Abstract
AIM: To investigate the expression pattern of gamma-aminobutyric acid A (GABAA) receptors in hepatocellular carcinoma (HCC) and indicate the relationship among gamma-aminobutyric acid (GABA), gamma-aminobutyric acid A receptor α3 subunit (GABRA3) and HCC.
METHODS: HCC cell line Chang, HepG2, normal liver cell line L-02 and 8 samples of HCC tissues and paired non-cancerous tissues were analyzed with semiquantitative polymerase chain reaction (PCR) for the expression of GABAA receptors. HepG2 cells were treated with gamma-aminobutyric acid (GABA) at serial concentrations (0, 1, 10, 20, 40 and 60 μmol/L), and their proliferating abilities were analyzed with the 3-(4, 5-methylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay, cell doubling time test, colon formation assay, cell cycle analysis and tumor planted in nude mice. Small interfering RNA was used for knocking down the endogenous GABRA3 in HepG2. Proliferating abilities of these cells treated with or without GABA were analyzed.
RESULTS: We identified the overexpression of GABRA3 in HCC cells. Knockdown of endogenous GABRA3 expression in HepG2 attenuated HCC cell growth, suggesting its role in HCC cell viability. We determined the in vitro and in vivo effect of GABA in the proliferation of GABRA3-positive cell lines, and found that GABA increased HCC growth in a dose-dependent manner. Notably, the addition of GABA into the cell culture medium promoted the proliferation of GABRA3-expressing HepG2 cells, but not GABRA3-knockdown HepG2 cells. This means that GABA stimulates HepG2 cell growth through GABRA3.
CONCLUSION: GABA and GABRA3 play important roles in HCC development and progression and can be a promising molecular target for the development of new diagnostic and therapeutic strategies for HCC.
Keywords: Hepatocellular carcinoma, Proliferation, Gamma-aminobutyric acid, Gamma-aminobutyric acid A receptor α3 subunit, RNAi
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the third leading cause of cancer-related death worldwide[1]. Although liver resection and local ablation are regarded as potentially curative treatment[2], its prognosis is poor. Up to 40% of patients are diagnosed at an advanced stage, and only palliative treatment can be offered.
To change this situation, the development of new molecular therapies against good targets is an urgent issue. To this direction, we previously used a method by combining the in silico screen and experimental verification to identify genes that are differently expressed in cancers compared with their corresponding normal tissues. Among genes that are overexpressed in HCC cells, we focused on the gene gamma-aminobutyric acid (GABA) A receptor α3 subunit (GABRA3). GABRA3 is a subunit of the GABAA receptors that may associate with other GABAA receptor subunits to form a functional chloride channel that mediates the inhibitory synaptic transmission in the mature central nervous system (CNS). GABA primarily functions as an inhibitory neurotransmitter in the mature CNS by activating the GABA receptor, but can also modulate the proliferation, migration and differentiation of neuronal cells during CNS development[3-5] and the proliferation of peripheral non-neuronal cells[6,7]. GABA and GABAA receptors are also present in peripheral tissues, including cancerous cells, but their precise functions are poorly defined.
This study demonstrates that GABRA3 overexpressed in HCC and GABA promoted the proliferation of cancer cells through GABRA3.
MATERIALS AND METHODS
Cell lines
HCC cell line Chang, HepG2 and normal liver cell line L-02 were maintained by our lab. All cell lines were cultured in DMEM supplemented with 10% FBS and antibiotics (100 units/mL penicillin and 100 μg/mL streptomycin). Cells were maintained at 37°C in an atmosphere of humidified air with 5% CO2.
Collection of tissues
All samples of HCC tissues and paired non-cancerous tissues (5 cm away from tumor) were obtained during surgical resection from the First Affiliated Hospital of Xiangya School of Medicine. Written consent was obtained from the patients, who agreed with the collection of tissue samples. The resected tissue samples were immediately cut into small pieces, and snap-frozen in liquid nitrogen until use. All tumor tissue and paired non-cancerous tissue samples were pathologically confirmed.
Semiquantitative polymerase chain reaction (PCR)
Total RNA from HepG2, Chang L-02 cell lines and liquid-nitrogen-frozen tissue samples were extracted using Trizol reagent (Invitrogen) according to the manufacturer’s instructions. First-strand cDNAs were synthesized from 2 μg of DNase I-treated total RNA using oligo (dT) primer with SuperscriptTM II reverse transcriptase (Invitrogen) for 60 min at 42°C. We prepared appropriate dilutions of each single-stranded cDNA for subsequent PCR amplification by monitoring β-actin as a quantitative control. The sets of primer for GABAA receptor subunits are shown in Table 1, 5′-AGCAAGAGAGGCATCCTCA-3′ and 5′-TCAGGCAGCTCGTAGCTCT-3′ for β-actin, 554bp. One μL of each cDNA product was amplified in a mixture containing 5 pmol primers, 200 μmol/L dNTPs, and 1 unit Taq DNA polymerase with reaction buffer in a final volume of 20 μL. PCR was performed using the following parameters: initial denaturation at 95°C for 1 min, 30 cycles of 30 s at 95°C, 30 s at 60°C, and 1 min 30 s at 68°C, followed by a final 10 min extension at 68°C. Reaction products were separated on 1.5% agarose gels containing ethidium bromide and the level of amplification was determined using a Phosphor Imager.
