Abstract
AIM: To present our experience with tuberculous peritonitis treated in our hospital from 2002-2007.
METHODS: We reviewed the medical records of 9 children with tuberculous peritonitis.
RESULTS: Nine patients (5 boys, 4 girls) of mean age 14.2 years were diagnosed with peritoneal tuberculosis. All patients presented with abdominal distention. Abdominal pain was seen in 55.5% and fever in 44.4% of the patients. Four cases had coexisting pleural effusion and two had pulmonary tuberculosis with parenchymal consolidation. Ultrasonography found ascites with septation in 7 patients. Two patients had only ascites without septation. Ascitic fluid analysis of 8 patients yielded serum-ascite albumin gradients of less than 1.1 gr/dL. Laparoscopy and laparotomy showed that whitish tuberculi were the most common appearance. Adhesions were also seen in three cases. The diagnosis of peritoneal tuberculosis was confirmed histo-pathologically in 7 patients and microbiologically in two. Two patients had been diagnosed by ascitic fluid diagnostic features and a positive response to antituberculous treatment. All patients completed the antituberculous therapy without any complications.
CONCLUSION: Tuberculous peritonitis has to be clinically suspected in all patients with slowly progressive abdominal distension, particularly when it is accompanied by fever and pain. Laparoscopy and peritoneal biopsy are still the most reliable, quick and safe methods for the diagnosis of tuberculous peritonitis.
Keywords: Child, Clinical presentation, Diagnosis, Tuberculous peritonitis
INTRODUCTION
Tuberculous peritonitis (TBP) is an uncommon presentation of tuberculosis (TB) especially in children without any other debilitating disease such as cirrhosis, diabetes and chronic renal failure on continuous ambulatory peritoneal dialysis[1]. It is estimated that TBP occurs in 0.1%-3.5 % of all patients with pulmonary TB and represents 4%-10% of all extrapulmonary TB[2,3]. Most of the cases are in their 30’s or 40’s and it is rarely seen in children[1,4]. It results from hematogenous spread or contagious spread from an abdominal focus or mesenteric lymph node[4,5]. Most patients have chronic abdominal complaints. Due to the protean nature of the manifestations, diagnosis is often delayed and the rate of complications and mortality increases. Therefore, clinicians should be aware of the disease for the early diagnosis. We aimed to review the clinical features of the TBP in children that were followed up in our center.
MATERIALS AND METHODS
In this retrospective study, we reviewed the medical records of 9 children with TBP followed by our Pediatric Department from 2002-2007. The presentation symptoms, history of TB exposure, biochemical tests, clinical and histological features of the patients were recorded. Other causes of ascite and chronic liver diseases were ruled out in all patients. None of the patients had any other chronic disease.
The tuberculin skin test (TST) was evaluated 48-72 h after intradermal injection of 5 tuberculin units of purified protein derivative. It was considered to be positive when the induration was greater than or equal to 15 mm in previously vaccinated patients and greater than or equal to 10 mm in patients who had never been vaccinated.
Diagnosis of TBP was based on either typical laparoscopic appearance with tubercles and histological presence of caseating granuloma or diagnostic features of the ascitic fluid and response of the antituberculous therapy (in the absence of tissue samples being available). Patients were treated with isoniazide (10 mg/kg per day) and rifampin (15 mg/kg per day) for 9 mo and pyrazinamide (30 mg/kg per day) and streptomycin (20-40 mg/kg per day) for the first 2 mo. Metilprednisolone (2 mg/kg per day) was also given to four patients (patients 1-3 and 5) for the first 6 wk. The data were expressed as mean ± SD.
RESULTS
Nine patients were diagnosed with peritoneal tuberculosis. They were 5 boys and 4 girls with a mean age of 14.2 years (range 11-16 years). At presentation, abdominal distention was a common complaint in all patients (100%). In addition, five had abdominal pain (55.5%), four had fever (44.4%), three had coughing, three had weight loss, and three had night sweating (33.3%). The mean duration of symptoms was 41.5 ± 30.2 (7-90) d. Six patients had a history of tuberculosis within the family (for details see Table 1).
