Abstract
The author reports a very rare case of sporadic primary multiple extragastrointestinal stromal tumors (EGISTs) of the omentum associated with different mutations of the exon 11 of the c-kit gene in a 75-year-old man with gastric cancer. During an operation for the cancer, two solid tumors (10 mm and 8 mm) were found in the omentum. Both tumors consisted of cellular spindle cells. Mitotic figures were two and three per 50 high power fields. The tumor cells were positive for KIT, CD34 and vimentin, but negative for desmin, S100 protein, α-smooth muscle actin and p53 protein. Ki67 labeling was 2% and 3%. The larger EGIST showed a deletion of codons 552-558 of exon 11 of the c-kit gene, while the smaller EGIST had a point mutation at codon 559 (GTT←GAT) in exon 11 of the c-kit gene. Exons 9, 13, and 17 of the c-kit gene, and exons 12 and 18 of the platelet derived growth factor receptor α genes showed no mutations. The case shows that sporadic multiple EGISTs can occur in the omentum.
Keywords: Omentum, Extragastrointestinal stromal tumors, c-kit, Platelet derived growth factor receptor, CD34
INTRODUCTION
Gastrointestinal stromal tumors (GIST) were once thought to be smooth muscle or neurogenic tumors, but recent advances in the study of the c-kit gene and platelet derived growth factor receptor α (PDGFRA) gene have revealed that GIST are associated with gain-of-function mutations of the c-kit gene and the PDGFRA gene[1,2]. KIT is a transmembrane receptor tyrosine kinase whose ligand is stem cell factor[3]. GIST is believed to be derived from interstitial cells of Cajal (ICC) (pacemaker cells) which are present in the muscular layer of the gastrointestinal walls[3]. ICC expresses KIT protein (CD117) and CD34[3]. Immunohistochemical demonstration of KIT and/or CD34 is a hallmark in the pathological diagnosis of GIST[3].
Mesenchymal tumors resembling GIST and positive for KIT have been found in the soft tissue[4] and less frequently in abdominal organs such as the liver[5], gall bladder[6,7], pancreas[8,9], and serosa[10]. Such tumors are called extragastrointestinal stromal tumors (EGIST). The present study presents a case having two EGISTs in the greater omentum. According to Todoroki et al[11], 28 cases of EGISTs in the omentum have been reported in the literature. However, primary multiple GISTs in the omentum have been reported only once, by Kim et al[12]. The author here reports this case with a comparison with normal omentum.
CASE REPORT
A 75-year-old Japanese man complained of epigastralgia. The patient did not have neurofibromatosis, and denied any family history of mesenchymal tumors of the gastrointestinal organs. An endoscopic examination showed a depressed lesion of the stomach, and a biopsy showed well-differentiated adenocarcinoma. No tumor formations were noted by various imaging modalities including CT. A gastrectomy was performed, and during the operation two small solid tumors (10 mm and 8 mm in diameter, respectively) were found in the greater omentum. The both tumors were not attached to the gastrointestinal organs. Both tumors were diagnosed as EGISTs as described below, and treated by imatinib. No recurrence was seen one year after the operation.
Cases of EGIST were reviewed from 27659 surgical and biopsy specimens performed from 2000 to 2007, in my laboratory. As a result, thee cases of EGIST were found. One was a uterine EGIST, one was an omental EGIST, and one was a mesenteric EGIST. In this case report, the author shows a case of omental EGIST. The patient’s clinical records were obtained. As controls, three cases of normal omentum, which were resected in cases of ovarian cancer, were used. The materials were fixed in 10% formalin and embedded in paraffin. Several 3-μm sections were cut from each paraffin block, and stained with hematoxylin and eosin.
Immunohistochemical studies were performed by the DAKO’s envision method as previously described[13]. The antibodies used were KIT (polyclonal, Dako Corp, Glustrup, Denmark), CD34 (QBEND10, Dako), vimentin (Vim 3B4, Dako), desmin (D33, Dako), α smooth muscle actin (1A4, Dako), S100 protein (polyclonal, Dako), p53 protein (DO7, Dako), and Ki-67 antigen (MIB1, Dako).
