectomy. Splenectomy could not increase the 5-year overall survival rate [RR = 1.17, 95% confidence interval (CI) 0.97-1.41]. The postoperative morbidity (RR = 1.76, 95% CI 0.82-3.80) or mortality (RR = 1.58, 95% CI 0.45-5.50) did not suggest any significant differences between the 2 groups. No significant differences were noted in terms of number of harvested lymph nodes, operation time, length of hospital stay and reoperation rate. Subgroup analyses showed splenectomy did not increase the survival rate for proximal and whole gastric cancer. No obvious differences were observed between the 2 groups when stratified by stage. Sensitivity analyses indicated no significant differences regarding the survival rates (P > 0.05).
CONCLUSION: Splenectomy did not show a beneficial effect on survival rates compared to splenic preservation. Routinely performing splenectomy should not be recommended.
Keywords: Gastric cancer, Splenectomy, Survival rate, Morbidity, Operative surgical procedure, Postoperative period, Treatment outcome
INTRODUCTION
Gastric cancer is a disease with a high incidence. It is estimated that approximately 21 500 new cases of gastric carcinomas and 10 880 deaths would occur in the United States in 2008[1]. There has been a trend toward proximal gastric carcinoma in Western countries[2,3]. In proximal gastric and gastroesophageal junction cancers, lymph node metastases are found more frequently in the splenic hilum[4].
Extended lymph node dissection is regarded as essential for the treatment of gastric cancer[5]. Splenectomy is performed for the purpose of effective lymph node dissection around the splenic artery and splenic hilum and for direct invasion of the splenic hilum or spleen; however, the effect of splenectomy on the prognosis is controversial. Previous reports suggested that gastrectomy with splenectomy resulted in better survival than gastrectomy alone in gastric cancer patients[6,7]. Some investigators have reported that splenectomy did not increase the survival rate[8-10]. In addition, the importance of the spleen as a part of the immune system and the immunological consequences of its removal have recently been stressed[11,12].
However, recent clinical trials showed that gastrectomy with splenectomy could result in higher postoperative morbidity and mortality[13-15].
The aim of this meta-analysis was to evaluate the impact of splenectomy on long-term survival of gastric cancer patients and to compare the postoperative morbidity and mortality of patients undergoing splenectomy with that of patients not undergoing splenectomy at the time of gastrectomy.
MATERIALS AND METHODS
Search strategy and study selection
We searched the electronic databases of PubMed (http://www.ncbi.nlm.nih.gov/sites/entrez/), the Cochrane Central Register of Controlled Trials (http://www.mrw.interscience.wiley.com/cochrane/cochrane_clcentral_articles_fs.html), the J-STAGE Database (Japan Science and Technology Information Aggregator, Electronic) (http://www.jstage.jst.go.jp/browse/), and CBMdisc (Chinese Biomedical Database) (http://dlib.edu.cnki.net/kns50/Navigator.aspx?ID=1). Other websites and conference proceedings were searched, including those of the National Cancer Institute (http://www.cancernet.nci.nih.gov/cancertopics), the European Organization for Research and Treatment of Cancer (http://www.eortc.be/), the Southwest Oncology Group (http://www.swog.org/), ClinicalTrials.gov (http://clinicaltrials.gov/), the American Society of Clinical Oncology (http://www.asco.org/portal/site/ASCO). Moreover, the reference lists from relevant articles were screened for study inclusion. Eligible unpublished papers were also considered to be included, if known from consultation with Prof. Chen ZX and Prof. Chen JP.
The search strategy of Medline was as follows and was applied to other databases also: [“Stomach Neoplasms” (Mesh) AND “Carcinoma” (Mesh)] AND [“splenectomy” (MeSH) OR “spleen dissection” (textword) OR “spleen resection” (textword) OR “splenic preservation” (textword)] AND [“Comparative Study” (Publication Type) OR “follow-up studies” (Mesh) OR “Clinical Trial” (Publication Type) OR “Evaluation Studies” (Publication Type) OR “Multicenter Study” (Publication Type) OR “Random allocation” (Subheading) OR “Randomized Controlled Trial” (Publication Type/subheading) OR “Controlled Clinical Trial” (Publication Type) or “Research design” (Subheading)]. The electronic search was up to December, 2008 with no limitations regarding publication date and language.
Inclusion and exclusion criteria
Only randomized controlled trials (RCTs) which compared the effectiveness or safety of splenectomy to those of non-splenectomy were eligible.
The patients had been confirmed with gastric carcinoma by endoscopy and biopsy preoperatively. There was no limitation in the location of the gastric carcinoma and surgical procedure. There was no distant metastasis, the primary tumors were resectable, and the patients could tolerate the operation. Patients treated with chemotherapy, immunotherapy, etc perioperatively were included. There was no limitation in age, gender and race. Patients with splenectomy induced by iatrogenic injury were included because of the small number. Curative or palliative gastrectomies were included, but patients with other kinds of gastric tumors, such as lymphoma, other organ tumors or multiple gastric tumors (i.e. adenosquamous carcinoma) were excluded. Trials with uncertain or marked inequality of characteristics between groups at baseline were excluded.
Selection, assessment and data extraction
In order to select studies for further assessment, 2 independent reviewers (Yang K, Zhang B) screened the title, abstract section and keywords of every record retrieved. Full articles were assessed if the information given suggested that the study conformed to our criteria described above. The final selection of studies was completed by 2 researchers (Yang K, Chen XZ). Any disagreements in quality assessment and data collection were discussed and resolved by a third reviewer (Hu JK) as the referee.
Data was extracted independently by 2 reviewers. Details of study sample (number in each arm), interventions (the details of splenectomy, as approach, as well as details of other treatments, such as adjuvant chemotherapy, immunotherapy, etc) and outcomes (5-year overall survival rate, postoperative mortality and morbidity and operation-related events) were extracted. Additionally, the year and country of study, the number and reason of withdrawals and dropouts and characteristics of patients were extracted.
