Abstract
Signal transducer and activator of transcription 4 (STAT4), a STAT family member, mediates interleukin 12 (IL12) signal transduction. IL12 is known to be related to calorie-restricted status. In the central nervous system, IL12 also enhances the production of nitric oxide (NO), which regulates food intake. In this study, the expression of neuronal NO synthase (Nos1), which is also related to food intake, was investigated in the hypothalamic areas of Stat4 knockout (KO) mice using nicotinamide adenine dinucleotide phosphate-diaphorase (NADPH-d) histochemistry, a marker for neurons expressing Nos1 enzyme. Western blots were also performed to evaluate Nos1 and Fos expression. Wild-type Balb/c (WT group, n=10 male) and Stat4 KO mice (Stat4 KO group, n=8 male) were used. The body weight and daily food intake in the WT group were 22.4±0.3 and 4.4 g per day, while those in the Stat4 KO group were 18.7±0.4 and 1.8 g per day, respectively. Stat4 mice had lower body weight and food intake than Balb/c mice. Optical intensities of NADPH-d-positive neurons in the paraventricular nucleus (PVN) and lateral hypothalamic area (LHA) of the Stat4 KO group were significantly higher than those of the WT group. Western blotting analysis revealed that the hypothalamic Nos1 and Fos expression of the Stat4 KO group was up-regulated, compared to that in the WT group. These results suggest that Stat4 may be related to the regulation of food intake and expression of Nos1 in the hypothalamus.
Keywords: Body weight, Food intake, Hypothalamus, Knockout mice, Nitric oxide synthase, Stat4
INTRODUCTION
The signal transducer and activator of transcription (STAT) family was first identified through biochemical purification of factors involved in interferon-regulated gene expression (Darnell et al., 1994, Darnell, 1997). The STAT family consists of seven members, STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B, and STAT6, which are genetically localized into three chromosomal regions (Copeland et al., 1995). The physiological role of each STAT protein can be examined through the study of "knockout (KO)" mice. Interleukin 12 (Il12)-mediated functions are impaired in Stat4 KO mice (Kaplan et al., 1996; Thierfelder et al., 1996). Il12 concentration in blood significantly increases in 30% calorie-restricted animal relative to control (Fenton et al., 2009). It is also known that Il12 activates macrophages and up-regulates nitric oxide (NO) production in response to infection (Reiss et al., 1996).
NO is made in many cells including neurons, endothelial cells, and certain immune system cells. NO is synthesized by three NO synthase (NOS) isoforms, named neuronal NOS (nNOS, NOS1), inducible NOS (iNOS, NOS2), and endothelial NOS (eNOS, NOS3) (Yang, 2001; Min, 2007). These enzymes generate NO from L-arginine and nicotinamide adenine dinucleotide phosphate (NADPH)-dependent reaction (Bredt, 1999). There is evidence that NO functions as a neurotransmitter and intracellular messenger in both the central and peripheral nervous systems (Dawson et al., 1992; Moncada, 1992). NO also plays an important role in mechanisms regulating feeding behaviors in animals under a number of conditions (Moncada et al., 1991). It has been shown that NO is positively correlated to increased food intake and body weight (Morley and Flood, 1991; Morley and Flood, 1992; Morley and Flood, 1994; Squadrito et al., 1994). However, the effects of Stat4 KO on food intake and Nos1 activity in mice have not been reported yet.
The objective of this study was to investigate the change of food intake and Nos1 expression in the hypothalamic regions of Stat4 KO mice. The expression of Nos1 was measured using NADPH-diaphorase (NADPH-d) histochemistry and Western blotting.
METHODS
Animals
Stat4 KO and Balb/c mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). Experimental procedures were performed in accordance with the animal care guidelines of the National Institute for Health (NIH) and the Korean Academy of Medical Sciences. All animals were housed in cages at room temperature (20~24℃) with a 12:12 h light-dark cycle. Water and food were made available ad libitum. Balb/c (WT group, n=10 male) and Stat4 KO (Stat4 KO group, n=8 male) mice were used after a 7-day adaptation period. Body weight and daily food intake of each mouse were measured.
