Abstract
The clinical characteristics of undifferentiated (embryonal) sarcoma of the liver (UESL) were investigated and the best treatment modalities were recommended. Both histology and immuno-histochemistry demonstrated the cellular features of this peculiar tumor. The tumor size was 12 cm × 9 cm × 8 cm in the right liver lobe. The patient underwent surgical resection of the tumor. The postoperative recovery was uneventful and she died eight months after diagnosis. The tumor showed mixed spindle and polygonal cells within the myxoid matrix. Some tumor cells contained eosinophilic hyaline globules that were positive for resistant diastase. Immunohistochemistry showed positive vimentin. Stellate and spindle cells were positively stained with alpha-1-antichymotrypsin (AACT) and CD68. This case indicates that UESL is not obviously differentiated in old-aged adults.
Keywords: Undifferentiated (embryonal) sarcoma, Old-aged adult, Immunohistochemistry, Liver tumor
INTRODUCTION
Undifferentiated (embryonal) sarcoma of the liver (UESL), first documented in 1978, is a rare and highly malignant hepatic neoplasm of mesenchymal origin and shows a divergent differentiation[1,2]. Although UESL is considered a relatively major entity in pediatric liver malignancies, its frequency in the adult population is extremely low. In fact, few reports have focused on the general features of adult cases[3]. Given that the majority of the populations are under the age of 30 years, adult cases over the age of 40 years are quite exceptional. Furthermore, the detailed pathological characteristics of adult cases based on particular immunohistochemistry are not yet clear. To our knowledge, no study has described the systemic pathology features of UESL. In the present study, we describe a UESL patient at the age of 61 years with no obvious mesenchymal differentiation such as smooth muscle phenotype.
CASE REPORT
A 61-year-old woman was admitted in November 2007 to the Department of Hepatobiliary Surgery because of a huge abdominal mass, located in the right hypochondrium. Her major complaint was abdominal pain. An abdominal ultrasound showed a lesion in the right liver lobe, which was avascular on angiographic examination, and subsequently confirmed by CT scan (Figure 1). However, her serum alpha-fetoprotein (AFP) level was normal. No pulmonary metastases were detected by chest X-ray examination. Medical and family histories were unremarkable, and the patient reported no contact with any hepatitis virus carrier. Right hepatic lobectomy and cholecystectomy were performed at a laparatomy. The postoperative period was uneventful, no adjuvant irradiation or chemotherapy was administered. The patient died after 8 mo.
Figure 1.
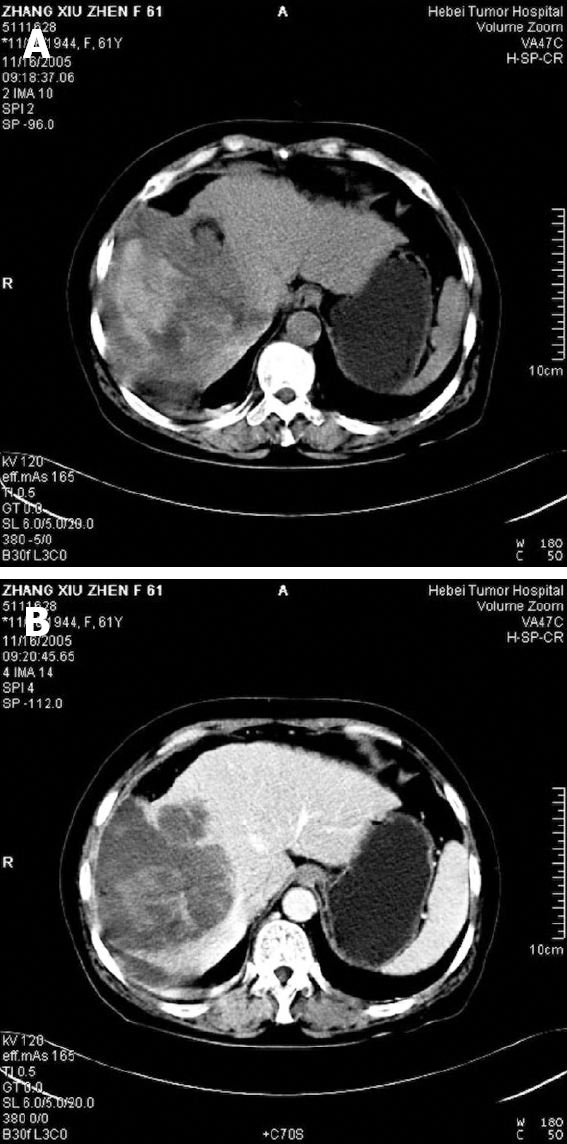
CT scan befor surgery showing a large mass (A) and a well-circumscribed and lowly-attenuated mass (B) in the right liver lobe.