Table 1.
Primers for GABAA receptor subunits
| Subunit | Primer | Product size (bp) |
| α1 | 902 | |
| Sense | 5'-TCGTCACCAGTTTCGGACC-3' | |
| Antisense | 5'-GGTTGCTGTTGGAGCGTAA-3' | |
| α2 | 722 | |
| Sense | 5'-TTCACAATGGGAAGAAATCAGTAG-3' | |
| Antisense | 5'-TGCATAAGCGTTGTTCTGTATCA-3' | |
| α3 | 561 | |
| Sense | 5'-TCGGTCTCTCCAAGTTTGTGC-3' | |
| Antisense | 5'-TTCCGTTGTCCACCAATCTGA-3' | |
| α4 | 750 | |
| Sense | 5'-TGAAATTCGGGAGTTATGCCTATC-3' | |
| Antisense | 5'-GGCTGAATGGGTTTGGACTG-3' | |
| α5 | 826 | |
| Sense | 5'-CACCATGCGCTTGACCATCTCT-3' | |
| Antisense | 5'-GCCGAACAAGACTGGGAATA-3' | |
| α6 | 764 | |
| Sense | 5'-TGAGGCTTACCATCAATGCTGA-3' | |
| Antisense | 5'-GACAGGTGTTGATTGTAAGATGGG-3' | |
| β1 | 603 | |
| Sense | 5'-GTTCTCTATGGACTCCGAATCACA-3' | |
| Antisense | 5'-ATTGGCACTCTGGTCTTGTTTG-3' | |
| β2 | 640 | |
| Sense | 5'-AGCTTAAGAGAAACATTGGCTACT-3' | |
| Antisense | 5'-CGATCTATGGCATTCACATCA-3' | |
| β3 | 633 | |
| Sense | 5'-AGTGCTGTATGGGCTCAGAATCAC-3' | |
| Antisense | 5'-CCCGGTTGCTTTCGCTCTT-3' | |
| γ1 | 262 | |
| Sense | 5'-GTGTTTTGCAGCCTTGATGG-3' | |
| Antisense | 5'-TGGCAATGCGTATGTGTATCCT-3' | |
| γ2 | 605 | |
| Sense | 5'-AAGTCCTCCGATTGAACAGCAACA-3' | |
| Antisense | 5'-CGCTGTGACATAGGAGACCTT-3' | |
| γ3 | 767 | |
| Sense | 5'-ACACTCCTGCCCGCTGATT-3' | |
| Antisense | 5'-TGTCTATGTGAATACGCCCTTTCC-3' | |
| Δ | 654 | |
| Sense | 5'-TCACCATCACCAGCTACCACTTCA-3' | |
| Antisense | 5'-GGGCGTAAATGTCAATGGTGTC-3' | |
| ε | 632 | |
| Sense | 5'-GCAGGCGGTTTGGCTATGT-3' | |
| Antisense | 5'-CGAGTAGTTATCCAGGCGGTAG-3' | |
| θ | 465 | |
| Sense | 5'-TCGAGTTCTCCTCTGCTGTG -3' | |
| Antisense | 5'-TATGCAGATCCAGGGACAA-3' | |
| π | 250 | |
| Sense | 5'-CGTCGAGGTCGGCAGAAGT-3' | |
| Antisense | 5'-GCGGGCATCCAGAGTGAAG-3' |
RNA interference
To knock down GABRA3 expression, we used pGCsi-U6/Neo/GFP vector encoding a small hairpin RNA directed against the target gene in HepG2. The target sequences for GABRA3 were 5'-GCACTGACAACATCACTAT-3' (Si-1), 5'-CTGAGACCAAGACCTACAA-3' (Si-2), 5'-GATCCTTCCACTGAACAAT-3' (Si-3). As a negative control, we used shRNA vector without hairpin oligonucleotides (Si-Mock). Human HCC cell line HepG2 was plated onto 6-well plates, and transfected with these small interfering RNA (siRNA) expression vectors using FuGENE6 (Roche) according to the instructions of the manufacturer, followed by 800 μg/mL of neomycin selection. The cells were harvested 10 d later to analyze the knockdown effect on GABRA3 by RT-PCR using the primers shown in Table 1 and by flow cytometry (FCM) using rabbit anti-human polyclone antibody against GABRA3 (Chemicon).
