Abstract
AIM: To assess the efficacy and advantages of 4-wk pegylated interferon α-2a (peg-IFN-α2a) monotherapy for chronic hepatitis C patients with strong predictors of sustained virologic response (SVR).
METHODS: Patients (n = 33) with genotype 2 and low viral load (< 100 KIU/mL), who became HCV RNA negative after 1 wk of IFN treatment, were randomly allocated to receive a 4- or 12-wk treatment course at a ratio of 2:1, respectively, with a subsequent 24-wk follow-up period. Peg-IFN-α2a was administered subcutaneously at a dose of 180 μg or 90 μg once weekly. SVR was defined as absence of serum HCV RNA at the end of the follow-up period.
RESULTS: All patients completed the treatment schedule, and more than half were symptom-free during the treatment. In the 4-wk treatment group, 20 of 22 (91%) patients achieved SVR. Two patients relapsed, but achieved SVR following re-treatment with peg-IFN-α2a alone. In the 12-wk treatment group, 11 of 11 (100%) patients attained SVR.
CONCLUSION: Our results show that a 4-wk course of peg-IFN-α2a monotherapy can achieve a high SVR rate in “IFN-sensitive” patients, without negatively affecting outcome.
Keywords: Chronic hepatitis C, Pegylated interferon alpha-2a monotherapy, Genotype 2, Low viral load, Randomized pilot study
INTRODUCTION
Pegylated interferon alpha (peg-IFN-α) in combination with ribavirin for 24 wk is currently recommended as a standard treatment for patients with hepatitis C virus (HCV) genotypes 2 and 3, because prolongation of treatment duration to 48 wk does not always provide a substantial gain in the sustained virologic response (SVR) rate[1-5]. In contrast, short-term combination treatment (i.e. less than 24 wk of duration) has been evaluated in genotype 2 and 3 patients with or without initial virological response[6-11]. Shorter treatment may lead to a substantial reduction in patients’ burden and to the avoidance of premature termination of treatment without adversely affecting the outcome. Still, the pros and cons of a shorter treatment have not been conclusively defined and treating patients for less than 24 wk is still controversial.
A recent trend in the treatment strategy for chronic hepatitis C is the development of tailored or individualized treatment regimens based on major strong predictors of SVR to IFN-based treatment, such as HCV genotype[2,3,12-17], pretreatment viral load[2,4,12,14,15,18], and initial virologic response to treatment[15,19-24]. Further subdivision or stratification of patients by combining these predictors may allow the development of more rational and optimal regimen without adversely affecting the outcome: e.g. treatment duration or combination with or without ribavirin. Specifically, a subgroup of genotype 2 patients with low viral load showed an excellent response even to conventional IFN monotherapy[12-14,18]. Furthermore, if initial virologic response is also taken into consideration in determining treatment regimen for the “IFN-sensitive” patient subgroup, even a shorter course of peg-IFN-α2a monotherapy might result in a high SVR rate without adversely affecting the outcome.
In the present study, we evaluated the efficacy and tolerability of a 4-wk peg-IFN-α2a monotherapy for “IFN-sensitive” patients with genotype 2 and low viral load, who became HCV RNA negative after 1 wk of treatment, in a pilot, randomized trial comparing a 4-wk versus a 12-wk treatment schedule. This study may allow us to substantially reduce the total dose and duration of peg-IFN-α treatment and to spare the use of ribavirin in the “IFN-sensitive” patient subgroup.