Genetic analyses for the c-kit gene (exons 9, 11, 13 and 17) and the PDGFRA gene (exons 12 and 18) were performed by direct sequencing of PCR products. The exons of both genes were selected because they are frequent mutation sites[3]. The primers are shown in Table 1. In brief, genomic DNA was extracted from the paraffin blocks by proteinase K digestion and phenol/chloroform extraction, and subjected to PCR for 40 cycles (94°C for one minute, 52°C for one minute, and 72°C for one minute), using a thermal cycler (GeneAmp PCR system 9700, Applied Biosystems, ABI, CA). The annealing temperature was 53°C. The PCR products were then subjected to electrophoresis in a 2% agarose gel with ethidium bromide, and the PCR products were diluted 1:1 in loading buffer (94% formamide, 10mg bromphenol blue), denatured by heating at 98°C for three minutes, and snap frozen, before being electrophoresed on a polyacrylamide gel. The products were extracted and sequenced on an ABI PRIZM 3100 Genetic analyzer (Applied Biosystems, ABI, CA).
Table 1.
Primer sequences
| Forward | Reverse |
| c-kit exon 9 | |
| 5’-TCCTAGAGTAAGCCAGGGCTT-3’ | 5’-TGGTAGACAGAGCCTAAACATCC-3’ |
| c-kit exon11 | |
| 5’-GATCTATTTTTCCCTTTCTC-3’ | 5’-AGCCCCTGTTTCATACTGAC-3’ |
| c-kit exon 13 | |
| 5’-GCTTGACATCAGTTTGCCAG-3’ | 5’-AAAGGCAGCTTGGACACGGCTTTA-3’ |
| c-kit exon 17 | |
| 5’-CTCCTCCAACCTAATAGTGT-3’ | 5’-GTCAAGCAGAGAATGGGTAC-3’ |
| PDGFRA exon12 | |
| 5’-TTGGATATTCACCAGTTACCTGTC-3’ | 5’-CAAGGGAAAAGCTCTTGG-3’ |
| PDGFRA exon 18 | |
| 5’-ACCATGGATCAGCCAGTCTT-3’ | 5’-TGAAGGAGGATGAGCCTGACC-3’ |
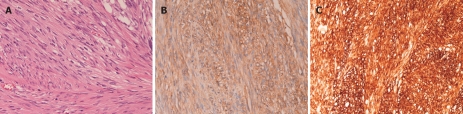
Histologically, the both tumors showed almost the same morphologies. Both tumors consisted of cellular spindle cells (Figure 1A). Mitotic figures were two in the smaller tumor and three in larger tumor, per 50 high power field (HPF). No epithelioid pattern was recognized and no necrotic areas were present. Immunohistochemically, the tumor cells of both tumors were strongly positive for KIT (Figure 1B), CD34 (Figure 1C) and vimentin, but negative for desmin, S100 protein, α-smooth muscle actin and p53 protein. Ki67 labeling was 2% in the smaller tumor and 3% in the larger tumor. Both tumors were diagnosed as EGIST. The normal omentum showed scattered mesenchymal cells positive for KIT and CD34 in the surface of the omentum. The stomach lesion was an early well-differentiated adenocarcinoma confined to the mucosa. The stomach showed no GISTs.
Figure 1.
Histological and immunohistochemical findings of the tumors. A: Histology of the tumor. The tumor is composed of cellular spindle cells. Hematoxylin and eosin (× 200). B: KIT immunostaining of the tumor. KIT is diffusely and strongly positive in the tumor cells (× 200). C: CD34 immunostaining of the tumor. CD34 is diffusely and strongly positive in the tumor cells (× 200).
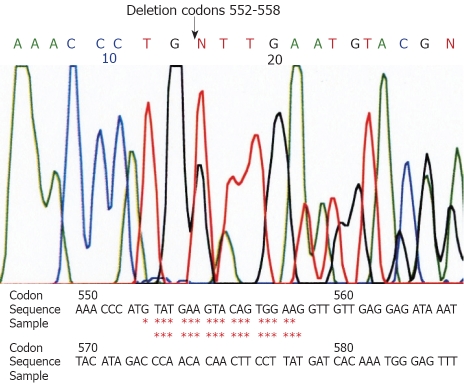
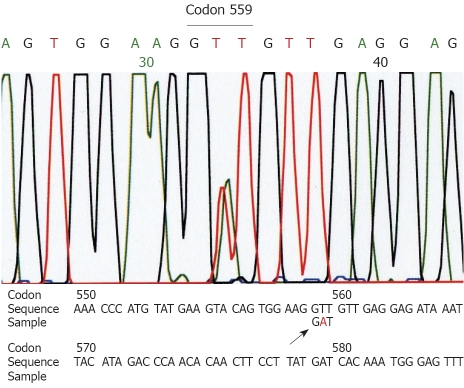
Genetic analyses for the c-kit gene (exons 9, 11, 13 and 17) and the PDGFRA (exons 12 and 18) showed a deletion at codon 552-558 of exon 11 of the c-kit gene in the larger tumor (Figure 2). The small tumor showed a point mutation (GTT→GAT) at codon 559 of exon 11 of the c-kit gene (Figure 3). Other exons of the c-kit gene showed no abnormalities. Exons 12 and 18 of the PDGFRA gene also showed no abnormalities in both tumors. The normal omentum showed no abnormalities in either of the two genes.