Table 1.
Summary of patients’ details, clinical presentations and outcome
| Patients | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| Age/sex (Yr) | 14/M | 14/F | 16/M | 16/M | 16/F | 11/M | 14/M | 12/F | 15/F |
| Clinical presentation | Abdominal distention | Abdominal distention | Abdominal distention | Abdominal distention | Abdominal distention | Abdominal distention | Abdominal distention | Abdominal distention | Abdominal distention |
| Abdominal | Abdominal | Weight loss | Weight loss | Abdominal | Abdominal | Abdominal | |||
| Pain | Pain | Night sweats | Fever | Pain | Pain | Pain | |||
| Night sweats | Night sweats | Fever | Fever | Fever | |||||
| Cough | Weight loss | Cough | Cough | ||||||
| Thoracic involvement | Consolidation | None | Pleural effusion | Pleural effusion | Pleural effusion | None | None | Pleural effusion | None |
| Consolidation | |||||||||
| Contact history | Father | None | Mother | Uncle | Mother | Grandfather | Father | None | None |
| Brother | Grandmother | ||||||||
| BCG /TST (scar/mm) | Neg/22 | Neg/17 | Neg/6 | Neg/10 | Pos/0 | Pos/20 | Neg/15 | Pos/13 | Pos/16 |
| ADA (IU/dL) | - | 121 | - | - | - | - | 94 | - | 102 |
| SAAG | - | 1.0 | 0.5 | 0.7 | 0.3 | 0.8 | 0.4 | 0.5 | 0.6 |
| Ascitic fluid AFB/culture | -/- | Neg/Pos | Neg/Neg | Neg/Neg | Neg/Pos | Neg/Neg | Neg/Neg | Pos/Neg | Neg/Neg |
| Abdominal USG | Minimal ascite | Ascites with septation | Ascites with septation | Ascites with septation | Ascites with septation | Ascites | Ascites with septation | Ascites with septation | Ascites with septation |
| LAP | Hepatomegaly | LAP | |||||||
| Laparoscopic/laparotomic appearance | Whitish tuberculi | Whitish tuberculi | - | - | Whitish tuberculi | Whitish tuberculi | Whitish tuberculi | Whitish tuberculi | Whitish tuberculi |
| Adhesions | Adhesions | Adhesions | |||||||
| Peritoneal histopathology | Caseating granuloma | Caseating granuloma | - | - | Caseating granuloma | Caseating granuloma | Caseating granuloma | Caseating granuloma | Caseating granuloma |
| Outcome | Alive | Alive | Alive | Alive | Alive | Alive | Alive | Alive | Alive |
-: Not obtained; BCG: Bacillus calmette-guerin vaccination; TST: Tuberculin skin test; SAAG: Serum-ascite albumin gradient; ADA: Adenosine deaminase activity; AFB: Acid fast bacilli; USG: Ultrasonography; LAP: Lymphadenopathy.
At admission, only four of the patients had two Bacillus Calmette-Guerin (BCG) scars. The tuberculin skin tests (TST) were positive in 6 patients with an induration ranging from 10-22 mm. Five cases had concomitant thoracic involvement (Table 1). Three patients had pleural effusion (Figure 1), one had parenchymal consolidation, and one (patient 4) had both of them. The most common abdominal ultrasonography (USG) findings were ascites with septation (found in 7 patients). Two had only ascites without septation (patients 1 and 6), one had hepatomegaly (patient 8) and two had intra-abdominal lymphadenopathies (patients 4 and 9) as well as ascites. In one patient (patient 1), peritoneal fluid could not be obtained. This patient had been previously operated on because of intestinal obstruction in another center, where they had seen multiple adhesions and whitish tuberculi on peritoneum. The patient was then referred to our center.
Figure 1.