MATERIALS AND METHODS
Study population
Thirty-seven patients chronically infected with HCV genotype 2 and low viral load were consecutively enrolled in this pilot randomized study. They received short-term peg-IFN-α2a monotherapy at the Department of Gastroenterology and Hepatology, Kashiwa Hospital, the Jikei University School of Medicine, between August 2006 and July 2007. Eligible patients had anti-HCV antibodies, infection with HCV genotype 2 confirmed by polymerase chain reaction (PCR)-based method[25], serum HCV RNA levels < 100 kilo-international units (KIU)/mL by a quantitative PCR assay (Amplicor HCV Monitor Version 2.0, Roche Diagnostics, Tokyo, Japan; lower detection limit, 0.5 KIU/mL) (defined as ‘low’ viral load) at the enrolment, persistently abnormal serum alanine transaminase (ALT) concentrations (> 30 IU/L) during the preceding 24 wk, platelet counts ≥ 50 × 103 /μL, neutrophil counts ≥ 750 /μL, and hemoglobin values ≥ 10 g/dL. All patients were ≥ 20 years of age and none had received prior IFN-containing treatment. Exclusion criteria were: liver cancer or decompensated liver cirrhosis; other forms of liver disease; coexisting serious psychiatric or medical illness; treatment with any other antiviral or immunomodulatory agent administered within the preceding 12 wk; hepatitis B surface antigen or hepatitis B core antibody; and pregnancy or lactation. The Local Ethics Committee of Jikei University School of Medicine approved the study. All patients provided informed consent before entry into the trial. Liver biopsy was performed between the enrolment and the beginning of treatment, and histopathologic evaluation was carried out using the ranking system for grading of necroinflammation activity and staging of fibrosis[26]. Steatosis was graded as mild (< 30% of hepatocytes contained lipid droplets in the cytoplasm), moderate (30%-60%), or severe (> 60%).
Study protocol
When patients showed virological response (VR) after 1 wk of treatment, patients were randomly allocated to receive a 4- or 12-wk treatment course at a ratio of 2:1 using a central randomization system, respectively, with a subsequent 24-wk follow-up period. Randomization was performed by means of sealed, opaque, numbered envelopes, each containing a sheet of paper assigning to either group, which were prepared independently by a medical statistician. The total sample size was estimated statistically (two-sided α = 0.10, β = 0.20). Patients without VR after 1 wk were excluded from the trial, and all received a 12-wk treatment course. VR was defined as undetectable serum HCV RNA, using a qualitative PCR assay (Amplicor HCV version 2.0, Roche Diagnostics) with a lower detection limit of 50 IU/mL. Peg-IFN-alpha-2a (Roche, Nutley, NJ) at a dose of 180 μg (platelet count > 90 × 103/μL and neutrophil count > 1500/μL) or 90 μg (< 90 × 103/μL and < 1500/μL, respectively) was administered subcutaneously once weekly. During the treatment, the dose of peg-IFN was adjusted based on platelet and/or neutrophil counts determined before each administration: Peg-IFN was reduced from 180 to 90 μg when platelet or neutrophil counts fell below 90 × 103/μL and 1500/μL, respectively, and from 90 to 45 or 30 μg when platelet or neutrophil counts fell below 70% of the values observed the previous week. Peg-IFN was discontinued when the platelet count, the neutrophil count, or the hemoglobin value dropped below 25 × 103 /μL, 500/μL, and 8.5 g/dL, respectively.
Clinical, laboratory and hematological data were assessed once weekly during the treatment and every 4 wk during the 24-wk follow-up period. Virological assessment was performed after 1 wk from the initiation of treatment, at the end of treatment, and every 4 wk during the follow-up period. Sustained virologic response (SVR) was defined as undetectable serum HCV RNA at the end of follow-up period. Safety was monitored clinically by careful interview and medical examination throughout the study.
Statistical analysis
The χ2 test, Fisher’s exact two-tail test, or Mann-Whitney test were used for statistical comparisons between groups, where appropriate. Treatment outcomes were analyzed on an intention-to-treat basis. All P values for statistical tests were two-tailed and values less than 0.05 were considered statistically significant. All calculations were performed using the SPSS 15.0 statistical package (SPSS, Chicago, IL).
RESULTS
Patient characteristics
Of 37 patients, 33 (89%) showed VR after 1 wk of treatment. The 33 patients were subsequently randomized to the 4-wk (n = 22) or the 12-wk (n = 11) treatment arms (Table 1, Figure 1). There was no statistically significant difference between the two groups as far as the baseline characteristics features were concerned. Irrespective of the treatment course, all 33 patients completed the treatment and were closely followed up as scheduled. None of the patients was lost to follow-up. None of the patients received growth factors for cytopenias, such as granulocyte-colony stimulating factor and erythropoietin. A liver biopsy was not available in 3 patients because of a bleeding disorder (n = 1, 4-wk treatment group) or because of patients’ refusal (n = 2, both for the 4-wk treatment group).
Table 1.