If only survival curves were reported, the overall 5-year survival rates were extracted and converted from the figures as accurately as possible[16].
When the trials had reported medians and ranges instead of means and standard deviations, we assumed medians were equal to means, and equated standard deviation to a quarter of the reported range. If neither a range nor any other measure of dispersion was reported, half of the mean or the median as standard deviation was used[17].
Seven items relevant to the quality appraisal were used for assessment[18]: (1) whether the method of allocation was truly random; (2) whether there was proper concealment of allocation; (3) whether there was equality between the 2 groups at baseline in terms of prognostic features; (4) whether the eligibility criteria were described; (5) whether blinding of the outcome assessors was performed; (6) whether loss to follow-up in each treatment arm was demonstrated, and (7) whether intention-to-treat analysis was considered. Seven or 6 items were required for a trial to be rated as high quality, 5 or 4 items as fair quality and 3 or fewer as low quality[18].
Outcomes of interest and definitions
The primary outcome measures were 5-year overall survival rate, overall hospital or postoperative 30 d mortality, and overall morbidity rate, while the secondary outcome measure was operation-related events, the number of harvested lymph nodes, operation time, length of hospital stay and reoperation rate. One or more outcome measures should be available in the trials, or they were excluded.
Statistical analysis
Weighted estimates of relative risks (RR) and weighted mean differences (WMD) with 95% confidence intervals (CI) were calculated for dichotomous data and continuous data respectively. The analyses were conducted using RevMan 4.3. A P-value < 0.05 was considered as statistically significant. Heterogeneities of treatment effect between trials were tested using a Chi-squared statistic with significance being set at P < 0.10, and the total variation across studies was estimated by I-square and divided into low, moderate and high levels, corresponding to the I-square of < 25%, 25%-50%, and > 50%[19]. If heterogeneities existed, one of the following techniques was undertaken to attempt to explain them: 1. Random effect model for meta-analyses; 2. Sub-group analyses; 3. Sensitivity analyses. Subgroup analyses stratified by the location of tumor and stage of tumor were performed. Sensitivity analyses were performed only in high quality trials to avoid errors caused by poor quality studies[20].
RESULTS
Included literature
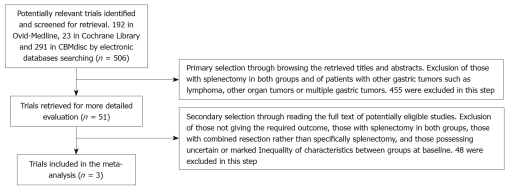
There were 506 papers found in total (192 in Medline, 23 in Cochrane Library, 291 in CBMdisc, no new findings in other databases) and the selection was performed according to the inclusion/exclusion criteria stated above. Four hundred and fifty five trials were excluded in the primary selection through browsing the retrieved titles and abstracts and 48 trials[7-10,14-15,21-62] were excluded in the secondary selection through reading the full texts of potentially eligible studies. The flow chart of study selection is summarized in Figure 1.
Figure 1.
Flow chart showing study selection procedure.
Only 3 RCT trials[6,63,64] comparing the effectiveness and safety of splenectomy with gastrectomy to gastrectomy alone in patients with histologically proven gastric adenocarcinoma met the inclusion criteria. The details of included trials are listed in Tables 1 and 2.
Table 1.
The characteristics of the included randomized trials
| Study | Participants | Interventions | Outcomes |
| Csendes et al[63], 2002 | 187 patients with gastric carcinoma entered this study. 97 patients with total gastrectomy and 90 patients with total gastrectomy and splenectomy | Total gastrectomy vs total gastrectomy plus splenectomy. The follow-up was at least 5 years | Five-year overall survival and survival by stage. Postoperative morbidity and mortality. Kaplan-Meier survival curve. Duration of operation and hospital stay |
| Toge et al[6], 1985 | The patients underwent total gastrectomy and had the main location of the tumor on lesser curvature region. They were divided into 2 groups at random: 41 in splenectomy (+) and 38 in splenectomy (-) groups | Splenectomy vs splenic preservation. The follow-up was at least 5 years | Kaplan-Meier survival curve. 5-year overall survival were from reported percentages data |
| Yu et al[64], 2006 | A total of 216 patients with proximal gastric cancer were randomized. 103 patients had the spleen preserved and 104 had a splenectomy | Splenectomy vs splenic preservation. Of the 207 patients, 7 were lost to follow-up (follow-up rate 96.6%) and mean duration of follow-up was 5.4 years | Harvested lymph nodes. Postoperative morbidity and mortality. Kaplan-Meier survival curve. 5-year overall survival were from reported percentages data |
Table 2.
The quality of the included randomized trials
| Study | Truly random | Concealed allocation | Baseline features | Eligibility criteria | Blinding assessment | Loss to follow-up | Intention to treat | Study quality |
| Csendes et al[63], 2002 | Yes | Unclear | Yes | Yes | Unclear | Yes | No | Fair |
| Toge et al[6], 1985 | Unclear | Unclear | No | No | Unclear | Unclear | Unclear | Poor |
| Yu et al[64], 2006 | Yes | Unclear | Yes | Yes | Unclear | Yes | Unclear | Fair |
A total of 466 patients were available for analysis, with 231 patients assigned treatment with gastrectomy plus splenectomy (treatment arm).