Cell proliferation test in vitro
Growth experiments of HepG2, HepG2/Si-1, and HepG2/Si-Mock were performed using the 3-(4,5-methylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay, cell doubling time test, soft agar cloning formation assay and were reconfirmed with cell cycle analysis, which was performed by flow cytometry.
In the MTT assay, cells were seeded with serum-free medium at a density of 103 cells/well in 96-well plates (n = 6), grown overnight, washed in PBS, and incubated with GABA (Sigma-Aldrich) at serial concentrations (0, 1, 10, 20, 40 and 60 μmol/L) in appropriate medium supplemented with 1% FBS. The samples were tested every 24 h for 6 d. MTT was added (50 μg/well) for 4 h. Formazan products were solubilized with DMSO, and the optical density was measured at 570 nm.
In the cell doubling time test, cells were seeded at a density of 104 cells/well in 24-well plates (n = 3), grown with serum-free medium overnight, and incubated with GABA at serial concentrations (0, 1, 10, 20, 40 and 60 μmol/L) in DMEM medium with 1% FBS. Cells were collected 6 d later, and cell densities were assessed by counting the cells in a hemocytometer. Cell doubling time (TD) was calculated with the following formula: TD=t × [lg2/(lgNt-lgNo)] [t: Period of culturing (hour); No: cell density when the cells were seeded; Nt: cell density when the cells were cultured “t” hours].
Soft agar colony formation assay was used to assess the anchorage-independent growth ability of cells. The cells were incubated with GABA at serial concentrations (0, 1, 10, 20, 40 and 60 μmol/L) in DMEM medium overnight. A total of 1000 single-cell suspension cells were resuspended in 1 mL growth media containing 0.3% low melting temperature agarose and GABA at different concentrations (0, 1, 10, 20, 40 and 60 μmol/L) respectively and were plated in triplicate on 6-well plates over a base layer of 1 mL growth media containing 0.6% low melting temperature agarose. Colonies > 50 μm were counted 14 d after plating.
For flow cytometry, cells were incubated with serum-free medium for 24 h, and then cultured in DMEM with 10% FBS in the presence or absence of 40 μmol/L of GABA for 48 h. Cells were harvested and resuspended in fixation fluid at a density of 106/mL, 1500 μL propidium iodide (PI) solution was added, and the cell cycle was detected by FACS Caliber (Becton Dickinson).
Tumor implanted in nude mice
Male 8-wk-old BALB/c nude (nu/nu) mice were purchased from Slac Laboratory Animal Co. Ltd (Shanghai, China). HepG2, HepG2/Mock and HepG2/Si-1 cells were treated with or without GABA (40 μmol/L) for 24 h first, and then the cells (3 × 106) were suspended in 0.2 mL of extracellular matrix gel and injected sc in the left back flank of these animals. The mice were divided into six groups: (a) the mice were injected with HepG2 and treated with 0.9% NaCl injection (150 μL) into the implanted tumor (HepG2, n = 5); (b) the mice were injected with HepG2 and treated with GABA injections (40 μmol/L in 150 μL of 0.9% NaCl) into the tumor (HepG2 + GABA, n = 5); (c) the mice were injected with HepG2/Mock and treated with 0.9% NaCl injection (150 μL) into the implanted tumor (HepG2/Si-Mock, n = 5); (d) the mice were injected with HepG2/Mock and treated with GABA injections (40 μmol/L in 150 μL of 0.9% NaCl) into the tumor (HepG2/Si-Mock + GABA, n = 5); (e) the mice were injected with HepG2/Si-1 and treated with 0.9% NaCl injection (150 μL) into the implanted tumor (HepG2/Si-1, n = 5); (f) the mice were injected with HepG2/Si-1 and treated with GABA injections (40 μmol/L in 150 μL of 0.9% NaCl) into the tumor (HepG2/Si-1 + GABA, n = 5). The same operator did the injections every other day starting from “day 0” when the tumors were implanted. Tumor variables were measured every three days by an electronic caliper, and volume was determined as tumor volume (mm3) = length (mm) × width2 (mm2) × 0.523. The measurement started from the first week, when the tumor mass was well established. A third operator, in a coded and blinded fashion, evaluated the morphometric variables.
Statistics
Results were expressed as mean ± SD. Student’s t test was used, and P < 0.05 was considered statistically significant.
RESULTS
Expression of GABAA receptors
We documented GABAA mRNA expression in 8 pairs of HCC and adjacent non-tumor tissues as well as HepG2, Chang, L-02 cell lines. The results of semiquantitative RT-PCR on a scale of + (limited expression) to +++ (highly expressed) are shown in Table 2.
Table 2.