Characteristics of treatment-naïve chronic hepatitis C patients with genotype 2 and low viral load at the start of treatment (mean ± SD)
| 4-wk treatment group (n = 22) | 12-wk treatment group (n = 11) | |
| Gender (M/F) | 10/12 | 6/5 |
| Age (yr) | 57 ± 10 | 56 ± 12 |
| Body weight (kg) | 58.5 ± 10.7 | 60.6 ± 10.4 |
| Body mass index (kg/m2) | 23.0 ± 2.9 | 21.8 ± 2.2 |
| History of transfusion (yes/no) | 10/12 | 3/8 |
| Initial dosage (180 μg/90 μg) | 20/2 | 9/2 |
| Aspartate transaminase (IU/L) | 53 ± 27 | 55 ± 32 |
| Alanine transaminase (IU/L) | 75 ± 35 | 77 ± 36 |
| γ-glutamyl transpeptidase (IU/L) | 52 ± 24 | 48 ± 30 |
| Albumin (g/dL) | 4.3 ± 0.3 | 4.2 ± 0.3 |
| Total cholesterol (mg/dL) | 169 ± 22 | 182 ± 35 |
| Platelet count (× 103/μL) | 157 ± 58 | 165 ± 62 |
| Hemoglobin (g/dL) | 13.3 ± 1.6 | 13.6 ± 1.8 |
| Leukocyte count (/μL) | 4700 ± 1500 | 5300 ± 900 |
| Neutrophil count (/μL) | 2490 ± 1140 | 2550 ± 613 |
| Liver histology | ||
| Stage (1/2/3/4) | 9/5/5/0 | 5/4/2/0 |
| Grade (mild/moderate/severe) | 11/8/0 | 8/3/0 |
| Steatosis (mild/moderate/severe) | 15/4/0 | 9/2/0 |
| Viral load (KIU/mL) | 24 ± 21 | 24 ± 22 |
Figure 1.
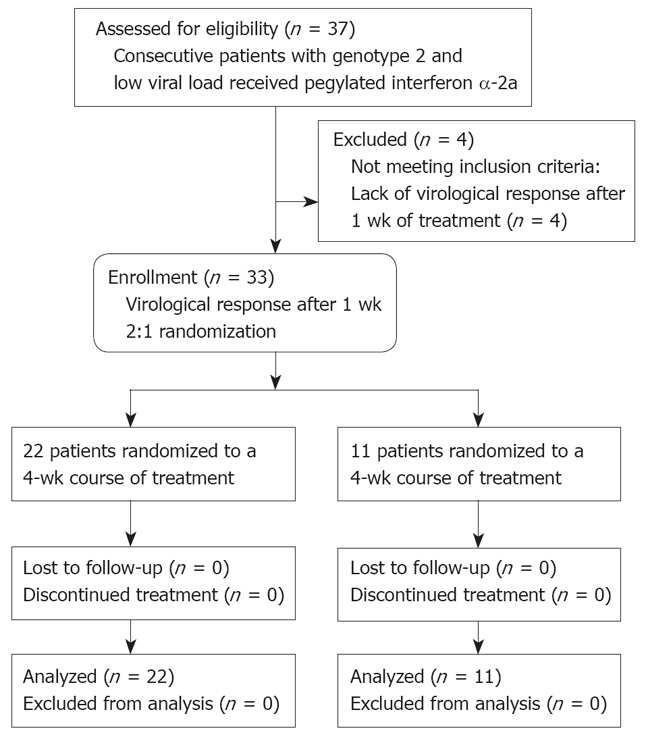
Flow diagram of patients included in the pilot study.
Sustained virological response rates
In the 4-wk treatment group, 20 of 22 (91%) patients achieved SVR. Two patients relapsed 8 wk after the end of treatment. In the 12-wk treatment group, 11 of 11 (100%) patients attained SVR. No statistical difference was observed between both groups as to the SVR rates. The two patients who relapsed were offered re-treatment (4-wk and 12-wk regimens, respectively): both agreed and achieved SVR.
Predictors of sustained virological response or relapse
Univariate analyses failed to identify factors (including patients’ age, body weight, and the presence of steatosis) associated with SVR or relapse.
Safety
Six patients in the 4-wk treatment group and five in the 12-wk treatment group complained of low-grade fever, headache, or malaise, but these symptoms were well tolerated during treatment. The dose of peg-IFN-α2a was reduced in three (all for thrombocytopenia) and four (two for neutropenia and two for thrombocytopenia) patients, respectively. Consequently, the cumulative dose of peg-IFN-α2a was 180 to 720 μg and 1350 to 2160 μg, respectively. All patients recovered completely from the adverse events after the completion of treatment.