Effectiveness
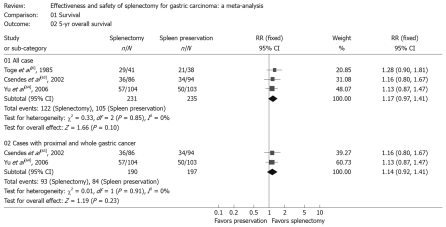
In this part, we used the number of patients alive as the number of events. The meta-analyses of trials showed that gastrectomy with splenectomy had no significant difference from splenic preservation on the 5-year overall survival rate, with RR of 1.17 (Table 3, Figure 2). The location and stage of the tumor had a major effect on the need for splenectomy to allow adequate hilar lymphadenectomy[10]. Thus we performed subgroup analyses with stratification by the 2 factors.
Table 3.
Outcomes of a meta-analysis of overall survival rates, safety, operation-related events and overall survival rates stratified by location of tumor
| No. of studies | Splenectomy (n1/N) | Splenic preservation (n1/N) | RR/WMD (95% CI) | P-value for effect size | P-value for heterogeneity | Effect model | |
| Overall survival rate stratified by different length of follow-up | |||||||
| 3 | 122/231 | 105/235 | 1.17 (0.97, 1.41) | 0.1 | 0.85 | Fixed | |
| Postoperative morbidity and mortality | |||||||
| Morbidity | 1 | 16/104 | 9/103 | 1.76 (0.82, 3.80) | 0.15 | NA | Fixed |
| Mortality | 2 | 6/194 | 4/200 | 1.58 (0.45, 5.50) | 0.47 | 0.82 | Fixed |
| Operation-related events | |||||||
| No. of harvested lymph nodes | 1 | 1042 | 1032 | 0.00 (-6.06, 6.06) | 1 | NA | Fixed |
| Operation time (min) | 1 | 902 | 972 | 10.00 (-14.37, 34.37) | 0.42 | NA | Fixed |
| Length of hospital stay (d) | 1 | 902 | 972 | 3.20 (-1.60, 8.00) | 0.19 | NA | Fixed |
| Reoperation | 1 | 10/90 | 9/97 | 1.20 (0.51, 2.81) | 0.68 | NA | Fixed |
| Overall survival rate stratified by location of tumor (proximal and whole stomach) | |||||||
| 2 | 93/190 | 84/197 | 1.14 (0.92, 1.41) | 0.23 | 0.91 | Fixed | |
Represents the patients alive;
The summed number of patients in each group; RR: Relative risk; WMD: Weighted mean differences; CI: Confidence interval; NA: Not applicable.
Figure 2.
Survival rate. Forest plot of RR of 5-year overall survival rate for all cases and cases with proximal and whole gastric carcinoma, with 95% CI. Data for a fixed-effects model are shown as there was no statistical heterogeneity.
In the subgroup analyses, we also found that, for proximal and whole gastric cancer, splenectomy could not facilitate prolongation of survival. The RR of the 5-year overall survival rate was 1.14, which indicated splenectomy had no significant influence on survival rate compared to splenic preservation for proximal and whole gastric cancer (Table 3, Figure 2).
Then we analyzed the overall survival rate stratified by stage. Because of the limited number of included trials in this step, only one RCT[63] was could be used. The 5-year overall survival rates of patients with stage I, stage II and stage III in this RCT[63] were not significantly different between the 2 groups (all P-values > 0.05).
Safety
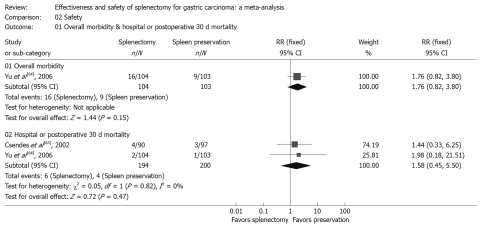
There was no clear and significant excess morbidity or mortality in the splenectomy group, with RR of 1.76 and 1.58 respectively, suggesting that postoperative morbidity and mortality did not occur more than in patients with splenic preservation. (Table 3, Figure 3).
Figure 3.
Morbidity and mortality. Forest plot of RR of postoperative morbidity and mortality, with 95% CI. Data for a fixed-effects model are shown as there was no statistical heterogeneity.
Operation-related events
Non-significantly more lymph nodes were excised from patients undergoing splenectomy (WMD = 0.00 nodes). Operative time and length of hospital stay were not significantly longer in the splenectomy group (WMD = 10.00 min and 3.20 d). There was no difference in reoperation rate between the 2 groups (RR = 1.20, Table 3).
Sensitivity analysis
The results of the sensitivity analysis, after excluding trials of low quality, are shown in Table 4. No significant differences were observed between the 2 arms in terms of the 5-year overall survival rate, and postoperative morbidity and mortality (RR = 1.14, 1.76 and 1.58, respectively).
Table 4.
Sensitivity results of meta-analysis of overall survival rates and safety (excluding the trial with low quality)
| No. of studies | Splenectomy (n1/N) | Splenic preservation (n1/N) | RR/WMD (95% CI) | P-value for effect size | P-value for heterogeneity | Effect model | |
| Overall survival rate stratified by different length of follow-up | |||||||
| 2 | 93/190 | 84/197 | 1.14 (0.92, 1.41) | 0.23 | 0.91 | Fixed | |
| Postoperative morbidity and mortality | |||||||
| Morbidity | 1 | 16/104 | 9/103 | 1.76 (0.82, 3.80) | 0.15 | NA | Fixed |
| Mortality | 2 | 6/194 | 4/200 | 1.58 (0.45, 5.50) | 0.47 | 0.82 | Fixed |
Represents the patients alive; NA: Not applicable.
