Abstract
We previously reported that glial cell line-derived neurotropic factor (GDNF) receptor α1 (GFRα1) is a direct target of apurinic/apyrimidinic endonuclease 1 (Ape1/Ref-1). In the present study, we further analyzed the physiological roles of Ape1/Ref-1-induced GFRα1 expression in Neuro2a mouse neuroblastoma cells. Ape1/Ref-1 expression caused the clustering of GFRα1 immunoreactivity in lipid rafts in response to GDNF. We also found that Ret, a downstream target of GFRα1, was functionally activated by GDNF in Ape1/Ref-1-expressing cells. Moreover, GDNF promoted the proliferation of Ape1/Ref-1-expressing Neuro2a cells. Furthermore, GFRα1-specific RNA experiments demonstrated that the downregulation of GFRα1 by siRNA in Ape1/Ref-1-expressing cells impaired the ability of GDNF to phosphorylate Akt and PLCγ-1 and to stimulate cellular proliferation. These results show an association between Ape1/Ref-1 and GDNF/GFRα signaling, and suggest a potential molecular mechanism for the involvement of Ape1/Ref-1 in neuronal proliferation.
Keywords: Ape1/Ref-1, GFRα1, GDNF, Lipid raft, Neuronal proliferation, Signal pathway
INTRODUCTION
Apurinic/apyrimidinic endonuclease 1/redox factor-1 (Ape1/Ref-1) is a ubiquitous and remarkably multifunctional protein (Fishel et al., 2008). It plays a central role in the base excision repair (BER) pathway for repairing damaged bases and DNA single-strand breaks induced by reactive oxygen species (ROS) and alkylating agents and also repairing apurinic/apyrimidinic (AP) sites that are generated spontaneously or after the excision of oxidized and alkylated bases by DNA glycosylases (Fung and Demple, 2005). AP or abasic sites are the most common form of DNA damage with about 20,000~50,000 sites produced in each cell/day. Ape1/Ref-1 specifically binds to abasic sites and cuts the 5' phosphodiester bond with its endonuclease activity to produce a DNA primer with 3' hydroxyl end, which is a required step in the base excision repair pathway (Fung et al., 2007). Therefore, Ape1/Ref-1 is an essential endonuclease and plays a central role in the repair of AP site of DNA lesions. Besides its repair function, mammalian Ape1/Ref-1 has transcriptional regulatory activity. It was independently identified as reductive activator of c-Jun in vitro and named Ref-1 (Xanthoudakis and Curran, 1992). Subsequently, several other transcription factors including NF-κB, hypoxia inducible factor 1-α, PAX5, PAX8, p53, Egr-1, and YB-1 were also shown to be activated by Ape1/Ref-1 (Liu et al., 2005; Chattopadhyay et al., 2008; Fantini et al., 2008).
Ape1/Ref-1 is essential for cell viability. Genetic knockout of Ape1/Ref-1 in mice causes postimplantation embryonic lethality and any attempt to isolate stable Ape1/Ref-1-knockout cell lines has been so far unsuccessful (Larsen et al., 2007). In human cancer cells as well as human lymphoblastoid cells, small interference RNA (siRNA) directed against Ape1/Ref-1 results in a decrease in proliferation, an increase in AP sites and increased levels of apoptosis (Fishel et al., 2008; Xiang et al., 2008). Dominant-negative forms of Ape1/Ref-1 leads to chemotherapeutic agent sensitization (Wang et al., 2004; McNeill and Wilson, 2007; McNeill et al., 2009), and targeted reduction of Ape1/Ref-1 protein by specific anti-sense oligonucleotides or siRNA renders mammalian cells hypersensitive to a variety of chemotherapeutic agents (Bobola et al., 2005; Yang et al., 2007).
Ape1/Ref-1 is highly expressed in select regions of the central nervous system (Ono et al., 1995; Wilson et al., 1996). Reduced Ape1/Ref-1 expression has been shown in the hippocampus following hypoxic-ischemic injury (Walton et al., 1997), in the cortex after compression injury (Lewen et al., 2001), and in the spinal cord following ischemia (Sakurai et al., 2003). In addition, overexpression of Ape1/Ref-1 in neuronal cultures using adenoviral constructs appears to be neuroprotective (Vasko et al., 2005). Moreover, alterations in Ape1/Ref-1 expression and mutations in the Ape1/Ref-1 gene have been detected in patients with a variety of neurodegenerative diseases (Edwards et al., 1998; Olkowski, 1998; Tan et al., 1998). Thus, Ape1/Ref-1 dysfunction may contribute to the development of neurodegenerative disease (Rass et al., 2007).