A mass measuring 12 cm × 9 cm × 8 cm was found in the right liver lobe. The tumor was well circumscribed and demarcated from the normal liver tissue. The cut surface revealed a white or yellowish soft mass with massive necrosis, hemorrhage and degeneration.
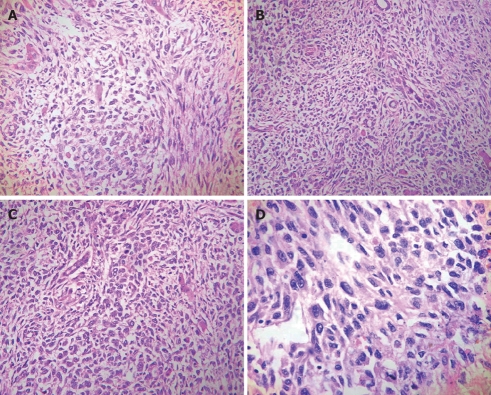
For light microscopy, fresh tissue was fixed in 10% formalin and embedded in paraffin. The tissue was cut into 4-μm thick sections which were stained with hematoxylin and eosin and periodic acid-Schiff (PAS) with and without diastase. The histopathological features of the neoplasm are presented in Figures 2 and 3. The primary tumor showed proliferation of atypical spindle cells mixed with stellated cells. These cells had obvious heteromorphism and were irregularly arranged. In some areas, the cells were differentiated into epithelium and had light eosinophilic cytoplasm. Cell membrane was fuzzy (Figure 2A). Some tumor cells contained eosinophilic hyaline globules, which were resistant to diastase and positive for PAS (Figure 2D, Figure 3B). Some ducts lined by a single layer of cuboidal cells resembling normal bile ducts were found in the tumor periphery (Figure 2B and C). Lymphocytes and plasma cells infiltrated around many thin-walled vessels in the background component of the tumor.
Figure 2.
UESL. A: Atypical spindle cells mixed with stellated cells (HE, × 200); B: Normal bile ducts in the tumor (HE, × 200); C: Normal hepatic cells in the tumor (HE, × 200); D: Giant cells containing eosinophilic hyaline globules in the cytoplasm (HE, × 400).
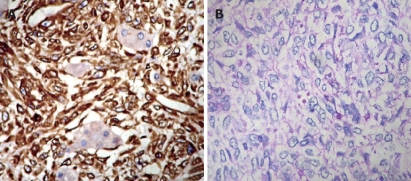
Figure 3.
Immunohistochemical expression of UESL. A: Strong reactivity for vimentin in spindle and polygonal or round cells (streptavidin-biotin method, × 200); B: Tumor cells containing eosinophilic hyaline globules with positive PAS.
Immunohistochemical and alkaline phosphatase staining was performed using a labeled streptavidin biotin system. Primary antibodies such as broad-spectrum vimentin, desmin, alpha-smooth muscle actin (SMA), muscle-specific actin, neuronspecific enolase (NSE), HMB-45, CD68, CD34, factor VIII, epithelial membrane antigen (EMA) and cytokeratins AE1/AE3 (Dako, Kyoto, Japan) were used. Immunostained sections were graded using a sliding scale of 1+ to 4+ according to the percentage of reactive cells (-: negative; 1+: < 10%; 2+: 10%-25%; 3+: > 25%-50%; 4+: > 50%). The immunohistochemical findings are summarized in Table 1. Most tumor cells were strongly reactive with vimentin (Figure 3A). Some stellate and spindle cells showed a granular cytoplasmic positivity for AACT and CD68. Lysozyme, alpha-sarcomeric actin, myoglobin, SMA, NSE, neurofilament, S-100 protein, HMB-45, CD34, factor VIII, EMA, AE1/AE3 and AFP were negatively stained. The tumor was diagnosed as an undifferentiated embryonal sarcoma (malignant mesenchymoma).