DISCUSSION
The incidence of proximal gastric cancers has increased[2,3]. Lymphography has demonstrated that the lymphatic flow from the left upper region of the stomach enters the lymph node in the splenic hilum and travels to the nodes around the celiac trunk along the splenic artery[65]. Thus it appears that splenectomy is more often performed for proximal gastric cancers[51], and for a curative gastrectomy it is necessary to dissect the lymph nodes in the splenic hilum and the lymph nodes along the splenic artery. The frequency of metastasis to lymph nodes at the splenic hilum or along the splenic artery, which is associated with stage and tumor location, reportedly ranges from 8% to 10%[4,66]. Splenectomy has been recommended to facilitate lymph node dissection. Direct invasion of the spleen by gastric carcinoma is an exception requiring splenectomy[25]. The possibility that splenectomy could increase the survival rate of patients with gastric cancer has attracted much attention. Some prospective randomized controlled trials and retrospective analyses have been done or are ongoing[67], but the main results are controversial.
When we searched for trials for the meta-analysis, we found that the search results consisted mostly of retrospective analyses with a high level of heterogeneity. From these retrospective analysis, we could see in those who underwent gastrectomy with splenectomy, the tumor was larger, the lesion was more commonly present in the upper stomach, grossly types 3 and 4 infiltration lesions were more frequent, depth of serosa invasion was greater, the rate of lymph node involvement was higher, and advanced stages were more frequent. Furthermore, in many retrospective papers, distal gastrectomy (naturally without splenectomy) for distal cancer and total gastrectomy with splenectomy for advanced proximal cancer were simply compared without any adjustment. So we excluded these kinds of trials for a meaningful result. With respect to the 5-year overall survival rate, our results failed to suggest that splenectomy could result in greater benefit to the patients. When stratified by proximal and whole gastric cancer, a similar result was observed. In the sensitivity analysis, after we excluded the trials of low quality, no significant differences could be detected in 5-year overall survival rates for all the cases or for cases with proximal and whole gastric cancer.
Although we included only RCTs to guarantee the reliability and validity of the results, the eligible number of patients was far too small. So we carefully selected some non-RCTs with good balanced baseline characteristics for a meta-analysis. The quality of non-randomized studies was assessed by using the Newcastle-Ottawa Scale[68] with some modifications to match the needs of this study. The quality of the studies was evaluated by examining patient selection methods, comparability of the study groups and assessment of outcome. Finally we included 7 non-RCTs with 895 patients available for analysis (453 patients were treated by splenectomy). This analysis also showed splenectomy had no significant influence on survival rates compared to splenic preservation for all the cases and for patients with proximal and whole gastric cancer, with an OR of 0.77 (95% CI: 0.57-1.04) and 0.54 (95% CI: 0.24-1.23). In the splenectomy group postoperative morbidity (OR = 3.75, 95% CI: 2.69-5.23), rather than mortality (OR = 1.38, 95% CI: 0.12-16.35), occurred more than that of splenic preservation. Based on the above results, we found splenectomy could show a trend for survival in randomized trials, and the data from non-RCTs showed an opposite trend. This discrepancy in the overall survival and morbidity between RCTs and non-RCTs may derive from the relatively uncertain quality of the non-RCTs although the included trials had balanced baselines. This arises because, in non-RCTs, splenectomy was selected for gastric cancer patients with more advanced tumors, while the spleen was preserved in earlier stage cancers. Also, in the included non-RCTs, gastric cancer requiring splenectomy was usually more extensive or originated from the gastric body. The majority were histologically diffuse type, while tumors treated by distal gastrectomy were more commonly intestinal type and had better prognosis. Furthermore, the extent of lymphadenectomy, the type of gastrectomy, the other organs resected, etc would affect the outcome. With respect to the availability of relatively few high quality RCTs, more well-designed RCTs are needed to explore the effectiveness of splenectomy, especially for proximal and whole gastric cancer.
Whether splenectomy could increase the survival rate in patients with lymph node metastasis at the splenic hilum or along the splenic artery, there is too little evidence. One randomized controlled trial[64] reported no patients could survive for 5 years if lymph nodes at the hilum of the spleen were positive, and the 5-year survival rate of positive lymph node metastasis along the splenic artery in the splenectomy arm or splenic preservation arm were 23.4% and 20.0%, respectively (P = 0.753). Zhang et al[52] found that splenectomy did not show superiority to splenic preservation in patients with positive No. 10 and No. 11 lymph nodes (P = 0.284). Kodera et al[39] reported that in patients who had histological evidence of metastasis to the splenic hilar nodes or the nodes along the splenic artery, pancreaticosplenectomy or splenectomy did not result in improved survival. As yet, there is no evidence to support that splenectomy could increase the survival rates of patients with metastasis to the lymph nodes at the splenic hilum or along the splenic artery.
Regarding the survival rates by stage, the included analyzable trials were too few. From previous reports[58,63], no obvious differences were observed between the 2 groups. Here, we also should note that there were distinct methods for staging at the different periods; furthermore, differences between UICC (Union Internationale Contre le Cancer) and Japanese gastric cancer parameters existed. Thus more unified trials should be done for future evaluation.
In addition, the spleen is an important component of the reticuloendothelial system and constitutes 25% of the total lymphoid mass[69]. There was a 12-fold increased risk of septicemia compared with the general population after splenectomy[63]. On the other hand, the role of the spleen in tumor immunology is still controversial[70]. Therefore the indication for splenectomy is debatable.
Recent European clinical trials of gastrectomy showed that splenectomy was an important risk factor for postoperative morbidity and mortality[13-15]. The common complications after splenectomy were pancreatitis, pleural effusion, abdominal abscess, wound infection, pancreatic leakage, ileus and anastomotic leakage[57]. Splenectomy could easily induce gastric remnant ischemia, possibly contributing to the high frequency of anastomotic leakage and mortality[25]. Resection of proximal gastric cancer was associated with a higher postoperative morbidity than that of distal gastric cancer, and splenectomy was more often performed for proximal gastric cancers[71,72]. However, our results failed to go against splenectomy in terms of postoperative morbidity and mortality. At the same time, with respect to the operation-related events, splenectomy showed no significant difference from splenic preservation in harvested lymph nodes, operation time, length of hospital stay and reoperation rate. All in all, as there were limitations in the trial quality and numbers of included trials, more high quality studies are needed.