NADPH-d histochemistry
Experimental animals were anesthetized and perfused with 0.05 M phosphate buffered saline (PBS, pH 7.4), followed by chilled 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. The brains were removed, postfixed in the same fixative for 2~3 h at 4℃, and incubated overnight in PBS containing 20% sucrose at 4℃. Coronal sections (40-µm in thickness) were prepared on a freezing microtome (Leica, Nuβloch, Germany). Sections were stained for NADPH-d activity as previously described (Scherer-Singler et al., 1983; Kim et al., 2000). In brief, free-floating sections were incubated at 37℃ for 60 min in 0.05 M PBS containing 0.3% Triton X-100, 0.1 mg/ml nitroblue tetrazolium (Sigma, MO, USA), and 0.1 mg/ml β-NADPH (Sigma). Sections were washed three times with 0.05 M PBS and mounted on gelatin-coated slides. The slides were air-dried overnight at room temperature, rinsed twice with distilled water, and dried again. Coverslips were mounted using permount solution. Brain sections were analyzed using the atlas by Paxinos and Watson. The staining intensities of sections stained specifically for NADPH-d were assessed quantitatively according to a microdensitometrical method based on optical density using an image analyzer (Multiscan, Fullerton, CA, USA) (Kim et al., 2000).
Western blotting
Western blotting was performed to confirm the up-regulation of Nos1 and Fos expression. Briefly, protein extracts were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a PVDF membrane. The membrane was incubated with the appropriate primary antibodies (Nos1 and Fos, 1:500, Santa Cruz, CA, USA) overnight at 4℃. Protein bands were then detected by incubation with horseradish peroxidase-conjugated antibodies, and immunoblotting signals were visualized by treatment with ECL reagent.
Statistical analysis
All data were presented as mean±SEM. Data from body weight, food intake, and NADPH-d histochemistry were analyzed by non-paired t-test using the statistical software SPSS (version 17.0; SPSS Inc., IL, USA). Differences were considered statistically significant at p<0.05.
RESULTS
Change of body weight and food intake
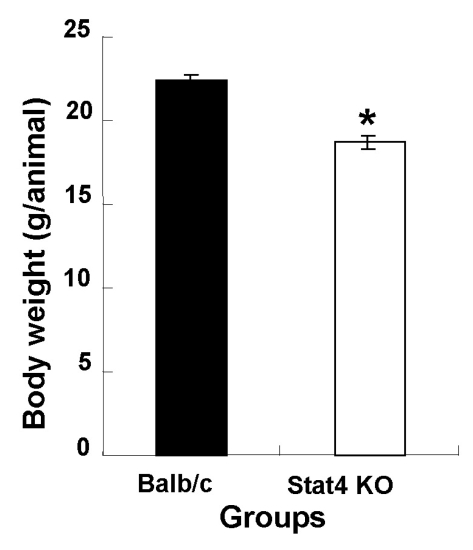
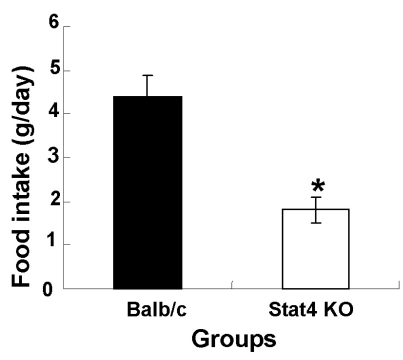
The average body weights of wild-type Balb/c (WT) and Stat4 KO mice were 22.4±0.3 and 18.7±0.4 g, respectively. Body weights in the Stat4 KO group were significantly lower than those of the WT group (Fig. 1). In Fig. 2, the daily food intake per animal in the WT and Stat4 KO groups was 4.4 and 1.8 g/day, respectively. The data showed that Stat4 KO mice consumed about 59% less food than WT mice.