GABAA receptor subunits mRNA expression in HepG2, Chang, L-02 and 8 sets of human HCC and adjacent nontumor tissues
| Set |
GABAA receptor subunits |
|||||||||||||||
| α1 | α2 | α3 | α4 | α5 | α6 | β1 | β2 | β3 | γ1 | γ2 | γ3 | Δ | ε | θ | π | |
| Cell line | ||||||||||||||||
| HepG2 | +++ | + | + | ++ | ++ | |||||||||||
| Chang | +++ | + | ++ | + | ||||||||||||
| L-02 | ++ | |||||||||||||||
| Tumor | ||||||||||||||||
| 1 | +++ | + | + | ++ | ||||||||||||
| 2 | ++ | + | + | + | + | |||||||||||
| 3 | + | +++ | + | ++ | ++ | |||||||||||
| 4 | + | + | ++ | |||||||||||||
| 5 | ++ | + | ++ | + | ||||||||||||
| 6 | ++ | + | + | + | + | |||||||||||
| 7 | + | ++ | + | |||||||||||||
| 8 | + | + | + | ++ | ||||||||||||
| Non-tumor | ||||||||||||||||
| 1 | + | ++ | + | |||||||||||||
| 2 | ++ | ++ | + | |||||||||||||
| 3 | + | ++ | ||||||||||||||
| 4 | + | + | + | |||||||||||||
| 5 | + | ++ | ||||||||||||||
| 6 | ++ | ++ | ++ | |||||||||||||
| 7 | + | + | ||||||||||||||
| 8 | ++ | + | ||||||||||||||
GABAA-α3, β3, δ, ε, and θ receptor subunits were detected in HepG2 and α3, δ, ε, and θ in Chang liver cells by RT-PCR. In L-02, only ε subunit was detected. In tissues adjacent to HCC, GABAA receptor expression was limited to GABAA-β3, ε and π. In HCC tissues, although GABAA-ε expression remained largely unchanged, GABAA-α3 mRNA expression was consistently (6/8) and significantly increased, and GABAA-δ and θ isoforms were also detected.
Effect of GABRA3-siRNA on the growth of HCC cells
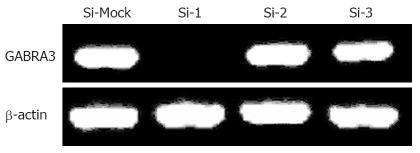
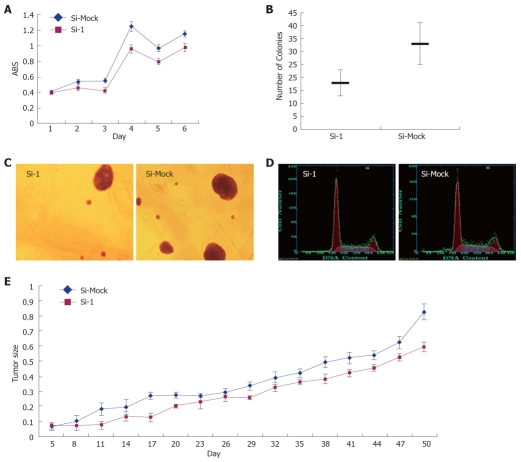
To investigate the biological significance of GABRA3 overexpression in HCC cells, we constructed three siRNA expression vectors (Si-1, Si-2, and Si-3) specific to GABRA3 transcripts and transfected them into HepG2 cells that endogenously expressed high levels of GABRA3 as shown in Table 2. A knockdown effect was observed by RT-PCR when we transfected Si-1, but not Si-2, Si-3 or a negative control Si-Mock (Figure 1). MTT assays (Figure 2A) and colony formation (Figure 2B and C) revealed a drastic reduction in the number of cells transfected with Si-1 compared with Si-Mock for which no knockdown effect was observed. The cell doubling time of the cells transfected with Si-1 was 8.7 ± 0.183 d, while Si-Mock was 5.3 ± 0.125 d (n =3, P < 0.05). Cell proliferation detected by flow cytometry is shown in Figure 2D and Table 3. This result was consistent with the results above. The tumor size of the nude mice injected with HepG2/Si-1 (Group e) was significantly smaller than the mice injected with HepG2/Si-Mock (Group c) (Figure 2E).
Figure 1.
RT-PCR confirmed the knockdown effect on GABRA3 expression by Si-1, but not by Si-2, Si-3 and a negative control Si-Mock in HepG2 cells. β-actin was used to quantify RNAs.
Figure 2.
Effect of GABRA3-siRNA on the growth of HCC cells. A: MTT assay of HepG2 cells transfected with Si-1 vectors to GABRA3 and a negative control vector (Si-Mock). Y-axis: Average value of absorbance (ABS) at 570 nm, measured with a microplate reader (n = 6, P < 0.05); B and C: Soft agar colony formation assay of HepG2/Si-1 and HepG2/Si-Mock. Same cells were incubated in low-melting agarose as described in Materials and Methods. Two weeks later, colonies were photographed and numbers of colonies were counted. (n = 3, P < 0.05); D: Cell cycle of HepG2/Si-1 and HepG2/Si-Mock measured by flow cytometry; E: Tumor volume in nude mice with HepG2/Si-1 or HepG2/Si-Mock injected (mm3).