Medication price
On the basis of current prices in Japan, the cost of medication (peg-IFN-α2a of 90 or 180 μg weekly) for 4-wk treatment course is between 536 and 1044 USD, and that for 12-wk treatment course is between 1608 and 3131 USD.
DISCUSSION
The results of this randomized pilot study suggest that a subgroup of patients could achieve a sufficiently high rate of SVR after only a 4-wk course of peg-IFN-α2a monotherapy. One of the most IFN-sensitive subgroups appears to include patients with genotype 2 and low viral load who exhibit VR after 1 wk of treatment.
Patients with genotype 2 or 3 are more sensitive to peg-IFN-α plus ribavirin treatment than those with genotype 1, and the current recommendation advocates a 24-wk treatment course, because more than 80% of the former group will attain SVR[3-5,7,11,27]. Recently, several studies suggested that the treatment duration could be shortened from 24 to between 12 and 16 wk without adversely affecting outcome in patients with genotype 2 or 3 who achieve VR after 4 wk of treatment[6-8,11]. In contrast, large trials indicated that shortening the treatment duration to 16 or 14 wk lessened the SVR rates[9,10]. It remains controversial whether the optimal treatment duration is 24 wk or less than 24 wk for patients with genotype 2 or 3. A shorter treatment may not to be suitable for every patient with genotype 2 or 3. There may be a need to investigate whether the 4 wk timepoint from the beginning of treatment is appropriate to predict the therapy outcome after 24-wk treatment course.
There is ample evidence that peg-IFN-α plus ribavirin treatment is more beneficial in patients with genotype 2 than those with genotype 3[4,7-10]. This suggests that treatment regimens should be tailored or individualized for each genotype, with a special emphasis on the duration of treatment. As for patients with genotype 2 alone, even high-dose conventional IFN-α monotherapy for 24 wk produced an SVR rate of 78%[14]. Furthermore, the inclusion of baseline viral load and initial virologic response, as strong predictors of SVR independent of genotype[4,6,7,10,11,15,16,19-24,27], may identify patients suitable for shorter treatment in a more accurate way. In a small size study, an SVR rate of 100% was reported in patients with genotype 2a and low viral load (< 100 KIU/mL) treated with IFN-α for 6 wk[28]. In another study, 89.5% and 100% of patients, without genotype 1 and high viral load (> 100 KIU/mL), who showed VR after 2 wk of treatment, achieved SVR with 8-wk and 24-wk peg-IFN-α2a monotherapy, respectively[29]. Although the sample size was very small, this pilot randomized trial suggested that the duration of peg-IFN-α2a monotherapy may be further shortened while maintaining a high SVR rate by dividing patients according to the presence or absence of strong predictors of response.
Common or characteristic adverse events are more severe and frequent in the combination treatment with ribavirin than in IFN-α or peg-IFN-α monotherapy, especially with prolonged treatment duration, leading to frequent withdrawals from treatment, because ribavirin accumulates in various tissues as well as in erythrocytes[2,15,24]. Therefore, only a minority of patients in need of therapy actually receives peg-IFN-α plus ribavirin treatment. Before participation in this trial, IFN-based treatment has been withheld from some patients for a variety of reasons such as advanced age, or the relatively low hemoglobin level and/or platelet count. Symptoms recorded in this study were mild or few, supporting the view that a short treatment course is safer and associated with few adverse events and less frequent withdrawal from treatment[1,2,7,8,11,30]. Our trial demonstrated that a 4-wk peg-IFN-α2a monotherapy was safe for such patients, and suggested that the indication for treatment could be extended to include patients considered otherwise unsuitable for the peg-IFN-α plus ribavirin combination therapy.
The combination treatment is costly, and the longer the treatment duration, the higher the cost of treatment. Currently, the cost of treatment of a person weighing 65 kg who receives peg-IFN-α2a at 180 μg weekly and ribavirin at 800 mg daily for 24 wk is approximately 11 253 USD in Japan. Thus, a 4-wk peg-IFN-α2a monotherapy would achieve a > 90% reduction in cost and drug exposure. When one adds the costs of medical consultation and laboratory tests, the 4-wk peg-IFN-α2a monotherapy provides a substantial saving in costs and inconvenience compared to the extended treatment.