In conclusion, splenectomy has not yet shown superiority on survival rates compared to splenic preservation. Routinely performing splenectomy should not be recommended and well-designed large-scale RCTs are required.
COMMENTS
Background
Splenectomy is performed for the purpose of effective lymph node dissection around the splenic artery and splenic hilum and for direct invasion of the splenic hilum or spleen in gastric cancer; however, the effect of splenectomy on prognosis has been controversial.
Research frontiers
Some studies compared the effectiveness or safety of splenectomy to those of non-splenectomy, but the main results were controversial. The aim of this meta-analysis was to evaluate the impact of splenectomy on long-term survival, postoperative morbidity and mortality of patients with gastric cancer.
Innovations and breakthroughs
The current study demonstrated that splenectomy could not yet show superiority on survival rates compared to splenic preservation.
Applications
Routinely performing splenectomy should not be recommended in gastric cancer surgery. However, well-designed large-scale RCTs are expected to investigate the effectiveness and safety of splenectomy further.
Peer review
This is an interesting article in a controversial area. An important meta-analysis that will contribute to the literature.
Footnotes
Supported by The Multi-disciplinary Treatment Project of Gastrointestinal Tumors, West China Hospital, Sichuan University, China, and the National Natural Science Foundation of China (NSFC), No. 30600591
Peer reviewers: Leonidas G Koniaris, Professor, Alan Livingstone Chair in Surgical Oncology, 3550 Sylvester Comprehensive Cancer Center (310T), 1475 NW 12th Ave., Miami, FL 33136, United States; Andrew V Biankin, BMedSc, MB, BS, PhD, Associate Professor, Cancer Research Program, Garvan Institute of Medical Research, 384 Victoria St, Darlinghurst, NSW 2010,
Australia
S- Editor Tian L L- Editor Cant MR E- Editor Lin YP
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Blot WJ, Devesa SS, Kneller RW, Fraumeni JF Jr. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991;265:1287–1289. [PubMed] [Google Scholar]
- 3.Powell J, McConkey CC. Increasing incidence of adenocarcinoma of the gastric cardia and adjacent sites. Br J Cancer. 1990;62:440–443. doi: 10.1038/bjc.1990.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mönig SP, Collet PH, Baldus SE, Schmackpfeffer K, Schröder W, Thiele J, Dienes HP, Hölscher AH. Splenectomy in proximal gastric cancer: frequency of lymph node metastasis to the splenic hilus. J Surg Oncol. 2001;76:89–92. doi: 10.1002/1096-9098(200102)76:2<89::aid-jso1016>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 5.Roder JD, Böttcher K, Siewert JR, Busch R, Hermanek P, Meyer HJ. Prognostic factors in gastric carcinoma. Results of the German Gastric Carcinoma Study 1992. Cancer. 1993;72:2089–2097. doi: 10.1002/1097-0142(19931001)72:7<2089::aid-cncr2820720706>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 6.Toge T, Kameda A, Kuroi K, Seto Y, Yamada H, Hattori T. [The role of the spleen in immunosuppression and the effects of splenectomy on prognosis in gastric cancer patients] Nippon Geka Gakkai Zasshi. 1985;86:1120–1123. [PubMed] [Google Scholar]
- 7.Orita K, Konaga E, Okada T, Kunisada K, Yumura M, Tanaka S. Effect of splenectomy in tumor-bearing mice and gastric cancer patients. Gann. 1977;68:731–736. [PubMed] [Google Scholar]
- 8.Kasakura Y, Fujii M, Mochizuki F, Kochi M, Kaiga T. Is there a benefit of pancreaticosplenectomy with gastrectomy for advanced gastric cancer? Am J Surg. 2000;179:237–242. doi: 10.1016/s0002-9610(00)00293-2. [DOI] [PubMed] [Google Scholar]
- 9.Saji S, Sakamoto J, Teramukai S, Kunieda K, Sugiyama Y, Ohashi Y, Nakazato H. Impact of splenectomy and immunochemotherapy on survival following gastrectomy for carcinoma: covariate interaction with immunosuppressive acidic protein, a serum marker for the host immune system. Tumor Marker Committee for the Study Group of Immunochemotherapy with PSK for Gastric Cancer. Surg Today. 1999;29:504–510. doi: 10.1007/BF02482344. [DOI] [PubMed] [Google Scholar]
- 10.Wanebo HJ, Kennedy BJ, Winchester DP, Stewart AK, Fremgen AM. Role of splenectomy in gastric cancer surgery: adverse effect of elective splenectomy on longterm survival. J Am Coll Surg. 1997;185:177–184. [PubMed] [Google Scholar]
- 11.Lockwood CM. Immunological functions of the spleen. Clin Haematol. 1983;12:449–465. [PubMed] [Google Scholar]
- 12.Llende M, Santiago-Delpín EA, Lavergne J. Immunobiological consequences of splenectomy: a review. J Surg Res. 1986;40:85–94. doi: 10.1016/0022-4804(86)90149-6. [DOI] [PubMed] [Google Scholar]
- 13.Bonenkamp JJ, Hermans J, Sasako M, van de Velde CJ, Welvaart K, Songun I, Meyer S, Plukker JT, Van Elk P, Obertop H, et al. Extended lymph-node dissection for gastric cancer. N Engl J Med. 1999;340:908–914. doi: 10.1056/NEJM199903253401202. [DOI] [PubMed] [Google Scholar]