Table 1.
The immunohistochemical findings
| Antibody | Expression |
| Vimentin | 4+ |
| AACT | 1+ |
| CD68 | 2+ |
| Lysozyme | 1+ |
| α-sarcomeric actin | - |
| Myoglobin | - |
| SMA | - |
| NSE | - |
| Factor VIII | - |
| EMA | - |
| AE1/AE3 | - |
| AFP | - |
| HMB-45 | - |
| S-100 | - |
| CD34 | - |
DISCUSSION
UESL has a predilection for children and young adults in the first 2 decades of their life. To our knowledge, few cases have been reported in patients over the age of 40 years[3]. The overall clinical outcome is poor with a long-term disease-free survival rate of less than 30% in all series. In 2 other series, the outcome was better[4]. In the first series of 9 patients, 4 were alive with no evidence of disease, whereas in the second series, 3 out of 4 patients were alive with no evidence of disease[4]. In all series, the long-term survival was achieved only in those whose tumors could be resected completely.
According to the literature, the principal pathological features of UESL in children include an expansive intrahepatic growth with massive necrosis, hemorrhage, and occasional gelatinous appearance[1,2]. Laboratory evaluations including alfa-fetoprotein showed no specific abnormality. Radiologic characteristics of USL are characterized by enhanced peripheral rim, some solid portions at the periphery or adjacent to the septa, and discrepancy in internal architecture between computed tomography (CT) and ultrasound scan[5].
UESL is histologically surrounded by a fibrous pseudocapsule with a slightly direct invasion of the adjacent hepatic parenchyma. The cellular component is composed of medium-large spindle or satellite cells with marked nuclear pleomorphism. At the periphery, trapped hepatocytes and abnormal bile duct cells are apparent[6]. In addition, there are prominent eosinophilic, PAS-positive, and diastase-resistant globules in the cytoplasm of tumor cells[1,7]. Generally, immunohistochemistry using various cell-specific markers reveals that UESL is highly positive for histiocytic markers such as AACT and CD68, and vimentin is commonly positive. A few case reports demonstrate that tumor cells are reactive to cytokeratin, S-100 protein, and NSE[1,7,8-11]. In our case, both vimentin and histiocytic markers were positive, whereas cytokeratins, S-100 protein, and NSE were negative. In some cases, the smooth muscle differentiation was confirmed by the positivity of desmin and alpha-SMA. However, our case did not exhibit any myogenic features. The differential diagnosis of UESL in old-aged adults includes malignant fibrous histiocytoma (MFH), leiomyosarcoma, liposarcoma, and angiomyolipoma (AML). MFH is characterized by fibroblastic, histiocytic, and pleomorphic tumor cells forming a storiform pattern. In the present study, the tumor was basically composed of primitive mesenchymal cells with a divergent differentiation toward fibroblastic, histiocytic lines. Although these cellular compositions are quite similar to those of MFH[7,12], it is possible to distinguish UESL from MFH on the basis of tissue pattern[13]. In our case, the diagnosis of leiomyosarcoma was inappropriate, because the tumor did not show any variable size of the foci and any fascicular pattern of the brightly eosinophilic spindle cells in hematoxylin, and SMA was negative. Lipoblastic differentiation has been suggested by some investigators on the basis of oil red O staining, and cells resembling lipoblasts seen at light microscopy[14]. However, no multivacuolated lipoblasts were observed in our case under light microscope, and the tumor cells were negative for S-100 protein. AML is characteristically composed of a varying heterogeneous mixture of 3 tissue components: blood vessels, smooth muscle cells, and adipose cells. However, the proportions of the 3 tissue components vary markedly from case to case and from area to area within the same tumor[15]. AML with spindle cells and pleomorphic features may be misdiagnosed as various sarcomas, including MFH, leiomyosarcoma, dedifferentiated liposarcoma, and undifferentiated sarcoma[16-20]. Recent studies indicate that HMB-45 is a promising marker for AML and can facilitate differentiation of this tumor from other lesions[20-22]. In our case, the tumor cells were negative for HMB-45. Whenever pathologists encounter spindle cell tumors in the liver, immunostaining with HMB-45 should be performed to distinguish AML from other neoplasms.