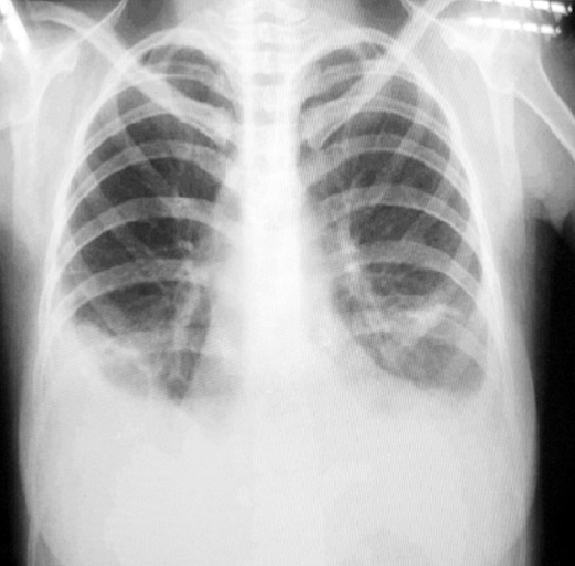
Chest roentgenogram showing pleural effusion on both sides (patient 5).
Ascitic fluid analysis was performed in 8 patients. All of the ascitic fluids were exudative, and the serum-ascite albumin gradient was less than 1.1 g/dL in all of them. Laboratory findings of serum and ascitic fluid are shown in Table 2. Direct examination of ascitic fluids revealed a predominance of lymphocytes. Acid fast bacilli (AFB) only found in one patient (patient 8). Positive cultures for M. tuberculosis were present in two patients. Three patients showed high adenosine deaminase activity (ADA) in their ascitic fluid. The clinical features of patients are shown in Table 1.
Table 2.
Laboratory findings of the patients at presentation
| Parameter | Mean ± SD (range) |
| Hemoglobin (g/dL) | 11.2 ± 1.3 (9.9-13) |
| White cell count (1012/L) | 6.4 ± 1.9 (4.1-9.0) |
| Erythrocyte sedimentation rate (mm/h) | 42.3 ± 21.0 (10-72) |
| C-reactive protein (g/dL) | 79.6 ± 63.7 (21-203) |
| Serum total protein (g/dL) | 7.4 ± 0.7 (6.5-8.5) |
| Serum albumin (g/dL) | 3.2 ± 0.5 (2.4-3.9) |
| Serum/ascites albumin gradient (g/dL) | 0.6 ± 0.2 (0.3-1.0) |
| Ascites LDH (IU/L) | 746.8 ± 327.1 (366-1331) |
| Ascites total protein (g/dL) | 5.2 ± 0.6 (4.7-6.0) |
| Ascites albumin (g/dL) | 2.6 ± 0.5 (1.9-3.4) |
| Ascites glucose (mg/dL) | 71.7 ± 15.1 (49-89) |
| Ascites polymorphonuclear leukocyte (per mm3) | 1915 ± 1578 (520-5200) |
| Ascites lymphocyte (per mm3) | 3135 ± 3165 (602-9400) |
LDH: Lactate dehydrogenase.
Laparoscopy was performed on 6 patients and laparotomy on one, and whitish tubercles were the most common findings in all. In three of the cases, adhesions were also seen. All biopsy samples obtained from 7 patients revealed caseating granuloma (Figure 2). The diagnosis of peritoneal tuberculosis was confirmed histo-pathologically in 7 patients, and two of these patients were also proved microbiologically. The remaining 2 patients had been diagnosed by ascitic fluid diagnostic features and a positive response to antituberculous treatment.
Figure 2.