Table 3.
Cell cycle of HepG2/Si-1 and HepG2/Si-Mock (mean ± SD)
| Si-1 | Si-Mock | |
| G1/G0% | 56.1 ± 3.36 | 50.9 ± 3.14 |
| G2/M% | 14.3 ± 1.72 | 14.2 ± 1.43 |
| S%1 | 29.5 ± 2.26 | 34.5 ± 2.37 |
n = 3, P < 0.05.
Effect of GABA on the growth of HCC cells
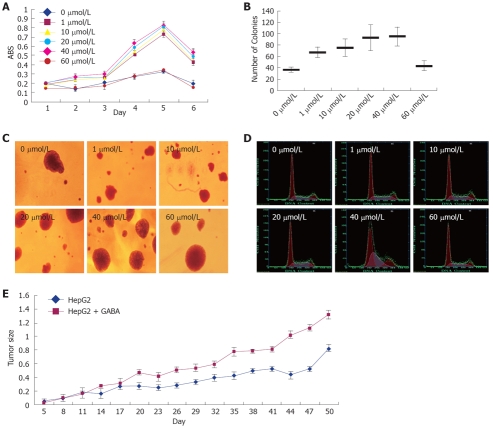
To examine the effect of GABA on the growth of GABRA3-expressing HCC cells, we treated GABRA3-positive HCC cells HepG2 with GABA at several concentrations. As shown in Figure 3 and Table 4, the addition of GABA in the culture media enhanced the proliferation of HepG2 cells in a dose-dependent manner. The promoting effect on HCC cell proliferation was more evident with the GABA concentration from 1 μmol/L to 40 μmol/L. When the GABA concentra-tion was up to 60 μmol/L, the promoting effect became insignificant. We also tried GABA at concentrations of 100 μmol/L, 200 μmol/L and 400 μmol/L, but there was no significant effect (data not shown). The effect of GABA persisted up to 5 d. The cell doubling times of HepG2 cells treated with 0, 1, 10, 20, 40 and 60 μmol/L were 5.1 ± 0.128, 2.73 ± 0.052, 2 ± 0.057, 1.51 ± 0.044, 1.3 ± 0.064 and 4.1 ± 0.085 d.
Figure 3.
Effect of GABA on the growth of HCC cells. HepG2 cells were treated with GABA at serial concentrations (0, 1, 10, 20, 40 and 60 μmol/L). A: MTT assay. Y-axis: Average value of absorbance (ABS) at 570 nm, measured with a microplate reader (n = 6, P < 0.05); B and C: Soft agar colony formation assay. Same cells were incubated in low-melting agarose as described in Materials and Methods. Two weeks later, colonies were photographed and numbers of colonies were counted. (n = 3, P < 0.05); D: Cell cycle measured by flow cytometry; E: Tumor volume in nude mice with HepG2 and GABA (0 μmol/L and 20 μmol/L) injected (mm3).
Table 4.
Cell cycle of HepG2 treated with GABA at serial concentrations (mean ± SD)
| 0 μmol/L | 1 μmol/L | 10 μmol/L | 20 μmol/L | 40 μmol/L | 60 μmol/L | |
| G1/G0% | 59.7 ± 3.82 | 58.5 ± 3.63 | 55.6 ± 3.46 | 56.5 ± 3.69 | 45.2 ± 3.07 | 57.6 ± 3.83 |
| G2/M% | 13.5 ± 1.14 | 18.6 ± 1.27 | 12.5 ± 1.15 | 7.6 ± 0.79 | 17.3 ± 1.48 | 12.7 ± 1.53 |
| S%1 | 27.2 ± 2.19 | 31.3 ± 2.24 | 32.4 ± 2.58 | 35.8 ± 2.47 | 37.6 ± 2.64 | 30.4 ± 2.32 |
n = 3, P < 0.05.
In the nude mice implanted with tumors (injected with HepG2 cells), a significant difference in tumor size was found in GABA-treated (at the concentration of 40 μmol/L) mice compared with mice injected with 0.9% NaCl only (Figure 3E).