The prognosis of UESL is poor. Even after complete resection of the tumor, few UESL patients can achieve a long-term, disease-free survival. Our patient died 8 mo after the tumor resection. Since 1990, long-term survivors after multiagent chemotherapy have been reported and their outcome appears to have improved substantially over the last decades[23,24]. The chance of cure depends on radical resection and vigorous multiple approaches including chemotherapy[23,24]. We hold that multiagent chemotherapy after resection can significantly improve the survival and the identified pathologic changes may offer important clues to the pathogenesis and development of UESL and other pediatric mesenchymal tumors.
Footnotes
Supported by The Key Oncologic Subject Foundation of Hebei Province (No. 200552), China
Peer reviewer: Ravi S Chari, MD, Associate Professor, Division of Hepatobiliary Surgery and Liver Transplantation, Departments of Surgery and Cancer Biology, 1313 21st Avenue South, Suite 801 Oxford House, Vanderbilt University Medical Center, Nashville, TN 37232-4753, United States
S- Editor Li LF L- Editor Wang XL E- Editor Lin YP
References
- 1.Stocker JT, Ishak KG. Undifferentiated (embryonal) sarcoma of the liver: report of 31 cases. Cancer. 1978;42:336–348. doi: 10.1002/1097-0142(197807)42:1<336::aid-cncr2820420151>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 2.Lack EE, Schloo BL, Azumi N, Travis WD, Grier HE, Kozakewich HP. Undifferentiated (embryonal) sarcoma of the liver. Clinical and pathologic study of 16 cases with emphasis on immunohistochemical features. Am J Surg Pathol. 1991;15:1–16. [PubMed] [Google Scholar]
- 3.Psatha EA, Semelka RC, Fordham L, Firat Z, Woosley JT. Undifferentiated (embryonal) sarcoma of the liver (USL): MRI findings including dynamic gadolinium enhancement. Magn Reson Imaging. 2004;22:897–900. doi: 10.1016/j.mri.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Leuschner I, Schmidt D, Harms D. Undifferentiated sarcoma of the liver in childhood: morphology, flow cytometry, and literature review. Hum Pathol. 1990;21:68–76. doi: 10.1016/0046-8177(90)90077-i. [DOI] [PubMed] [Google Scholar]
- 5.Moon WK, Kim WS, Kim IO, Yeon KM, Yu IK, Choi BI, Han MC. Undifferentiated embryonal sarcoma of the liver: US and CT findings. Pediatr Radiol. 1994;24:500–503. doi: 10.1007/BF02015012. [DOI] [PubMed] [Google Scholar]
- 6.Walker NI, Horn MJ, Strong RW, Lynch SV, Cohen J, Ong TH, Harris OD. Undifferentiated (embryonal) sarcoma of the liver. Pathologic findings and long-term survival after complete surgical resection. Cancer. 1992;69:52–59. doi: 10.1002/1097-0142(19920101)69:1<52::aid-cncr2820690111>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 7.Aoyama C, Hachitanda Y, Sato JK, Said JW, Shimada H. Undifferentiated (embryonal) sarcoma of the liver. A tumor of uncertain histogenesis showing divergent differentiation. Am J Surg Pathol. 1991;15:615–624. doi: 10.1097/00000478-199107000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Miettinen M, Kahlos T. Undifferentiated (embryonal) sarcoma of the liver. Epithelial features as shown by immunohistochemical analysis and electron microscopic examination. Cancer. 1989;64:2096–2103. doi: 10.1002/1097-0142(19891115)64:10<2096::aid-cncr2820641021>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 9.Leuschner I, Schmidt D, Harms D. Undifferentiated sarcoma of the liver in childhood: morphology, flow cytometry, and literature review. Hum Pathol. 1990;21:68–76. doi: 10.1016/0046-8177(90)90077-i. [DOI] [PubMed] [Google Scholar]