Fig. 1.
Body weight in wild-type Balb/c and Stat4 knockout (KO) mice. Values are mean±SEM. *p<0.05 vs. Balb/c mice.
Fig. 2.
Daily food intake in wild-type Balb/c and Stat4 knockout (KO) mice. Values are mean±SEM. *p<0.05 vs. Balb/c mice.
Change of NADPH-d-positive neurons
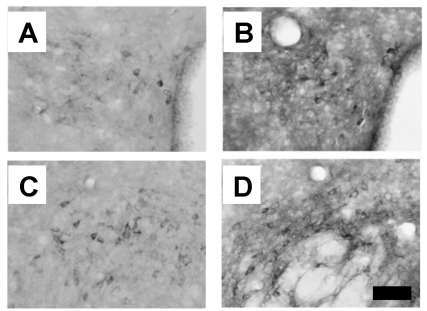
Staining intensities of NADPH-d-positive neurons in the paraventricular nucleus (PVN) and lateral hypothalamic area (LHA) of WT mice were noticeably lower than those of Stat4 KO mice (Fig. 3). In the PVN of WT mice, several stained neurons and fibers were found in the magnocellular part, and more such neurons and fibers were seen in the ventral part, while neurons of the medial area exhibited weaker reactions (Fig. 3A). The number of NADPH-d-positive cells in the PVN of Stat4 KO mice was significantly higher than in WT mice (Fig. 3B). In the LHA of WT mice, NADPH-d-positive cells and fibers were strongly stained, while medium-sized stained neurons and fibers were scattered over the general area of the LHA (Fig. 3C). The number of NADPH-d-positive cells in the LHA of the Stat4 KO group was also significantly higher than that in the WT group (Fig. 3D).
Fig. 3.
Distribution of NADPH-diaphorase-positive neurons in the hypothalamic regions. (A) The paraventricular nucleus of Balb/c mice (WT group). (B) The paraventricular nucleus of Stat4 knockout mice (Stat4 KO group). (C) The lateral hypothalamic area of the WT group. (D) The lateral hypothalamic area of the Stat4 KO group. Scale bar represents 80 µm.
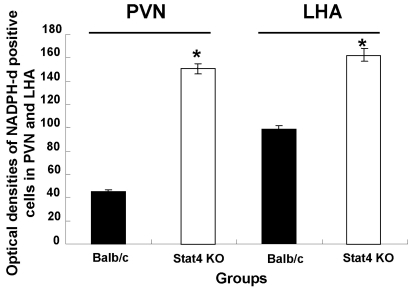
As shown in Fig. 4, the optical densities of NADPH-d-positive neurons in the PVN and LHA of Stat4 KO mice were significantly increased, compared to those of WT mice (p<0.05). In the PVN, the optical densities of NADPH-d-positive neurons of WT and Stat4 KO mice were 45.2±1.2 and 150.5±4.1, respectively. In the LHA, the optical densities of NADPH-d-positive neurons of WT and Stat4 KO mice were 96.6±2.2 and 159.6±5.2, respectively. These results indicate that WT and Stat4 KO mice have different patterns of NADPH-d-positive neurons in the hypothalamic areas.
Fig. 4.
Optical density of NADPH-diaphorase-positive neurons in the hypothalamic regions. Values are mean±SEM. NADPH-d, nicotinamide adenine dinucleotide phosphate-diaphorase; PVN, paraventricular nucleus; LHA, lateral hypothalamic area; Stat4 KO, Stat4 knockout mice. *p<0.05 vs. Balb/c mice.