GABA stimulated HCC cell proliferation through GABRA3
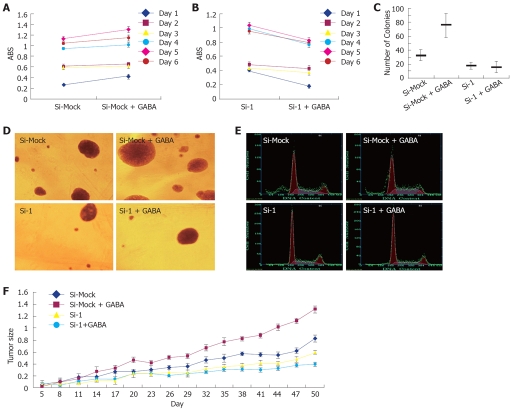
To examine the function of GABRA3 as a GABA receptor on the growth of GABRA3-expressing HCC cells, we treated HepG2/Si-1 and HepG2/Si-Mock cells with or without GABA (40 μmol/L). As shown in Figure 4 and Table 5, GABA enhanced the growth of HepG2/Si-Mock compared with the HepG2/Si-Mock without GABA. On the other hand, the proliferating ability of HepG2/Si-1, which did not express GABRA3, was not enhanced but lowered by GABA. In the nude mice injected with HepG2/Si-Mock, the tumor size of the mice treated with GABA was much larger than the mice treated without GABA, while the result was opposite in the mice injected with HepG2/Si-1.
Figure 4.
GABA stimulated HCC cell proliferation through GABRA3. HepG2/Si-1 and HepG2/Si-Mock cells were treated with or without GABA (20 μmol/L). A: MTT assay of HepG2/Si-Mock cells treated with or without GABA. (n = 6, P < 0.05); B: MTT assay of HepG2/Si-1 cells treated with or without GABA. (n = 6); C and D: Soft agar colony formation assay. (n = 6, P < 0.05 for HepG2/Si-Mock vs HepG2/Si-Mock + GABA); E: Cell cycle measured by flow cytometry; F: Tumor volume in nude mice (mm3).
Table 5.
Cell cycle of HepG2/Si-Mock and HepG2/Si-1 treated with or without GABA at a concentration of 40 μmol/L (mean ± SD)
| HepG2/Si-Mock | HepG2/Si-Mock+GABA | HepG2/Si-1 | HepG2/Si-1+GABA | |
| G1/G0% | 51.7 ± 3.53 | 49.3 ± 3.23 | 54.5 ± 3.37 | 59.4 ± 3.85 |
| G2/M% | 15.7 ± 1.75 | 7.2 ± 0.92 | 17.6 ± 1.64 | 15.6 ± 1.69 |
| S% | 32.4 ± 2.12 | 43.6 ± 2.841 | 27.6 ± 1.92 | 25.5 ± 2.06 |
HepG2/Si-Mock vs HepG2/Si-Mock + GABA, n = 3, P < 0.05.
DISCUSSION
Although GABA and GABA receptors function as an inhibitory neurotransmitter in the mature CNS, abnormal levels of gene and protein expression of some GABA receptor subunits have been detected in many malignant tumors, such as π subunit in pancreatic cancer[8]. This indicates that GABAergic system may play an important role in the pathogenesis and development of malignant tumors, and their expression levels are important for prognosis. In this study, we found the overexpression of GABRA3 in more than half of the HCC tissues compared with the adjacent non-tumor liver tissues, and GABRA3 was expressed in malignant liver cell lines HepG2 and Chang, but not in normal cell line L-02, implicating that GABRA3 may be a good molecular target for the diagnosis of HCC.
GABAA-β3 receptor expression is absent in malignant hepatic cell lines, and the proliferating ability of Chang cells, which were stably transfected with GABAA-β3 receptor, was significantly decreased[9]. On the other hand, GABAB receptors were increased and GABAB receptor agonist promoted proliferation of hepatocytes[10]. It means that even in HCC, different GABA receptor subunits may have different functions. Functional analysis using siRNA of GABRA3 strongly supported its involvement in the development and progression of HCC. In our study, the proliferation rate of HepG2 cells after GABRA3 knockdown was significantly reduced, whereas proliferation of HepG2/Si-Mock cells was not inhibited. This result indicated that GABRA3 may increase the proliferating ability of hepatocytes.
Biju et al [10] reported that, in N-nitrosodiethylamine-induced neoplasia in the rat liver, GABAB receptors were increased and the GABAB receptor agonist baclofen increased EGF-mediated DNA synthesis in hepatocytes. Thus, GABA-associated pathways also could act positively in the regulation of cancer cell behavior. Our findings in this study also support the theory that GABA and GABRA3 promoted HepG2 cell proliferation in vivo and in vitro. Although GABA usually induces hyperpolarization in adult neurons, GABA has been shown to exert depolarizing responses in the immature CNS structures and CNS tumors[11,12]. And Minuk et al[13] reported that human HCC tissues were depolarized compared with adjacent non-tumor tissues. In particular, GABA increased the proliferation of immature cerebellar granule cells through the activation of GABAA receptors and voltage-dependent calcium channels[14]. Takehara et al[14] reported that GABA stimulated pancreatic cancer growth through GABRP by increased intracellular Ca2+ levels and activating the mitogenactivated protein kinase/extracellular signal-regulated kinase (MAPK/Erk) cascade[8]. From the results above, we deduce that GABA may promote the HepG2 cell proliferation through GABRA3 by voltage-dependent calcium channels. Interestingly, GABAA receptor antagonist bicuculline methiodide also could promote the proliferation of HepG2 cells (data not shown), indicating that it might activate some other signal transductions.