- 10.Parham DM, Kelly DR, Donnelly WH, Douglass EC. Immunohistochemical and ultrastructural spectrum of hepatic sarcomas of childhood: evidence for a common histogenesis. Mod Pathol. 1991;4:648–653. [PubMed] [Google Scholar]
- 11.Weiss SW, Enzinger FM. Malignant fibrous histiocytoma: an analysis of 200 cases. Cancer. 1978;41:2250–2266. doi: 10.1002/1097-0142(197806)41:6<2250::aid-cncr2820410626>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 12.Keating S, Taylor GP. Undifferentiated (embryonal) sarcoma of the liver: ultrastructural and immunohistochemical similarities with malignant fibrous histiocytoma. Hum Pathol. 1985;16:693–699. doi: 10.1016/s0046-8177(85)80154-4. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto I, Oshiro Y, Fukuda T, Tsuneyoshi M. Pleomorphic leiomyosarcoma of the soft parts: a reassessment by histology and immunohistochemistry of pleomorphic soft tissue sarcomas. Oncol Rep. 1999;6:533–537. doi: 10.3892/or.6.3.533. [DOI] [PubMed] [Google Scholar]
- 14.Cozzutto C, De Bernardi B, Comelli A, Soave F. Malignant mesenchymoma of the liver in children: a clinicopathologic and ultrastructural study. Hum Pathol. 1981;12:481–485. doi: 10.1016/s0046-8177(81)80033-0. [DOI] [PubMed] [Google Scholar]
- 15.Nonomura A, Mizukami Y, Kadoya M, Matsui O, Shimizu K, Izumi R. Angiomyolipoma of the liver: Its clinical and pathological diversity. J Hepatobiliary Pancr Surg. 1996;3:122–132. [Google Scholar]
- 16.Kimura N, Kubota M, Nagura H. A hepatic tumor associated with bilateral renal angiomyolipomas: a variant of angiomyolipoma? Pathol Int. 1994;44:540–547. doi: 10.1111/j.1440-1827.1994.tb02605.x. [DOI] [PubMed] [Google Scholar]
- 17.Nonomura A, Mizukami Y, Muraoka K, Yajima M, Oda K. Angiomyolipoma of the liver with pleomorphic histological features. Histopathology. 1994;24:279–281. doi: 10.1111/j.1365-2559.1994.tb00524.x. [DOI] [PubMed] [Google Scholar]
- 18.Nonomura A, Mizukami Y, Shimizu K, Kadoya M, Matsui O. Angiomyolipoma mimicking true lipoma of the liver: report of two cases. Pathol Int. 1996;46:221–227. doi: 10.1111/j.1440-1827.1996.tb03602.x. [DOI] [PubMed] [Google Scholar]
- 19.Salisbury JR, Portmann BC. Oncocytic liver cell adenoma. Histopathology. 1987;11:533–539. doi: 10.1111/j.1365-2559.1987.tb02661.x. [DOI] [PubMed] [Google Scholar]
- 20.Tsui WM, Yuen AK, Ma KF, Tse CC. Hepatic angiomyolipo-mas with a deceptive trabecular pattern and HMB-45 reactivity. Histopathology. 1992;21:569–573. doi: 10.1111/j.1365-2559.1992.tb00446.x. [DOI] [PubMed] [Google Scholar]
- 21.Pea M, Bonetti F, Zamboni G, Martignoni G, Riva M, Colombari R, Mombello A, Bonzanini M, Scarpa A, Ghimenton C. Melanocyte-marker-HMB-45 is regularly expressed in angiomyolipoma of the kidney. Pathology. 1991;23:185–188. doi: 10.3109/00313029109063563. [DOI] [PubMed] [Google Scholar]
- 22.Weeks DA, Malott RL, Arnesen M, Zuppan C, Aitken D, Mierau G. Hepatic angiomyolipoma with striated granules and positivity with melanoma--specific antibody (HMB-45): a report of two cases. Ultrastruct Pathol. 1991;15:563–571. doi: 10.3109/01913129109016264. [DOI] [PubMed] [Google Scholar]
- 23.O'Sullivan MJ, Swanson PE, Knoll J, Taboada EM, Dehner LP. Undifferentiated embryonal sarcoma with unusual features arising within mesenchymal hamartoma of the liver: report of a case and review of the literature. Pediatr Dev Pathol. 2001;4:482–489. doi: 10.1007/s10024001-0047-9. [DOI] [PubMed] [Google Scholar]
- 24.Walker NI, Horn MJ, Strong RW, Lynch SV, Cohen J, Ong TH, Harris OD. Undifferentiated (embryonal) sarcoma of the liver. Pathologic findings and long-term survival after complete surgical resection. Cancer. 1992;69:52–59. doi: 10.1002/1097-0142(19920101)69:1<52::aid-cncr2820690111>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]