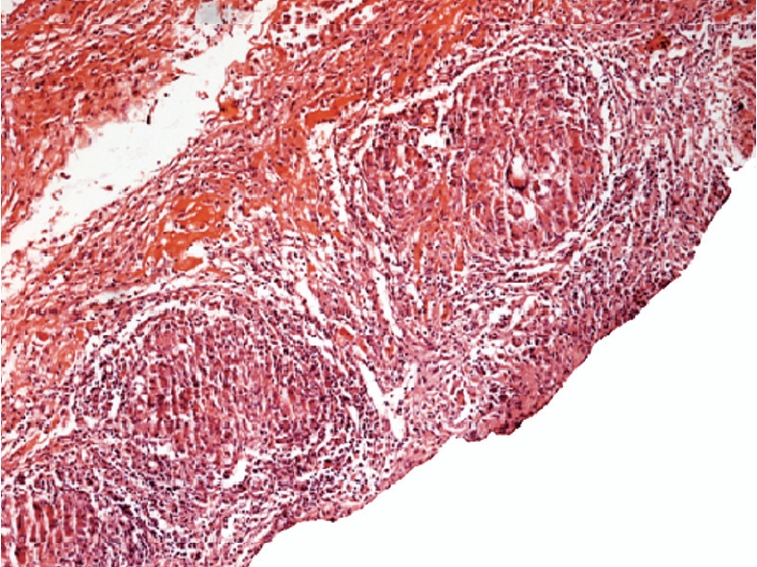
Histological appearance of caseating granuloma (patient 5, haematoxylin and eosin stain, original magnification × 10).
All patients completed the 9 mo course of therapy and recovered without any complications. Patients were followed for 6 to 15 mo after the end of therapy and all were in good health.
DISCUSSION
Tuberculous infection is still a significant cause of morbidity and mortality in the world. It is estimated that the incidence of TBP among all forms of TB varies from 0.1 % to 0.7 % worldwide and it is seen most commonly in patients between 35 and 45 years of age[4]. It is seen in children with a lower frequency. Recently, Forssbohm et al[6] reported that in Germany, only 5% of peritoneal TB cases were children under 14 years old. In the US, peritoneal TB accounts 0.3% of all TB cases in children less than 20 years and median age in children was 13 years[7]. Our review showed that TBP is also seen in lower frequency in our local area, the Black Sea region of Turkey. We have followed up only 9 children with TBP in a 5 year period. Mean age in this study was 14.2 years, in accordance with the literature. There was only one patient of 11 years old, the others were between 14-16 years. However, younger children with TBP have been reported in two studies from Turkey[8,9].
The initial symptoms of TBP cases are nonspecific, such as abdominal distention, pain, fever and weight loss and the occurrence of these events take a long time[5,8-10]. The clinical presentation of our patients was similar and ascites, causing abdominal distention was present in all cases. Constitutional symptoms of abdominal pain was seen in 55.5% of the patients, fever in 44.4%, and weight loss, night sweating and coughing were seen in 33.3% of the patients. One patient (patient 1) presented with small-bowel obstruction due to chronic peritoneal inflammation and adhesions. Intestinal obstruction developed in patients with TBP is also reported in the other studies[4,11-13].
Due to the nonspecific symptoms and physical findings, diagnosis is often delayed. The gold standard of diagnosis of TB in children is the triad of contact with a patient with active TB, positive TST and compatible physical and radiological findings. Clinicians must be aware of this serious health problem and inquire of familial history of TB. In our series, 66.7 % of patients have a contact history within the family and therefore we consider that family history is an important factor to take into account when making the diagnosis. In addition, TST is a helpful tool in diagnosis of TB and applied frequently. However, its diagnostic value in TBP can be variable. The frequency of TST positivity was higher (66.7%) in our study population than reported as 18%-27% in the other studies[8,14,15].
Chest radiographs are abnormal in 50-75% of patients with TBP[5]. It has been reported that 12%-63% of patients with TBP also had pleural effusion in different studies[8,16,17]. Similarly, in our study, 5 patients (55.5%) had thoracic involvement, 4 of them had pleural effusion and 2 had consolidation. In most studies, direct examination of AFB and culture positivity of the peritoneal fluid are rarely seen[2,9,14,18]. In the present study, ARB were seen in only one case (patient 8) and culture of peritoneal fluid was positive in two cases (patients 2 and 5).