- 14.Cuschieri A, Fayers P, Fielding J, Craven J, Bancewicz J, Joypaul V, Cook P. Postoperative morbidity and mortality after D1 and D2 resections for gastric cancer: preliminary results of the MRC randomised controlled surgical trial.The Surgical Cooperative Group. Lancet. 1996;347:995–999. doi: 10.1016/s0140-6736(96)90144-0. [DOI] [PubMed] [Google Scholar]
- 15.Bonenkamp JJ, Songun I, Hermans J, Sasako M, Welvaart K, Plukker JT, van Elk P, Obertop H, Gouma DJ, Taat CW. Randomised comparison of morbidity after D1 and D2 dissection for gastric cancer in 996 Dutch patients. Lancet. 1995;345:745–748. doi: 10.1016/s0140-6736(95)90637-1. [DOI] [PubMed] [Google Scholar]
- 16.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 17.Hosono S, Arimoto Y, Ohtani H, Kanamiya Y. Meta-analysis of short-term outcomes after laparoscopy-assisted distal gastrectomy. World J Gastroenterol. 2006;12:7676–7683. doi: 10.3748/wjg.v12.i47.7676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.NHS Centre for Reviews and Dissemination. Undertaking systematic reviews of research on effectiveness: CRD's guidance for carrying out or commissioning reviews (Report No 4). 2nd ed. Vol. 12. York: NHS CRD; 2001. [Google Scholar]
- 19.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dixon E, Hameed M, Sutherland F, Cook DJ, Doig C. Evaluating meta-analyses in the general surgical literature: a critical appraisal. Ann Surg. 2005;241:450–459. doi: 10.1097/01.sla.0000154258.30305.df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nanthakumaran S, Fernandes E, Thompson AM, Rapson T, Gilbert FJ, Park KG. Morbidity and mortality rates following gastric cancer surgery and contiguous organ removal, a population based study. Eur J Surg Oncol. 2005;31:1141–1144. doi: 10.1016/j.ejso.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 22.Wu CW, Chang IS, Lo SS, Hsieh MC, Chen JH, Lui WY, Whang-Peng J. Complications following D3 gastrectomy: post hoc analysis of a randomized trial. World J Surg. 2006;30:12–16. doi: 10.1007/s00268-005-7951-5. [DOI] [PubMed] [Google Scholar]
- 23.Biffi R, Chiappa A, Luca F, Pozzi S, Lo Faso F, Cenciarelli S, Andreoni B. Extended lymph node dissection without routine spleno-pancreatectomy for treatment of gastric cancer: low morbidity and mortality rates in a single center series of 250 patients. J Surg Oncol. 2006;93:394–400. doi: 10.1002/jso.20495. [DOI] [PubMed] [Google Scholar]
- 24.Roukos DH, Paraschou P, Lorenz M. Distal gastric cancer and extensive surgery: a new evaluation method based on the study of the status of residual lymph nodes after limited surgery. Ann Surg Oncol. 2000;7:719–726. doi: 10.1007/s10434-000-0719-0. [DOI] [PubMed] [Google Scholar]
- 25.Furukawa H, Hiratsuka M, Ishikawa O, Ikeda M, Imamura H, Masutani S, Tatsuta M, Satomi T. Total gastrectomy with dissection of lymph nodes along the splenic artery: a pancreas-preserving method. Ann Surg Oncol. 2000;7:669–673. doi: 10.1007/s10434-000-0669-6. [DOI] [PubMed] [Google Scholar]
- 26.Sano T, Martin IG. Lymphadenectomy and pancreatico-splenectomy in gastric cancer surgery. Lancet. 1996;348:195–196. doi: 10.1016/s0140-6736(05)66140-5. [DOI] [PubMed] [Google Scholar]
- 27.Kunisaki C, Makino H, Suwa H, Sato T, Oshima T, Nagano Y, Fujii S, Akiyama H, Nomura M, Otsuka Y, et al. Impact of splenectomy in patients with gastric adenocarcinoma of the cardia. J Gastrointest Surg. 2007;11:1039–1044. doi: 10.1007/s11605-007-0186-z. [DOI] [PubMed] [Google Scholar]
- 28.Barry JD, Blackshaw GR, Edwards P, Lewis WG, Murphy P, Hodzovic I, Thompson IW, Allison MC. Western body mass indices need not compromise outcomes after modified D2 gastrectomy for carcinoma. Gastric Cancer. 2003;6:80–85. doi: 10.1007/s10120-002-0212-5. [DOI] [PubMed] [Google Scholar]
- 29.Karaki Y, Fujimaki M, Yamada A, Katoh H, Hokari I, Sakamoto T. Surgical treatment of carcinoma of the esophagogastric junction. Int Surg. 1991;76:205–208. [PubMed] [Google Scholar]
- 30.Weitz J, D'Angelica M, Gonen M, Klimstra D, Coit DG, Brennan MF, Karpeh MS. Interaction of splenectomy and perioperative blood transfusions on prognosis of patients with proximal gastric and gastroesophageal junction cancer. J Clin Oncol. 2003;21:4597–4603. doi: 10.1200/JCO.2003.12.136. [DOI] [PubMed] [Google Scholar]
- 31.Gao ZQ. Combined organ resection for treatment of advanced gastric cancer. Zhonghua Weichang Waike Zazhi. 2003;6:123. [Google Scholar]
- 32.Kitamura K, Nishida S, Ichikawa D, Taniguchi H, Hagiwara A, Yamaguchi T, Sawai K. No survival benefit from combined pancreaticosplenectomy and total gastrectomy for gastric cancer. Br J Surg. 1999;86:119–122. doi: 10.1046/j.1365-2168.1999.00967.x. [DOI] [PubMed] [Google Scholar]