Figure 2.
Computer analysis of a part of exon 11 of the c-kit gene in the larger tumor. Deletion of codons 552-558 is noted. Asterisks show the deletion.
Figure 3.
Computer analysis of a part of exon 11 of the c-kit gene in the smaller tumor. A mutation at codon 559 (GTT→GAT) is recognized (arrow).
DISCUSSION
According to Todoroki et al[11], there are 28 cases of EGIST of the omentum in the literature. Most of them are single EGIST[10,11], and multiple EGISTs of the omentum have been reported only once[12]. The present study reports a second case of primary multiple EGIST of the omentum. The diagnosis of EGIST of the present case was conclusive. The present study showed scattered KIT-positive ICC-like cells in surface of the omentum. Sakurai et al[14] also reported KIT-positive ICC-like mesenchymal cells in the omentum. Therefore, the present case may be derived from the ICC-like cells physiologically present in the omentum.
GIST is thought to be a potentially malignant tumor[3]. The malignant potential has been accessed by various parameters, such as tumor size, mitotic figures, necrosis, cell type, and Ki-67 labeling[15-17]. However, the parameters and the results varied from one observer to another. For example, Hasegawa et al[17] reported that tumor size of less than 5 cm was low risk, 5-10 cm posed an intermediate risk, and more than 10 cm posed a high risk. The present case is probably low risk due to the tumor size. GIST with mitotic figures less than 5 per 50 high power fields are low risk, 5-10 are intermediate risk, and more than 10 are high risk. The present case is low risk according to the mitotic count. The presence of necrosis is a high risk for malignancy, therefore the absence of necrosis in the present case also suggests it is low risk. Ki-67 labeling is also related to the GIST risk. In the present study, the Ki-67 was 2% and 3%, respectively, indicating low risk. Taken together, these data suggest that the present EGIST case has a low risk of malignancy.
The present study examined the c-kit and PDGFRA genes and found that the two EGISTs of the omentum had different mutations in exon 11 of the c-kit gene. It has been found that mutations ratio of the c-kit gene is 80% in exon 11, 15 % in exon 9, and less than 2% in exons 13 and 17[3]. Mutations ratio of the PDGFRA gene is 10% in exon 18 and less than 2% in exons 12 and 14[3]. Therefore, the present case showed a common mutation pattern. In addition, the present case shows that the two EGISTs are not identical tumors, but individual tumors with different pathogenesis.
Imatinib has been developed to treat GIST[3]. The present case also used Imanitib. Imanitib is effective in GIST cases with c-kit mutations in exon 11[3] and in present case it may have been be effective, because neither recurrence nor metastasis has been seen one year after the operation.
In summary, the author showed a very rare case of primary multiple EGISTs of the omentum with different mutations in exon 11 of the c-kit gene.