Expression of Fos and Nos1 proteins
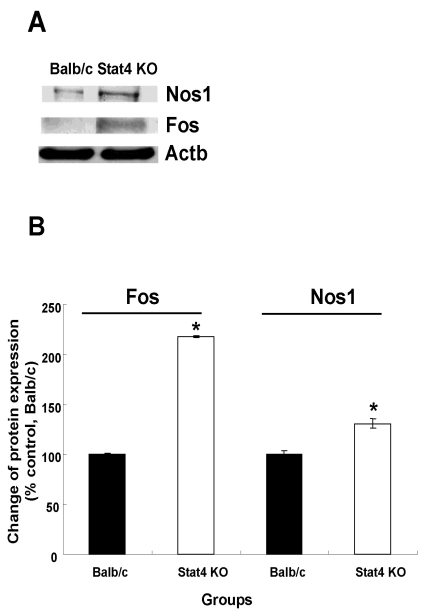
The Fos protein has been used as a marker of neuronal activation in the central nervous system (CNS). The expression levels of Fos protein in WT and Stat4 KO mice were 100±1.4 and 217.9±1.0% and those of Nos1 protein in WT and Stat4 KO mice were 100±3.8 and 130.9±4.7%, respectively (Fig. 5). The levels of Fos and Nos1 expression in the hypothalamic areas of the Stat4 KO group were significantly higher than those of the WT group.
Fig. 5.
Western blotting analysis of Nos1 and Fos immunoreactivity. (A) Immunoreactive bands on western blots. (B) Expression of Nos1 and Fos proteins was calculated by densitometer and normalized to that of Actb (internal control). Values are mean±SEM. Nos1, nitric oxide synthase 1, neuronal; Fos, FBJ osteosarcoma oncogene; Actb, actin, beta; Stat4 KO, Stat4 knockout mice. *p<0.05 vs. Balb/c mice.
DISCUSSION
Seven members of the STAT protein family have been identified in mammalian cells (Copeland et al., 1995). They play important roles in cytokine signaling in immunocompetent cells (Kaplan et al., 1996). Stat4 is involved in Il12 signaling and is expressed in T cells, B cells, natural killer cells, dendritic cells, and macrophages (Frucht et al., 2000). Stat4 activates some genes related to Th1 immune responses (Hoey et al., 2003). Raman et al. (2003) reported that the STAT4 signaling pathway regulates inflammation and airway physiology in allergic airway inflammation. Kataoka et al. (2005) demonstrated that Stat4 plays important roles in Th1 immune responses in the connective tissue-type mast cells in mice. Th1 immune responses are also related to food intake. Furthermore, Fenton et al. (2009) reported that Il12 concentration is closely associated with nutritional status in C57BL/6N mice. Reiss et al. (1996) also showed that Il12 up-regulates the production of NO in the CNS.
NO is also known to regulate molecules that affect food intake such as leptin, and there may be possible associations with the action of STAT4. However, changes of body weight and food intake have not been reported in Stat4 KO mice. Our results showed that the daily food intake of Stat4 KO mice was reduced to 59% of the WT mice. NO is synthesized by NOS in the brain and other mammalian tissues (Dawson et al., 1992; Moncada, 1992). NO is known to be an important regulator of food intake (Morley and Mattammal, 1996; Kataoka et al., 2005). Squadrito et al. (1993) demonstrated that obese Zucker rats exhibited a decrease in food intake and body weight when treated with a Nos inhibitor for 24 days. These results showed that NO plays a major role in the hyperphagia of obesity and that NO might be a physiological mediator in the mechanism controlling feeding behavior in obese Zucker rats. In the present study, the optical densities of NADPH-d-positive cells in the hypothalamic PVN and LHA of Stat4 KO mice were significantly increased, compared to WT mice. Since Stat4 mice showed lower body weight and food intake than WT mice, we expected the Nos1 expression to be decreased in the hypothalamus of Stat4 KO mice, but the result was opposite. Furthermore, the expressions of Nos1 and Fos protein were increased in the hypothalamus of Stat4 KO mice, compared to those in WT mice. It is possible that weight reduction in Stat4 KO mice and reduction of food intakes may originate from other factors related to satiety signals. Therefore, further studies on not only the immune function, but also the food intake regulation, in Stat4 KO mice are strongly needed. However, this marks the first report on Nos1 expression in Stat4 KO mice using NADPH-d histochemistry.