- 33.Iriyama K, Ohsawa T, Tsuchibashi T, Noji M, Miki C, Ilunga K, Suzuki H. Results of combined resection of invaded organs in patients with potentially curable, advanced gastric cancer. Eur J Surg. 1994;160:27–30. [PubMed] [Google Scholar]
- 34.Maruyama K, Sasako M, Kinoshita T, Sano T, Katai H, Okajima K. Pancreas-preserving total gastrectomy for proximal gastric cancer. World J Surg. 1995;19:532–536. doi: 10.1007/BF00294714. [DOI] [PubMed] [Google Scholar]
- 35.Brady MS, Rogatko A, Dent LL, Shiu MH. Effect of splenectomy on morbidity and survival following curative gastrectomy for carcinoma. Arch Surg. 1991;126:359–364. doi: 10.1001/archsurg.1991.01410270105017. [DOI] [PubMed] [Google Scholar]
- 36.Erturk S, Ersan Y, Cicek Y, Dogusoy G, Senocak M. Effect of simultaneous splenectomy on the survival of patients undergoing curative gastrectomy for proximal gastric carcinoma. Surg Today. 2003;33:254–258. doi: 10.1007/s005950300057. [DOI] [PubMed] [Google Scholar]
- 37.Fatouros M, Roukos DH, Lorenz M, Arampatzis I, Hottentrott C, Encke A, Kappas AM. Impact of spleen preservation in patients with gastric cancer. Anticancer Res. 2005;25:3023–3030. [PubMed] [Google Scholar]
- 38.Griffith JP, Sue-Ling HM, Martin I, Dixon MF, McMahon MJ, Axon AT, Johnston D. Preservation of the spleen improves survival after radical surgery for gastric cancer. Gut. 1995;36:684–690. doi: 10.1136/gut.36.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kodera Y, Yamamura Y, Shimizu Y, Torii A, Hirai T, Yasui K, Morimoto T, Kato T, Kito T. Lack of benefit of combined pancreaticosplenectomy in D2 resection for proximal-third gastric carcinoma. World J Surg. 1997;21:622–627; discussion 627-628. doi: 10.1007/s002689900283. [DOI] [PubMed] [Google Scholar]
- 40.Koga S, Kaibara N, Kimura O, Nishidoi H, Kishimoto H. Prognostic significance of combined splenectomy or pancreaticosplenectomy in total and proximal gastrectomy for gastric cancer. Am J Surg. 1981;142:546–550. doi: 10.1016/0002-9610(81)90422-0. [DOI] [PubMed] [Google Scholar]
- 41.Kwon SJ. Prognostic impact of splenectomy on gastric cancer: results of the Korean Gastric Cancer Study Group. World J Surg. 1997;21:837–844. doi: 10.1007/s002689900314. [DOI] [PubMed] [Google Scholar]
- 42.Lee KY, Noh SH, Hyung WJ, Lee JH, Lah KH, Choi SH, Min JS. Impact of splenectomy for lymph node dissection on long-term surgical outcome in gastric cancer. Ann Surg Oncol. 2001;8:402–406. doi: 10.1007/s10434-001-0402-0. [DOI] [PubMed] [Google Scholar]
- 43.Lu HM. The treatment value of total gastrectomy with splenectomy for gastric cancer. Nantong Yixueyuan Yuebao. 2001;21:288. [Google Scholar]
- 44.Maehara Y, Moriguchi S, Yoshida M, Takahashi I, Korenaga D, Sugimachi K. Splenectomy does not correlate with length of survival in patients undergoing curative total gastrectomy for gastric carcinoma. Univariate and multivariate analyses. Cancer. 1991;67:3006–3009. doi: 10.1002/1097-0142(19910615)67:12<3006::aid-cncr2820671213>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 45.Martin RC 2nd, Jaques DP, Brennan MF, Karpeh M. Extended local resection for advanced gastric cancer: increased survival versus increased morbidity. Ann Surg. 2002;236:159–165. doi: 10.1097/00000658-200208000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Otsuji E, Yamaguchi T, Sawai K, Ohara M, Takahashi T. End results of simultaneous splenectomy in patients undergoing total gastrectomy for gastric carcinoma. Surgery. 1996;120:40–44. doi: 10.1016/s0039-6060(96)80239-x. [DOI] [PubMed] [Google Scholar]
- 47.Schmid A, Thybusch A, Kremer B, Henne-Bruns D. Differential effects of radical D2-lymphadenectomy and splenectomy in surgically treated gastric cancer patients. Hepatogastroenterology. 2000;47:579–585. [PubMed] [Google Scholar]
- 48.Shiu MH, Perrotti M, Brennan MF. Adenocarcinoma of the stomach: a multivariate analysis of clinical, pathologic and treatment factors. Hepatogastroenterology. 1989;36:7–12. [PubMed] [Google Scholar]
- 49.Tamada R, Sugimachi K, Okamura T, Hiramoto Y, Notsuka T, Korenaga D, Inokuchi K. [Evaluation of splenectomy in total gastrectomy for gastric cancer] Nippon Geka Gakkai Zasshi. 1985;86:1124–1127. [PubMed] [Google Scholar]
- 50.Weitz J, Jaques DP, Brennan M, Karpeh M. Association of splenectomy with postoperative complications in patients with proximal gastric and gastroesophageal junction cancer. Ann Surg Oncol. 2004;11:682–689. doi: 10.1245/ASO.2004.03.048. [DOI] [PubMed] [Google Scholar]
- 51.Yoshino K, Yamada Y, Asanuma F, Aizawa K. Splenectomy in cancer gastrectomy: recommendation of spleen-preserving for early stages. Int Surg. 1997;82:150–154. [PubMed] [Google Scholar]
- 52.Zhang CH, Zhan WH, He YL, Chen CQ, Huang MJ, Cai SR. Spleen preservation in radical surgery for gastric cardia cancer. Ann Surg Oncol. 2007;14:1312–1319. doi: 10.1245/s10434-006-9190-x. [DOI] [PubMed] [Google Scholar]