We recently showed that glial cell line-derived neurotropic factor (GDNF) receptor α1 (GFRα1) is a direct target of Ape1/Ref-1 (Kim et al., 2009). We found that Ape1/Ref-1-mediated increases in GFRα1 expression contributed to neurite outgrowth as well as to neuronal survival in response to β-amyloid and H2O2 exposure. It seems likely that Ape1/Ref-1 is important for promoting stress resistance and regulating neuronal cell life span under normal conditions. Thus, we initiated the effect of Ape1/Ref-1 and GDNF/GFRα signaling on the neuronal cell proliferation, and examined biochemical analyses to elucidate the mechanism of Ape1/Ref-1-mediated neuronal survival and proliferation, with a focus on possible interplay between Ape1/Ref-1 and GFRα. Our data suggest that Ape1/Ref-1 protein is crucial in the regulation of neuronal cell proliferation through GDNF/GFRα signaling pathway and is a rational therapeutic target of drug development in the treatment of neurodegenerative diseases.
METHODS
Reagents and cell culture
GDNF was purchased from Sigma (St. Louis, MO, USA). Mouse GFRα1 small interfering RNA (siRNA; sc-35470) and scrambled siRNA (sc-37007) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Neuro2a (mouse) and SK-N-SH (human) neuroblastoma cells were cultured in Eagle's minimum essential medium (EMEM) containing 10% fetal bovine serum (FBS). SN4741 (a mouse neuron-like dopaminergic cell line) cells were grown in Dulbecco's modified Eagle's medium (DMEM) containing 10% FBS. All cells were maintained in cell-specific media at 37℃ in a humidified atmosphere of 5% CO2. The Neuro2a and SK-N-SH cell lines were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA). SN4741 was provided by Dr. H. S. Jeon (Chosun University).
Western blotting and immunoprecipitation
Cells were lysed in M-PER buffer (Mammalian Protein Extraction Reagent; Pierce, Rockford, IL) with protease inhibitors (Roche Diagnostic Corp., Indianapolis, IN, USA). Equal amounts of protein were separated by 6~15% SDSPAGE followed by electrotransfer onto a polyvinylidene difluoride membrane (Millipore, Bedford, MA, USA). The membranes were blocked for 1 h with TBS-t (10 mM Tris-HCl [pH 7.4], 150 mM NaCl, and 0.1% tween-20) containing 5% non-fat milk and then incubated at room temperature with primary antibodies against Ret (sc-167G), c-Src (sc-8056), Ape1/Ref-1 (sc-13104), and β-actin (sc-47778; Santa Cruz Biotechnology, Santa Cruz, CA, USA); p-Ret (Tyr905) (3221), p-AKT (Ser473) (9271), AKT (9272), p-PLC γ-1 (Tyr783) (2821) and PLCγ-1 (2822; Cell Signaling Technology, Danvers, MA, USA); and human GFRα-1 (AF714) and rat GFRα1 (MAB560; R&D Systems, Minneapolis, MN, USA). The blots were washed four times for 15 min with 0.1% Tween 20-containing TBS-t and then incubated for 1 h with peroxidase-conjugated secondary antibodies (1:5,000, Jackson ImmunoResearch Inc., West Grove, PA, USA). The membranes were washed four more times and developed using an enhanced chemiluminescence detection system (ECL; GE Healthcare, Buckinghamshire, UK).