In conclusion, our results suggest that Stat4 may be related to the changes of body weight and food intake, and the hypothalamic Nos1 expression.
ACKNOWLEDGEMENTS
This work was supported by a grant from Kyung Hee University Post-doctoral Fellowship in 2009 (no. KHU-20090501).
ABBREVIATIONS
- KO
knockout
- LHA
lateral hypothalamic area
- NADPH-d
nicotinamide adenine dinucleotide phosphate-diaphorase
- NO
nitric oxide
- NOS
nitric oxide synthase
- PVN
paraventricular nucleus
- STAT4
signal transducer and activator of transcription 4
References
- 1.Bredt DS. Endogenous nitric oxide synthesis: biological functions and pathophysiology. Free Radic Res. 1999;31:577–596. doi: 10.1080/10715769900301161. [DOI] [PubMed] [Google Scholar]
- 2.Copeland NG, Gilbert DJ, Schindler C, Zhong Z, Wen Z, Darnell JE, Jr, Mui AL, Miyajima A, Quelle FW, Ihle JN, Jenkins NA. Distribution of the mammalian Stat gene family in mouse chromosomes. Genomics. 1995;29:225–228. doi: 10.1006/geno.1995.1235. [DOI] [PubMed] [Google Scholar]
- 3.Darnell JE., Jr STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 4.Darnell JE, Jr, Kerr IM, Stark GM. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 5.Dawson TM, Dawson VL, Snyder SH. A novel neuronal messenger molecule in brain: the free radical, nitric oxide. Ann Neurol. 1992;32:297–311. doi: 10.1002/ana.410320302. [DOI] [PubMed] [Google Scholar]
- 6.Fenton JI, Nuñez NP, Yakar S, Perkins SN, Hord NG, Hursting SD. Diet-induced adiposity alters the serum profile of inflammation in C57BL/6N mice as measured by antibody array. Diabetes Obes Metab. 2009;11:343–354. doi: 10.1111/j.1463-1326.2008.00974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frucht DM, Aringer M, Galon J, Danning C, Brown M, Fan S, Centola M, Wu CY, Yamada N, El Gabalawy H, O'Shea JJ. Stat4 is expressed in activated peripheral blood monocytes, dendritic cells, and macrophages at sites of Th1-mediated inflammation. J Immunol. 2000;164:4659–4664. doi: 10.4049/jimmunol.164.9.4659. [DOI] [PubMed] [Google Scholar]
- 8.Hoey T, Zhang S, Schmidt N, Yu Q, Ramchandani S, Xu X, Naeger LK, Sun YL, Kaplan MH. Distinct requirements for the naturally occurring splice forms Stat4alpha and Stat4beta in IL-12 responses. EMBO J. 2003;22:4237–4248. doi: 10.1093/emboj/cdg393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaplan MH, Sun YL, Hoey T, Grusby MJ. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature. 1996;382:174–177. doi: 10.1038/382174a0. [DOI] [PubMed] [Google Scholar]
- 10.Kataoka TR, Komazawa N, Morii E, Oboki K, Nakano T. Involvement of connective tissue-type mast cells in Th1 immune responses via Stat4 expression. Blood. 2005;105:1016–1020. doi: 10.1182/blood-2004-07-2811. [DOI] [PubMed] [Google Scholar]
- 11.Kim MJ, Kim Y, Choe BK, Kim SA, Lee HJ, Kim JW, Huh Y, Kim C, Chung JH. Differential expression of nicotinamide adenine dinucleotide phosphate-diaphorase in hypothalamic areas of obese Zucker rats. Neurosci Lett. 2000;292:60–62. doi: 10.1016/s0304-3940(00)01428-2. [DOI] [PubMed] [Google Scholar]