- 53.Zhang J, Zhou YM. The option of spleen in D3 lymphadenectomy for gastric cancer (87 cases) Yixue Wenxuan. 2000;19:484–485. [Google Scholar]
- 54.Stipa S, Di Giorgio A, Ferri M, Botti C. Results of curative gastrectomy for carcinoma. J Am Coll Surg. 1994;179:567–572. [PubMed] [Google Scholar]
- 55.Chen JQ, Zhan WH, Cai SR, Lu YF. Effect of splenectomy combined with radical total gastrectomy on the survival of patients with proximal gastric carcinoma. Zhongguo Putong Waike Zazhi. 2005;14:161–164. [Google Scholar]
- 56.Han FH, Zhan WH, Li YM, He YL, Peng JS, Ma JP, Wang Z, Chen ZX, Zheng ZQ, Wang JP, et al. [Analysis of long-term results of radical gastrectomy combining splenectomy for gastric cancer] Zhonghua Waike Zazhi. 2005;43:1114–1117. [PubMed] [Google Scholar]
- 57.Adachi Y, Kamakura T, Mori M, Maehara Y, Sugimachi K. Role of lymph node dissection and splenectomy in node-positive gastric carcinoma. Surgery. 1994;116:837–841. [PubMed] [Google Scholar]
- 58.Cho MY, Kroh MD, Joh YG, Suh SO. Impact of splenectomy on circulating T-lymphocyte subsets in stage III gastric cancer. ANZ J Surg. 2002;72:411–416. doi: 10.1046/j.1445-2197.2002.02429.x. [DOI] [PubMed] [Google Scholar]
- 59.Miwa H, Kojima K, Ono F, Kobayashi T, Tsurumi T, Hatosaki A, Iijima T, Orita K. Effect of splenectomy and immunotherapy on advanced gastric cancer associated with total gastrectomy. Jpn J Surg. 1983;13:20–24. doi: 10.1007/BF02469685. [DOI] [PubMed] [Google Scholar]
- 60.Suehiro S, Nagasue N, Ogawa Y, Sasaki Y, Hirose S, Yukaya H. The negative effect of splenectomy on the prognosis of gastric cancer. Am J Surg. 1984;148:645–648. doi: 10.1016/0002-9610(84)90343-x. [DOI] [PubMed] [Google Scholar]
- 61.Sugimachi K, Kodama Y, Kumashiro R, Kanematsu T, Noda S, Inokuchi K. Critical evaluation of prophylactic splenectomy in total gastrectomy for the stomach cancer. Gann. 1980;71:704–709. [PubMed] [Google Scholar]
- 62.Suo J, Chen Y, Wang Q. Retrospective analysis of effectiveness of spleen preserving total gastrectomy for elderly patients with gastric cancer. Zhongguo Laonianxue Zazhi. 2006;26:845–846. [Google Scholar]
- 63.Csendes A, Burdiles P, Rojas J, Braghetto I, Diaz JC, Maluenda F. A prospective randomized study comparing D2 total gastrectomy versus D2 total gastrectomy plus splenectomy in 187 patients with gastric carcinoma. Surgery. 2002;131:401–407. doi: 10.1067/msy.2002.121891. [DOI] [PubMed] [Google Scholar]
- 64.Yu W, Choi GS, Chung HY. Randomized clinical trial of splenectomy versus splenic preservation in patients with proximal gastric cancer. Br J Surg. 2006;93:559–563. doi: 10.1002/bjs.5353. [DOI] [PubMed] [Google Scholar]
- 65.Maruyama K, Okabayashi K, Kinoshita T. Progress in gastric cancer surgery in Japan and its limits of radicality. World J Surg. 1987;11:418–425. doi: 10.1007/BF01655804. [DOI] [PubMed] [Google Scholar]
- 66.Maruyama K, Gunvén P, Okabayashi K, Sasako M, Kinoshita T. Lymph node metastases of gastric cancer. General pattern in 1931 patients. Ann Surg. 1989;210:596–602. doi: 10.1097/00000658-198911000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sano T, Yamamoto S, Sasako M. Randomized controlled trial to evaluate splenectomy in total gastrectomy for proximal gastric carcinoma: Japan clinical oncology group study JCOG 0110-MF. Jpn J Clin Oncol. 2002;32:363–364. doi: 10.1093/jjco/hyf085. [DOI] [PubMed] [Google Scholar]
- 68.Athanasiou T, Al-Ruzzeh S, Kumar P, Crossman MC, Amrani M, Pepper JR, Del Stanbridge R, Casula R, Glenville B. Off-pump myocardial revascularization is associated with less incidence of stroke in elderly patients. Ann Thorac Surg. 2004;77:745–753. doi: 10.1016/j.athoracsur.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 69.Ellison EC, Fabri PJ. Complications of splenectomy. Etiology, prevention, and management. Surg Clin North Am. 1983;63:1313–1330. doi: 10.1016/s0039-6109(16)43191-9. [DOI] [PubMed] [Google Scholar]
- 70.Okuno K, Tanaka A, Shigeoka H, Hirai N, Kawai I, Kitano Y, Yasutomi M. Suppression of T-cell function in gastric cancer patients after total gastrectomy with splenectomy: implications of splenic autotransplantation. Gastric Cancer. 1999;2:20–25. doi: 10.1007/s101200050016. [DOI] [PubMed] [Google Scholar]
- 71.Harrison LE, Karpeh MS, Brennan MF. Proximal gastric cancers resected via a transabdominal-only approach. Results and comparisons to distal adenocarcinoma of the stomach. Ann Surg. 1997;225:678–683; discussion 683-685. doi: 10.1097/00000658-199706000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Viste A, Haùgstvedt T, Eide GE, Søreide O. Postoperative complications and mortality after surgery for gastric cancer. Ann Surg. 1988;207:7–13. doi: 10.1097/00000658-198801000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]