In conclusion, the present study showed high SVR rates in genotype 2 patients with low viral load who had undetectable serum HCV RNA after 1 wk of treatment, treated with a 4-wk course of peg-IFN-α2a monotherapy. Tailoring or individualizing treatment to individual patients would considerably reduce both patients’ and society burdens, without adversely affecting the clinical outcome.
COMMENTS
Background
Hepatitis C virus (HCV) genotype, pretreatment viral load and initial virologic response are major predictors of the treatment outcome in interferon (IFN)-based treatment for chronic hepatitis C. Patients infected with genotype 2 or 3 are currently recommended to receive a 24-wk course of pegylated IFN alpha (peg-IFN-α) plus ribavirin combination therapy. However, treatment regimens should be modified for those subgroups of genotype 2 patients with favorable predictors.
Research frontiers
To improve the treatment outcome while reducing the adverse effects and treatment costs, peg-IFN-α plus ribavirin combination treatment regimens for chronic hepatitis C are rationally tailored or reasonably individualized based on major predictors of response. The duration of combination therapy for genotype 1 or 4 patients is modified from 24 wk to 48 or 72 wk according to virological response after 4 wk, 12swk and 24 wk after treatment. In contrast, it remains controversial whether the treatment duration for genotype 2 or 3 patients could be shortened from 24 wk to 12, 14 or 16 wk according to the virological response after 4 wk of therapy.
Innovations and breakthroughs
This pilot, randomized study demonstrates that further stratification of patients by combining strong predictors may shorten the treatment duration and allow peg-IFN-α monotherapy without adversely affecting the outcome, leading to the substantial reduction in both patients’ and society burdens. Moreover, this study questions whether the virological response at 4 wk after treatment is appropriate for efficiently discriminating the treatment outcome in a 24-wk treatment course for genotype 2 patients.
Applications
This randomized pilot study suggests that further stratification by combining strong predictors may be extended to individuals infected with other genotypes in tailoring or individualizing more rational and optimal regimens.
Terminology
Virological response is defined as undetectable serum HCV RNA (< 50 IU/mL). Treatment outcome means sustained virologic response, defined as undetectable serum HCV RNA at the end of the 24-wk follow-up period.
Peer review
This is an interesting and well-written manuscript describing an important issue in hepatitis C treatment.
Footnotes
Supported by Clinical Research Funds from Department of Gastroenterology and Hepatology, Kashiwa Hospital, Jikei University School of Medicine
Peer reviewer: Br Carla W Brady, MD, MHS, Duke University Medical Center, Division of Gastroenterology, DUMC Box 3913, Durham, NC 27705, United States
S- Editor Cheng JX L- Editor Negro F E- Editor Lin YP
References
- 1.McHutchison JG, Gordon SC, Schiff ER, Shiffman ML, Lee WM, Rustgi VK, Goodman ZD, Ling MH, Cort S, Albrecht JK. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339:1485–1492. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- 2.Poynard T, Marcellin P, Lee SS, Niederau C, Minuk GS, Ideo G, Bain V, Heathcote J, Zeuzem S, Trepo C, et al. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT) Lancet. 1998;352:1426–1432. doi: 10.1016/s0140-6736(98)07124-4. [DOI] [PubMed] [Google Scholar]
- 3.Hadziyannis SJ, Sette H Jr, Morgan TR, Balan V, Diago M, Marcellin P, Ramadori G, Bodenheimer H Jr, Bernstein D, Rizzetto M, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346–355. doi: 10.7326/0003-4819-140-5-200403020-00010. [DOI] [PubMed] [Google Scholar]