Analysis of ascitic fluid often shows exudative features with lymphocytic predominance and serum-ascite albumin gradient lesser than 1.1 gr/dL[18]. The ascite samples acquired from eight patients in our study had lymphocytic predominance and low serum-ascite albumin gradient, therefore we also suggest that the ascitic fluid features mentioned above might be a good indicator for diagnosis.
High levels of ADA in the ascitic fluid has been shown to be compatible with the diagnosis of TBP with high sensitivity (100%) and specificity (97%), but the analysis of ADA activity is expensive and may not be available everywhere[18-20]. We were able to assess ADA activity in ascitic fluid in only three patients and their values were high.
Bacillus can reach the peritoneum through the gastrointestinal tract via mesenteric lymph nodes or directly from the blood[5]. The most common form of disease is wet peritonitis, both visceral and parietal peritoneal layers are affected with the formation of multiple tuberculous nodules and ascites[21]. Abdominal USG is a non-invasive and easy available method of detecting abdominal fluid and lymphadenopathy. So it can be used for the diagnosis of TBP as a first step investigation. The most specific sonographic findings of TBP are ascites with fine septations and lymphadenopathy with hypoechogenic centers indicating caseating necrosis[18]. Abdominal USG of the present patients revealed ascites as the most common findings in all patients, also with fine septations in 7 of them, intra-abdominal LAP was seen in two patients, and hepatomegaly in one.
A thickened peritoneum, whitish tubercles and adhesions are the most common appearance of TBP in laparoscopy or laparotomy. Besides specific appearance, these procedures allow us to take a peritoneal biopsy, which is the gold standard for diagnosis[22]. In our series, 6 patients had undergone laparoscopy and one had a laparotomy and whitish tubercles on peritoneum were seen in all and adhesions in two. This pathognomonic appearance was proved by the observation of caseating granuloma on the histology. No complications were encountered in our patients.
Some authors suggest that corticosteroid admini-stration combined with antituberculosis treatment can reduce the complications and morbidity rate, however, there is a controversy about the benefit[23-25]. In the present study, we added metilprednisolone to antituberculosis therapy in four patients, with positive results.
In conclusion, TB is still a major cause of mortality and morbidity worldwide. Although TBP is uncommon in childhood, it needs to be considered in all patients presenting with ascites, particularly when it is accompanied by fever and abdominal pain. Due to its high fatality rate, if not diagnosed in time, early diagnosis is very important. Laparoscopy and peritoneal biopsy are still the most reliable, quick and safe methods for the diagnosis of TBP.
COMMENTS
Background
Tuberculous peritonitis is an uncommon presentation of tuberculosis in children. Its diagnosis is more difficult in children because of nonspecificity of symptoms and difficulty in confirming the diagnosis.
Research frontiers
Tuberculous peritonitis is seen most commonly in adults and seen in children with a lower frequency. Due to the insidious nature of the manifestations, diagnosis is often delayed and the rate of complications and mortality increases. Chronic abdominal complaints, ascidic fluid investigations and laparoscopy are important in diagnosis.
Innovations and breakthroughs
Abdominal distention due to ascites was present in all of our cases and fever and abdominal pain were seen in almost half of them. In addition, serum-ascite albumin gradient less than 1.1 gr/dL, laparoscopic and histological findings were important in the diagnosis.
Applications
Although the clinical signs and symptoms suggest the disease, the laparoscopy and biopsy are still the most reliable methods for the definite diagnosis of tuberculous peritonitis and they are easy to apply.
Terminology
Low serum-ascite albumin gradient (< 1.1 gr/dL) is a better distinguishing marker for separating ascites related to non-portal hypertension origin from ascites with portal hypertension.