By comparing the proliferative activity of the GABRA3-knockdown HepG2 cells treated with GABA, we found that GABA stimulated HepG2 cell growth through GABRA3. The proliferating ability of the cells treated with GABA was not enhanced compared with the cells without GABA treatment. And interestingly, GABA inhibited the growth of the GABRA3-knockdown HepG2 cells. It indicates that GABA activates some other receptors to inhibit the proliferation without GABRA3, which is identical to some previous reports[15,16,17]. But GABA primarily activates GABRA3 if it exists.
Regarding the expression of other GABAA receptor subtypes/isoforms, the GABAA-δ expression detected in the majority of HCC tissues has not been previously described, and the significance of this finding has yet to be elucidated. Although GABAA-α1 (1 case), α3 (1 case), β3, γ2 (2 cases), ε and π expression was also detectable, it is unlikely that expression of these isoforms results in the formation of functional GABA-gated receptors with GABRA3. The reason is that GABA could produce opposite effects on HepG2 cells with or without GABRA3 expression, which means that GABA could activate GABA receptors. Moreover, the detection of the subunits was not unexpected, because previous reports have documented that the spectrum of GABAA receptor isoform expression correlates with the state of cell differentiation[18,19].
In conclusion, relative to adjacent non-tumor tissues, HCC tissues have increased GABAA-α3 receptor expression. Knockdown of GABRA3 expression in receptor expressing malignant hepatocytes results in attenuated in vivo and in vitro tumor growth. Moreover, GABA promotes hepatocyte proliferation through GABRA3. These findings highlight the importance of elucidating the role of GABAergic activity in the pathogenesis of HCC. They also raise the potential for new therapeutic and diagnostic approaches to HCC in humans.
COMMENTS
Background
Gamma-aminobutyric acid A receptor α3 subunit (GABRA3) is a subunit of the gamma-aminobutyric acid A (GABAA) receptors that may associate with other GABAA receptor subunits to form a functional chloride channel that mediates the inhibitory synaptic transmission in the mature central nervous system (CNS). GABA functions as an inhibitory neurotransmitter for activating GABA receptors.
Research frontiers
Recently, abnormal levels of gene and protein expression of some GABA receptor subunits have been detected in many malignant tumors. This indicates that GABAergic system may play an important role in the pathogenesis and development of malignant tumors.
Innovations and breakthroughs
This study demonstrated the overexpression of GABRA3 in Hepatocellular carcinoma (HCC) tissues, which has not been previously described, and illustrated that GABA stimulated HCC cell proliferation through GABRA3.
Applications
The findings raise the potential for new therapeutic and diagnostic approaches to HCC in humans.
Terminology
GABA stands for gamma-aminobutyric acid, which is an inhibitory neurotransmitter. GABRA3 stands for gamma-aminobutyric acid A receptor α3 subunit.
Peer review
In this work, the authors have explored the expression pattern of GABAA receptors in hepatocellular carcinoma cells, identifying the overexpression of GABRA3 in HCC cells and tissues.They also demonstrate that GABA increases proliferation of GABRA3 positive cells, which is cancelled when GABRA-3 expression is knock-down. Results are new and show relevance to understand the molecular mechanisms that control hepatocarcinogenesis.