For immunoprecipitation, cells were harvested 10 min after 30 ng/ml GDNF treatment and washed with ice-cold PBS before being lysed with lysis buffer (50 mM Tris-HCl, pH 8.0, 1 mM EDTA, 150 mM NaCl, 0.5% Na-deoxycholate, 0.02% Na-azide, 1 mM NaF, 1 mM Na-vanadate, 1 mM phenylmethylsulfonyl fluoride [PMSF], 1% Nonidet P-40, 1 mM dithiothreitol [DTT], 0.1% SDS, 2 µg/ml pepstatin, 2 µg/ml leupeptin, and 2 µg/ml aprotinin). After incubation for 1 h at 4℃, cellular debris was removed by centrifugation at 14,000×g for 30 min. The cell lysates were immunoprecipitated using anti-Ret antibodies and protein G-agarose beads (Santa Cruz Biotechnology) for 6 h at 4℃. The beads were then washed extensively with lysis buffer, boiled in SDS sample buffer, fractionated by SDS-PAGE, and analyzed by Western blotting with anti-phospho-Ret, anti-GFR α1, anti-c-Src or anti-Ret antibodies.
Lipid raft labeling
Membrane lipid raft microdomains were fluorescently labeled using a Vybrant® Lipid Raft Labeling kit (V-34403; Molecular Probes, Eugene, OR, USA), following the manufacturer's instructions. Staining for GFRα1 was performed using goat anti-rat GFRα1 antibodies (R&D Systems) for either 2 h on ice prior to fixation of the cells with 4% paraformaldehyde or after extraction with 1% Triton X-100. The cells were then washed in phosphate-buffered saline (PBS) and incubated on ice in buffer (2 mM MgCl2, 10 mM EGTA, and 60 mM PIPES, pH 7.0). After washing, the cells were incubated with Alexa fluor 647 chicken anti-goat IgG. For visualization of the lipid raft marker GM1, the cells were labeled for 45 min with 0.1 µg/ml fluorescein-conjugated cholera toxin B fragment (Sigma) and anti-CT-B rabbit serum in PBS containing 0.1% BSA, washed with PBS, and mounted in mounting solution. The cells were then visualized by fluorescence microscopy.
Proliferation assays
Vector/Neuro2a cells and Neuro2a/Ape1cells were cultured in serum-free medium for 12 h and then treated with or without 30 ng/ml GDNF in serum-free medium for the indicated times. Cellular proliferation was determined by counting the cells under a light microscope.
Statistical analysis
All values are expressed as the mean±standard deviation (SD). Where indicated, we performed statistical analyses using two-tailed Student's t-tests. We considered p<0.05 (indicated by * in the Figs) as significant and p<0.01 (**) as highly significant.
RESULTS
Ape1/ Ref-1 causes the clustering of GFRα1 immunoreactivity in lipid rafts
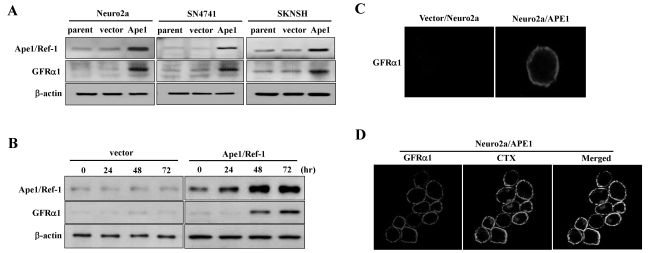
GDNF signals through a multi-component receptor complex, which consists of a glycosyl-phosphatidylinositol (GPI)-binding subunit and a transmembrane receptor tyrosine kinase (Ret), which is a major component of the signaling cascade activated by members of the GDNF family (Airaksinen and Saarma, 2002). We previously showed that GPI-anchored GFRα1 is a downstream target of Ape1/Ref-1 (Kim et al., 2009). To further elucidate the biological relevance of GDNF using Ape1/Ref-1-expressing neuronal cells, we tested whether GFRα1 expression could be attributed to Ape1/Ref-1 in various neuronal cell types. We found that GFRα1 expression in Ape1/Ref-1 expression vector-transfected Neuro2a, SN4741, and SKNSH cells was significantly higher than in empty vector-transfected cells (Fig. 1A). The induction of GFRα1 was observed in Neuro2a cells as early as 48 h after Ape1/Ref-1 expression vector transfection (Fig. 1B).
Fig. 1.