- 4.Zeuzem S, Hultcrantz R, Bourliere M, Goeser T, Marcellin P, Sanchez-Tapias J, Sarrazin C, Harvey J, Brass C, Albrecht J. Peginterferon alfa-2b plus ribavirin for treatment of chronic hepatitis C in previously untreated patients infected with HCV genotypes 2 or 3. J Hepatol. 2004;40:993–999. doi: 10.1016/j.jhep.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Strader DB, Wright T, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004;39:1147–1171. doi: 10.1002/hep.20119. [DOI] [PubMed] [Google Scholar]
- 6.Dalgard O, Bjoro K, Hellum KB, Myrvang B, Ritland S, Skaug K, Raknerud N, Bell H. Treatment with pegylated interferon and ribavarin in HCV infection with genotype 2 or 3 for 14 weeks: a pilot study. Hepatology. 2004;40:1260–1265. doi: 10.1002/hep.20467. [DOI] [PubMed] [Google Scholar]
- 7.von Wagner M, Huber M, Berg T, Hinrichsen H, Rasenack J, Heintges T, Bergk A, Bernsmeier C, Haussinger D, Herrmann E, et al. Peginterferon-alpha-2a (40KD) and ribavirin for 16 or 24 weeks in patients with genotype 2 or 3 chronic hepatitis C. Gastroenterology. 2005;129:522–527. doi: 10.1016/j.gastro.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Mangia A, Santoro R, Minerva N, Ricci GL, Carretta V, Persico M, Vinelli F, Scotto G, Bacca D, Annese M, et al. Peginterferon alfa-2b and ribavirin for 12 vs. 24 weeks in HCV genotype 2 or 3. N Engl J Med. 2005;352:2609–2617. doi: 10.1056/NEJMoa042608. [DOI] [PubMed] [Google Scholar]
- 9.Shiffman ML, Suter F, Bacon BR, Nelson D, Harley H, Sola R, Shafran SD, Barange K, Lin A, Soman A, et al. Peginterferon alfa-2a and ribavirin for 16 or 24 weeks in HCV genotype 2 or 3. N Engl J Med. 2007;357:124–134. doi: 10.1056/NEJMoa066403. [DOI] [PubMed] [Google Scholar]
- 10.Dalgard O, Bjoro K, Ring-Larsen H, Bjornsson E, Holberg-Petersen M, Skovlund E, Reichard O, Myrvang B, Sundelof B, Ritland S, et al. Pegylated interferon alfa and ribavirin for 14 versus 24 weeks in patients with hepatitis C virus genotype 2 or 3 and rapid virological response. Hepatology. 2008;47:35–42. doi: 10.1002/hep.21975. [DOI] [PubMed] [Google Scholar]
- 11.Yu ML, Dai CY, Huang JF, Hou NJ, Lee LP, Hsieh MY, Chiu CF, Lin ZY, Chen SC, Hsieh MY, et al. A randomised study of peginterferon and ribavirin for 16 versus 24 weeks in patients with genotype 2 chronic hepatitis C. Gut. 2007;56:553–559. doi: 10.1136/gut.2006.102558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshioka K, Kakumu S, Wakita T, Ishikawa T, Itoh Y, Takayanagi M, Higashi Y, Shibata M, Morishima T. Detection of hepatitis C virus by polymerase chain reaction and response to interferon-alpha therapy: relationship to genotypes of hepatitis C virus. Hepatology. 1992;16:293–299. doi: 10.1002/hep.1840160203. [DOI] [PubMed] [Google Scholar]
- 13.Tsubota A, Chayama K, Ikeda K, Yasuji A, Koida I, Saitoh S, Hashimoto M, Iwasaki S, Kobayashi M, Hiromitsu K. Factors predictive of response to interferon-alpha therapy in hepatitis C virus infection. Hepatology. 1994;19:1088–1094. [PubMed] [Google Scholar]
- 14.Tsubota A, Kumada H, Chayama K, Arase Y, Saitoh S, Koida I, Murashima N, Suzuki Y, Kobayashi M, Takagi K, et al. Relationship between pretreatment viremia level and response to interferon-alpha therapy in chronic hepatitis C differs in viral type 1 and 2 infections. Dig Dis Sci. 1996;41:1925–1932. doi: 10.1007/BF02093591. [DOI] [PubMed] [Google Scholar]
- 15.Davis GL, Esteban-Mur R, Rustgi V, Hoefs J, Gordon SC, Trepo C, Shiffman ML, Zeuzem S, Craxi A, Ling MH, et al. Interferon alfa-2b alone or in combination with ribavirin for the treatment of relapse of chronic hepatitis C. International Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339:1493–1499. doi: 10.1056/NEJM199811193392102. [DOI] [PubMed] [Google Scholar]