Peer review
Tuberculosis peritonitis (TP) even if it is rare in developed countries; it remains frequent in developing countries. This retrospective study includes 9 cases. Even if the number of cases is small, it is necessary to stay in touch with this disease since it still affects children. This study is well done even though is retrospective. The different observations are well documented. This study confirms that, regardless of enormous progress in imagery, laparoscopy is still the most reliable method for the diagnosis of TP.
Footnotes
Peer reviewer: Abdellah Essaid, Professor, Hospital Ibn Sina, Rabat 10100, Morocco
S- Editor Li LF L- Editor Stewart GJ E- Editor Lin YP
References
- 1.Lazarus AA, Thilagar B. Abdominal tuberculosis. Dis Mon. 2007;53:32–38. doi: 10.1016/j.disamonth.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Demir K, Okten A, Kaymakoglu S, Dincer D, Besisik F, Cevikbas U, Ozdil S, Bostas G, Mungan Z, Cakaloglu Y. Tuberculous peritonitis--reports of 26 cases, detailing diagnostic and therapeutic problems. Eur J Gastroenterol Hepatol. 2001;13:581–585. doi: 10.1097/00042737-200105000-00019. [DOI] [PubMed] [Google Scholar]
- 3.Sochocky S. Tuberculous peritonitis. A review of 100 cases. Am Rev Respir Dis. 1967;95:398–401. doi: 10.1164/arrd.1967.95.3.398. [DOI] [PubMed] [Google Scholar]
- 4.Sanai FM, Bzeizi KI. Systematic review: tuberculous peritonitis--presenting features, diagnostic strategies and treatment. Aliment Pharmacol Ther. 2005;22:685–700. doi: 10.1111/j.1365-2036.2005.02645.x. [DOI] [PubMed] [Google Scholar]
- 5.Cruz AT, Starke JR. Clinical manifestations of tuberculosis in children. Paediatr Respir Rev. 2007;8:107–117. doi: 10.1016/j.prrv.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Forssbohm M, Zwahlen M, Loddenkemper R, Rieder HL. Demographic characteristics of patients with extrapulmonary tuberculosis in Germany. Eur Respir J. 2008;31:99–105. doi: 10.1183/09031936.00020607. [DOI] [PubMed] [Google Scholar]
- 7.Starke JS, Smith KC. Tuberculosis. In: Feigin RD, Cherry JD, Demmler GD, Kaplan SL, eds , et al., editors. Textbook of Pediatric Infectious Diseases. 5th ed. Philadelphia: Saunders; 2004. pp. 1337–1379. [Google Scholar]
- 8.Tanrikulu AC, Aldemir M, Gurkan F, Suner A, Dagli CE, Ece A. Clinical review of tuberculous peritonitis in 39 patients in Diyarbakir, Turkey. J Gastroenterol Hepatol. 2005;20:906–909. doi: 10.1111/j.1440-1746.2005.03778.x. [DOI] [PubMed] [Google Scholar]
- 9.Gurkan F, Ozates M, Bosnak M, Dikici B, Bosnak V, TasMA , Haspolat K. Tuberculous peritonitis in 11 children: clinical features and diagnostic approach. Pediatr Int. 1999;41:510–513. doi: 10.1046/j.1442-200x.1999.01114.x. [DOI] [PubMed] [Google Scholar]
- 10.Maltezou HC, Spyridis P, Kafetzis DA. Extra-pulmonary tuberculosis in children. Arch Dis Child. 2000;83:342–346. doi: 10.1136/adc.83.4.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ozbey H, Tireli GA, Salman T. Abdominal tuberculosis in children. Eur J Pediatr Surg. 2003;13:116–119. doi: 10.1055/s-2003-39588. [DOI] [PubMed] [Google Scholar]