Footnotes
Peer reviewer: Jiro Fujimoto, Professor, First Department of Surgery, Hyogo College of Medicine, 1-1 Mukogawa-cho, Nishinomiya, Hyogo 663-8501, Japan
S- Editor Li JL L- Editor Stewart GJ E- Editor Lin YP
References
- 1.Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 2.Hirota S, Ohashi A, Nishida T, Isozaki K, Kinoshita K, Shinomura Y, Kitamura Y. Gain-of-function mutations of platelet-derived growth factor receptor alpha gene in gastrointestinal stromal tumors. Gastroenterology. 2003;125:660–667. doi: 10.1016/s0016-5085(03)01046-1. [DOI] [PubMed] [Google Scholar]
- 3.Hirota S, Isozaki K. Pathology of gastrointestinal stromal tumors. Pathol Int. 2006;56:1–9. doi: 10.1111/j.1440-1827.2006.01924.x. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto H, Oda Y, Kawaguchi K, Nakamura N, Takahira T, Tamiya S, Saito T, Oshiro Y, Ohta M, Yao T, et al. c-kit and PDGFRA mutations in extragastrointestinal stromal tumor (gastrointestinal stromal tumor of the soft tissue) Am J Surg Pathol. 2004;28:479–488. doi: 10.1097/00000478-200404000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Hu X, Forster J, Damjanov I. Primary malignant gastrointestinal stromal tumor of the liver. Arch Pathol Lab Med. 2003;127:1606–1608. doi: 10.5858/2003-127-1606-PMGSTO. [DOI] [PubMed] [Google Scholar]
- 6.Ortiz-Hidalgo C, de Leon Bojorge B, Albores-Saavedra J. Stromal tumor of the gallbladder with phenotype of interstitial cells of Cajal: a previously unrecognized neoplasm. Am J Surg Pathol. 2000;24:1420–1423. doi: 10.1097/00000478-200010000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Mendoza-Marin M, Hoang MP, Albores-Saavedra J. Malignant stromal tumor of the gallbladder with interstitial cells of Cajal phenotype. Arch Pathol Lab Med. 2002;126:481–483. doi: 10.5858/2002-126-0481-MSTOTG. [DOI] [PubMed] [Google Scholar]
- 8.Yamaura K, Kato K, Miyazawa M, Haba Y, Muramatsu A, Miyata K, Koide N. Stromal tumor of the pancreas with expression of c-kit protein: report of a case. J Gastroenterol Hepatol. 2004;19:467–470. doi: 10.1111/j.1440-1746.2003.02891.x. [DOI] [PubMed] [Google Scholar]
- 9.Daum O, Klecka J, Ferda J, Treska V, Vanecek T, Sima R, Mukensnabl P, Michal M. Gastrointestinal stromal tumor of the pancreas: case report with documentation of KIT gene mutation. Virchows Arch. 2005;446:470–472. doi: 10.1007/s00428-004-1200-4. [DOI] [PubMed] [Google Scholar]
- 10.Miettinen M, Monihan JM, Sarlomo-Rikala M, Kovatich AJ, Carr NJ, Emory TS, Sobin LH. Gastrointestinal stromal tumors/smooth muscle tumors (GISTs) primary in the omentum and mesentery: clinicopathologic and immunohistochemical study of 26 cases. Am J Surg Pathol. 1999;23:1109–1118. doi: 10.1097/00000478-199909000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Todoroki T, Sano T, Yamada S, Hirahara N, Toda N, Tsukada K, Motojima R, Motojima T. Clear cell carcinoid tumor of the distal common bile duct. World J Surg Oncol. 2007;5:6. doi: 10.1186/1477-7819-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JH, Boo YJ, Jung CW, Park SS, Kim SJ, Mok YJ, Kim SD, Chae YS, Kim CS. Multiple malignant extragastrointestinal stromal tumors of the greater omentum and results of immunohistochemistry and mutation analysis: a case report. World J Gastroenterol. 2007;13:3392–3395. doi: 10.3748/wjg.v13.i24.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terada T, Kawaguchi M, Furukawa K, Sekido Y, Osamura Y. Minute mixed ductal-endocrine carcinoma of the pancreas with predominant intraductal growth. Pathol Int. 2002;52:740–746. doi: 10.1046/j.1440-1827.2002.01416.x. [DOI] [PubMed] [Google Scholar]
- 14.Sakurai S, Hishima T, Takazawa Y, Sano T, Nakajima T, Saito K, Morinaga S, Fukayama M. Gastrointestinal stromal tumors and KIT-positive mesenchymal cells in the omentum. Pathol Int. 2001;51:524–531. doi: 10.1046/j.1440-1827.2001.01224.x. [DOI] [PubMed] [Google Scholar]
- 15.Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29:52–68. doi: 10.1097/01.pas.0000146010.92933.de. [DOI] [PubMed] [Google Scholar]
- 16.Singer S, Rubin BP, Lux ML, Chen CJ, Demetri GD, Fletcher CD, Fletcher JA. Prognostic value of KIT mutation type, mitotic activity, and histologic subtype in gastrointestinal stromal tumors. J Clin Oncol. 2002;20:3898–3905. doi: 10.1200/JCO.2002.03.095. [DOI] [PubMed] [Google Scholar]
- 17.Hasegawa T, Matsuno Y, Shimoda T, Hirohashi S. Gastrointestinal stromal tumor: consistent CD117 immunostaining for diagnosis, and prognostic classification based on tumor size and MIB-1 grade. Hum Pathol. 2002;33:669–676. doi: 10.1053/hupa.2002.124116. [DOI] [PubMed] [Google Scholar]