- 16.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL Jr, Haussinger D, Diago M, Carosi G, Dhumeaux D, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 17.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 18.Lau JY, Simmonds P, Urdea MS. Implications of variations of "conserved" regions of hepatitis C virus genome. Lancet. 1995;346:425–426. doi: 10.1016/s0140-6736(95)92786-7. [DOI] [PubMed] [Google Scholar]
- 19.Neumann AU, Lam NP, Dahari H, Gretch DR, Wiley TE, Layden TJ, Perelson AS. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-alpha therapy. Science. 1998;282:103–107. doi: 10.1126/science.282.5386.103. [DOI] [PubMed] [Google Scholar]
- 20.Zeuzem S, Lee JH, Franke A, Ruster B, Prummer O, Herrmann G, Roth WK. Quantification of the initial decline of serum hepatitis C virus RNA and response to interferon alfa. Hepatology. 1998;27:1149–1156. doi: 10.1002/hep.510270433. [DOI] [PubMed] [Google Scholar]
- 21.Lee WM, Reddy KR, Tong MJ, Black M, van Leeuwen DJ, Hollinger FB, Mullen KD, Pimstone N, Albert D, Gardner S. Early hepatitis C virus-RNA responses predict interferon treatment outcomes in chronic hepatitis C. The Consensus Interferon Study Group. Hepatology. 1998;28:1411–1415. doi: 10.1002/hep.510280533. [DOI] [PubMed] [Google Scholar]
- 22.Neumann AU, Lam NP, Dahari H, Davidian M, Wiley TE, Mika BP, Perelson AS, Layden TJ. Differences in viral dynamics between genotypes 1 and 2 of hepatitis C virus. J Infect Dis. 2000;182:28–35. doi: 10.1086/315661. [DOI] [PubMed] [Google Scholar]
- 23.Bekkering FC, Stalgis C, McHutchison JG, Brouwer JT, Perelson AS. Estimation of early hepatitis C viral clearance in patients receiving daily interferon and ribavirin therapy using a mathematical model. Hepatology. 2001;33:419–423. doi: 10.1053/jhep.2001.21552. [DOI] [PubMed] [Google Scholar]
- 24.Bjoro K, Bell H, Hellum KB, Skaug K, Raknerud N, Sandvei P, Doskeland B, Maeland A, Lund-Tonnesen S, Myrvang B. Effect of combined interferon-alpha induction therapy and ribavirin on chronic hepatitis C virus infection: a randomized multicentre study. Scand J Gastroenterol. 2002;37:226–232. doi: 10.1080/003655202753416920. [DOI] [PubMed] [Google Scholar]
- 25.Okamoto H, Sugiyama Y, Okada S, Kurai K, Akahane Y, Sugai Y, Tanaka T, Sato K, Tsuda F, Miyakawa Y. Typing hepatitis C virus by polymerase chain reaction with type-specific primers: application to clinical surveys and tracing infectious sources. J Gen Virol. 1992;73(Pt 3):673–679. doi: 10.1099/0022-1317-73-3-673. [DOI] [PubMed] [Google Scholar]
- 26.Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513–1520. [PubMed] [Google Scholar]
- 27.Davis GL, Wong JB, McHutchison JG, Manns MP, Harvey J, Albrecht J. Early virologic response to treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C. Hepatology. 2003;38:645–652. doi: 10.1053/jhep.2003.50364. [DOI] [PubMed] [Google Scholar]
- 28.Tabaru A, Narita R, Hiura M, Abe S, Otsuki M. Efficacy of short-term interferon therapy for patients infected with hepatitis C virus genotype 2a. Am J Gastroenterol. 2005;100:862–867. doi: 10.1111/j.1572-0241.2005.40826.x. [DOI] [PubMed] [Google Scholar]
- 29.Jeong S, Kawakami Y, Kitamoto M, Ishihara H, Tsuji K, Aimitsu S, Kawakami H, Uka K, Takaki S, Kodama H, et al. Prospective study of short-term peginterferon-alpha-2a monotherapy in patients who had a virological response at 2 weeks after initiation of interferon therapy. J Gastroenterol Hepatol. 2008;23:541–545. doi: 10.1111/j.1440-1746.2008.05356.x. [DOI] [PubMed] [Google Scholar]
- 30.McHutchison JG, Manns M, Patel K, Poynard T, Lindsay KL, Trepo C, Dienstag J, Lee WM, Mak C, Garaud JJ, et al. Adherence to combination therapy enhances sustained response in genotype-1-infected patients with chronic hepatitis C. Gastroenterology. 2002;123:1061–1069. doi: 10.1053/gast.2002.35950. [DOI] [PubMed] [Google Scholar]