- 12.Akcakaya A, Sahin M, Coskun A, Demiray S. Comparison of mechanical bowel obstruction cases of intra-abdominal tumor and non-tumoral origin. World J Surg. 2006;30:1295–1299. doi: 10.1007/s00268-005-0440-z. [DOI] [PubMed] [Google Scholar]
- 13.Saczek KB, Schaaf HS, Voss M, Cotton MF, Moore SW. Diagnostic dilemmas in abdominal tuberculosis in children. Pediatr Surg Int. 2001;17:111–115. doi: 10.1007/s003830000455. [DOI] [PubMed] [Google Scholar]
- 14.Muneef MA, Memish Z, Mahmoud SA, Sadoon SA, Bannatyne R, Khan Y. Tuberculosis in the belly: a review of forty-six cases involving the gastrointestinal tract and peritoneum. Scand J Gastroenterol. 2001;36:528–532. doi: 10.1080/003655201750153412. [DOI] [PubMed] [Google Scholar]
- 15.Sotoudehmanesh R, Shirazian N, Asgari AA, Malekzadeh R. Tuberculous peritonitis in an endemic area. Dig Liver Dis. 2003;35:37–40. doi: 10.1016/s1590-8658(02)00010-5. [DOI] [PubMed] [Google Scholar]
- 16.Wang HK, Hsueh PR, Hung CC, Chang SC, Luh KT, Hsieh WC. Tuberculous peritonitis: analysis of 35 cases. J Microbiol Immunol Infect. 1998;31:113–118. [PubMed] [Google Scholar]
- 17.Uygur-Bayramicli O, Dabak G, Dabak R. A clinical dilemma: abdominal tuberculosis. World J Gastroenterol. 2003;9:1098–1101. doi: 10.3748/wjg.v9.i5.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rasheed S, Zinicola R, Watson D, Bajwa A, McDonald PJ. Intra-abdominal and gastrointestinal tuberculosis. Colorectal Dis. 2007;9:773–783. doi: 10.1111/j.1463-1318.2007.01337.x. [DOI] [PubMed] [Google Scholar]
- 19.Riquelme A, Calvo M, Salech F, Valderrama S, Pattillo A, Arellano M, Arrese M, Soza A, Viviani P, Letelier LM. Value of adenosine deaminase (ADA) in ascitic fluid for the diagnosis of tuberculous peritonitis: a meta-analysis. J Clin Gastroenterol. 2006;40:705–710. doi: 10.1097/00004836-200609000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Hillebrand DJ, Runyon BA, Yasmineh WG, Rynders GP. Ascitic fluid adenosine deaminase insensitivity in detecting tuberculous peritonitis in the United States. Hepatology. 1996;24:1408–1412. doi: 10.1002/hep.510240617. [DOI] [PubMed] [Google Scholar]
- 21.Aston NO. Abdominal tuberculosis. World J Surg. 1997;21:492–499. doi: 10.1007/pl00012275. [DOI] [PubMed] [Google Scholar]
- 22.Bedioui H, Ksantini R, Nouira K, Mekni A, Daghfous A, Chebbi F, Rebai W, Fteriche F, Jouini M, Kacem M, et al. Role of laparoscopic surgery in the etiologic diagnosis of exsudative ascites: a prospective study of 90 cases. Gastroenterol Clin Biol. 2007;31:1146–1149. doi: 10.1016/s0399-8320(07)78354-9. [DOI] [PubMed] [Google Scholar]
- 23.Alrajhi AA, Halim MA, al-Hokail A, Alrabiah F, al-Omran K. Corticosteroid treatment of peritoneal tuberculosis. Clin Infect Dis. 1998;27:52–56. doi: 10.1086/514627. [DOI] [PubMed] [Google Scholar]
- 24.Haas DW. Is adjunctive corticosteroid therapy indicated during tuberculous peritonitis? Clin Infect Dis. 1998;27:57–58. doi: 10.1086/514628. [DOI] [PubMed] [Google Scholar]
- 25.Bukharie H. Paradoxical response to anti-tuberculous drugs: resolution with corticosteroid therapy. Scand J Infect Dis. 2000;32:96–97. doi: 10.1080/00365540050164326. [DOI] [PubMed] [Google Scholar]