- 12.Min SS. Effects of nos inhibitors on arthritis and arthritic pain in rats. Korean J Physiol Pharmacol. 2007;11:253–257. [Google Scholar]
- 13.Moncada S. The 1991 Ulf von Euler Lecture. The L-arginine: nitric oxide pathway. Acta Physiol Scand. 1992;145:201–227. doi: 10.1111/j.1748-1716.1992.tb09359.x. [DOI] [PubMed] [Google Scholar]
- 14.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology,pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 15.Morley JE, Flood JF. Competitive antagonism of nitric oxide synthase causes weight loss in mice. Life Sci. 1992;51:1285–1289. doi: 10.1016/0024-3205(92)90018-k. [DOI] [PubMed] [Google Scholar]
- 16.Morley JE, Flood JF. Effect of competitive antagonism of NO synthase on weight and food intake in obese and diabetic mice. Am J Physiol. 1994;266:R164–R168. doi: 10.1152/ajpregu.1994.266.1.R164. [DOI] [PubMed] [Google Scholar]
- 17.Morley JE, Flood JF. Evidence that nitric oxide modulates food intake in mice. Life Sci. 1991;49:707–711. doi: 10.1016/0024-3205(91)90102-h. [DOI] [PubMed] [Google Scholar]
- 18.Morley JE, Mattammal MB. Nitric oxide synthase levels in obese Zucker rats. Neurosci Lett. 1996;209:137–139. doi: 10.1016/0304-3940(96)12622-7. [DOI] [PubMed] [Google Scholar]
- 19.Raman K, Kaplan MH, Hogaboam CM, Berlin A, Lukacs NW. STAT4 signal pathways regulate inflammation and airway physiology changes in allergic airway inflammation locally via alteration of chemokines. J Immunol. 2003;170:3859–3865. doi: 10.4049/jimmunol.170.7.3859. [DOI] [PubMed] [Google Scholar]
- 20.Reiss CS, Komatsu T, Barna M, Bi Z. Interleukin-12 promotes enhanced recovery from viral infection of neurons in the central nervous system. Ann NY Acad Sci. 1996;795:257–265. doi: 10.1111/j.1749-6632.1996.tb52675.x. [DOI] [PubMed] [Google Scholar]
- 21.Scherer-Singler U, Vincent SR, Kimura H, McGeer EG. Demonstration of a unique population of neurons with NADPH-diaphorase histochemistry. J Neurosci Methods. 1983;9:229–234. doi: 10.1016/0165-0270(83)90085-7. [DOI] [PubMed] [Google Scholar]
- 22.Squadrito F, Calapai G, Altavilla D, Cucinotta D, Zingarelli B, Campo GM, Arcoraci V, Sautebin L, Mazzaglia G, Caputi AP. Food deprivation increases brain nitric oxide synthase and depresses brain serotonin levels in rats. Neuropharmacology. 1994;33:83–86. doi: 10.1016/0028-3908(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 23.Squadrito F, Calapai G, Cucinotta D, Altavilla D, Zingarelli B, Ioculano M, Urna G, Sardella A, Campo GM, Caputi AP. Anorectic activity of NG-nitro-L-arginine, an inhibitor of brain nitric oxide synthase, in obese Zucker rats. Eur J Pharmacol. 1993;230:125–128. doi: 10.1016/0014-2999(93)90422-e. [DOI] [PubMed] [Google Scholar]
- 24.Thierfelder WE, van Deursen JM, Yamamoto K, Tripp RA, Sarawar SR, Carson RT, Sangster MY, Vignali DA, Doherty PC, Grosveld GC, Ihle JN. Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature. 1996;382:171–174. doi: 10.1038/382171a0. [DOI] [PubMed] [Google Scholar]
- 25.Yang EK. Role of angiotensin II and nitric oxide in the rat paraventricular nucleus. Korean J Physiol Pharmacol. 2001;5:41–46. [Google Scholar]