Footnotes
Peer reviewer: Isabel Fabregat, PhD, Associate Professor, Laboratori d’Oncologia Molecular, Institut d’Investigación Biomèdica de Bellvitge, Gran Via, Km 2,7, L’Hospitalet, 08907 Barcelona, Spain
S- Editor Li LF L- Editor Ma JY E- Editor Lin YP
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Song TJ, Ip EW, Fong Y. Hepatocellular carcinoma: current surgical management. Gastroenterology. 2004;127:S248–S260. doi: 10.1053/j.gastro.2004.09.039. [DOI] [PubMed] [Google Scholar]
- 3.Haydar TF, Wang F, Schwartz ML, Rakic P. Differential modulation of proliferation in the neocortical ventricular and subventricular zones. J Neurosci. 2000;20:5764–5774. doi: 10.1523/JNEUROSCI.20-15-05764.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behar TN, Schaffner AE, Scott CA, Greene CL, Barker JL. GABA receptor antagonists modulate postmitotic cell migration in slice cultures of embryonic rat cortex. Cereb Cortex. 2000;10:899–909. doi: 10.1093/cercor/10.9.899. [DOI] [PubMed] [Google Scholar]
- 5.Meier J, Akyeli J, Kirischuk S, Grantyn R. GABA(A) receptor activity and PKC control inhibitory synaptogenesis in CNS tissue slices. Mol Cell Neurosci. 2003;23:600–613. doi: 10.1016/s1044-7431(03)00079-4. [DOI] [PubMed] [Google Scholar]
- 6.Tamayama T, Maemura K, Kanbara K, Hayasaki H, Yabumoto Y, Yuasa M, Watanabe M. Expression of GABA(A) and GABA(B) receptors in rat growth plate chondrocytes: activation of the GABA receptors promotes proliferation of mouse chondrogenic ATDC5 cells. Mol Cell Biochem. 2005;273:117–126. doi: 10.1007/s11010-005-8159-6. [DOI] [PubMed] [Google Scholar]
- 7.Erlander MG, Tobin AJ. The structural and functional heterogeneity of glutamic acid decarboxylase: a review. Neurochem Res. 1991;16:215–226. doi: 10.1007/BF00966084. [DOI] [PubMed] [Google Scholar]
- 8.Takehara A, Hosokawa M, Eguchi H, Ohigashi H, Ishikawa O, Nakamura Y, Nakagawa H. Gamma-aminobutyric acid (GABA) stimulates pancreatic cancer growth through overexpressing GABAA receptor pi subunit. Cancer Res. 2007;67:9704–9712. doi: 10.1158/0008-5472.CAN-07-2099. [DOI] [PubMed] [Google Scholar]
- 9.Sun D, Gong Y, Kojima H, Wang G, Ravinsky E, Zhang M, Minuk GY. Increasing cell membrane potential and GABAergic activity inhibits malignant hepatocyte growth. Am J Physiol Gastrointest Liver Physiol. 2003;285:G12–G19. doi: 10.1152/ajpgi.00513.2002. [DOI] [PubMed] [Google Scholar]
- 10.Biju MP, Pyroja S, Rajeshkumar NV, Paulose CS. Enhanced GABA(B) receptor in neoplastic rat liver: induction of DNA synthesis by baclofen in hepatocyte cultures. J Biochem Mol Biol Biophys. 2002;6:209–214. doi: 10.1080/10258140290018667. [DOI] [PubMed] [Google Scholar]
- 11.Ganguly K, Schinder AF, Wong ST, Poo M. GABA itself promotes the developmental switch of neuronal GABAergic responses from excitation to inhibition. Cell. 2001;105:521–532. doi: 10.1016/s0092-8674(01)00341-5. [DOI] [PubMed] [Google Scholar]
- 12.Labrakakis C, Patt S, Hartmann J, Kettenmann H. Functional GABA(A) receptors on human glioma cells. Eur J Neurosci. 1998;10:231–238. doi: 10.1046/j.1460-9568.1998.00036.x. [DOI] [PubMed] [Google Scholar]
- 13.Minuk GY, Zhang M, Gong Y, Minuk L, Dienes H, Pettigrew N, Kew M, Lipschitz J, Sun D. Decreased hepatocyte membrane potential differences and GABAA-beta3 expression in human hepatocellular carcinoma. Hepatology. 2007;45:735–745. doi: 10.1002/hep.21562. [DOI] [PubMed] [Google Scholar]
- 14.Fiszman ML, Borodinsky LN, Neale JH. GABA induces proliferation of immature cerebellar granule cells grown in vitro. Brain Res Dev Brain Res. 1999;115:1–8. doi: 10.1016/s0165-3806(99)00035-8. [DOI] [PubMed] [Google Scholar]
- 15.Zhang M, Gong Y, Assy N, Minuk GY. Increased GABAergic activity inhibits alpha-fetoprotein mRNA expression and the proliferative activity of the HepG2 human hepatocellular carcinoma cell line. J Hepatol. 2000;32:85–91. doi: 10.1016/s0168-8278(00)80193-2. [DOI] [PubMed] [Google Scholar]
- 16.Tatsuta M, Iishi H, Baba M, Nakaizumi A, Ichii M, Taniguchi H. Inhibition by gamma-amino-n-butyric acid and baclofen of gastric carcinogenesis induced by N-methyl-N'-nitro-N-nitrosoguanidine in Wistar rats. Cancer Res. 1990;50:4931–4934. [PubMed] [Google Scholar]
- 17.Tatsuta M, Iishi H, Baba M, Yano H, Uehara H, Nakaizumi A. Effect of selective and non-selective muscarinic blockade on baclofen inhibition of gastric carcinogenesis induced by N-methyl-N'-nitro-N-nitrosoguanidine in Wistar rats. Carcinogenesis. 1996;17:293–296. doi: 10.1093/carcin/17.2.293. [DOI] [PubMed] [Google Scholar]
- 18.Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neelands TR, Zhang J, Macdonald RL. GABA(A) receptors expressed in undifferentiated human teratocarcinoma NT2 cells differ from those expressed by differentiated NT2-N cells. J Neurosci. 1999;19:7057–7065. doi: 10.1523/JNEUROSCI.19-16-07057.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]