GFRα1 colocalizes with a lipid raft marker in Ape1/Ref-1-expressing Neuro2a cells. (A) Neuro2a, SN4741, and SKNSH cells were transfected with empty vector (vector) or Ape1/Ref-1 expression vector (Ape1) then harvested 48 h later. Total cell extracts were prepared for immunoblotting as indicated. (B) Neuro2a cells were transfected with empty or Ape1/Ref-1 expression vector and then harvested at the indicated times after transfection. Total cell extracts were prepared for immunoblotting as indicated. (C) Neuro2a/Ape1 and vector/Neuro2a cells were immunostained using polyclonal anti-GFRα1 antibodies tagged with Alexa fluor. (D) Neuro2a/Ape1 cells were stained with anti-GFRα1 antibodies and cholera toxin (CTX), which specifically binds the lipid raft ganglioside GM1. The images show the colocalization of GFRα1 and CTX.
GFRα1 is attached to the outer membrane by a GPI modification. The glycolipid moiety of GPI-anchored receptors is known to have affinity for specialized regions of the plasma membrane known as lipid rafts. These rafts are liquid-ordered phase microdomains produced by the lateral packing of sphingolipids and cholesterol scattered within the fluid, disordered phase of the lipid bilayer (Tsui-Pierchala et al., 2002). To test the possible involvement of Ape1/Ref-1 in the clustering of GFRα1 in response to GDNF, we established Neuro2a cells that stably expressed Ape1/Ref-1 (Neuro2a/Ape1) or vector alone (vector/Neuro2a), because Neuro2a cells express endogenous Ret, but not GFRα1 (Coulpier et al., 2002). We then tested the effect of GDNF on the localization of GFRα1 in the plasma membrane. Control and Ape1/Ref-1-expressing Neuro2a cells were treated for 4 h with GDNF or left untreated; subsequently, the medium was removed and the cells were incubated with anti-GFRα1 antibodies on ice prior to fixation and staining for the receptor at the surface of the living cells. As shown in Fig. 1C, no GFRα1 immunostaining was detected in the vector/Neuro2a cells, whereas the Neuro2a/Ape1 cells exhibited receptor clusters. This is in accordance with the finding that GFRα1 was predominantly located in lipid rafts at the plasma membrane in our stable Ape1/Ref-1 transfectants. The localization of GFRα1 in lipid rafts was visualized by double labeling with anti-GFRα1 antibodies and FITC-coupled cholera toxin B, which specifically binds the lipid raft marker GM1, a cell-surface ganglioside (Iwamori et al., 1985). Triton extraction of the cells was used to visualize the detergent-insoluble membrane compartments, as described previously (Paratcha et al., 2001), and we found that GFRα1 was highly colocalized with GM1 in Neuro2a/Ape1cells (Fig. 1D). These results suggest that GDNF induces the clustering of GFRα1 in lipid microdomains on the surface of Neuro2a/Ape1cells.
GDNF activates GFRα1 in association with Ret and induces Ret phosphorylation in Ape1/ Ref-1-expressing Neuro2a cells
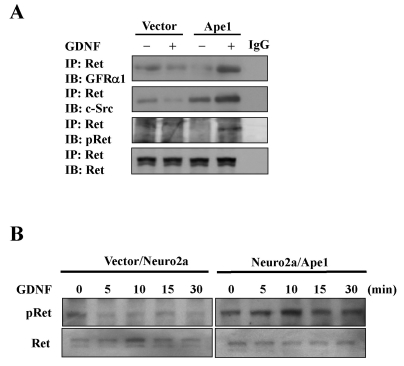
Lipid rafts are thought to represent specialized signaling organelles within the plasma membrane because of the enrichment of many adaptor and signaling molecules (Anderson, 1998). GDNF signals via a receptor complex consisting of Ret and a GPI-anchored ligand-binding subunit, GFRα1. As expected from its GPI anchorage, GFRα1 is located in lipid rafts (Tsui-Pierchala et al., 2002). In the absence of GDNF, GFRα1 and Ret do not associate with each other and Ret is not present in lipid rafts. In contrast, upon ligand stimulation, GDNF/GFRα1 complexes recruit Ret into lipid rafts. Translocation to the rafts is essential for Ret function because manipulations that render GFRα1 incapable of recruiting Ret into rafts or treatments that disrupt lipid rafts have been shown to compromise downstream signaling (Tansey et al., 2000). GFRα1 binds Ret in response to GDNF in neurons, leading to the phosphorylation of Ret tyrosine kinase, which subsequently associates with and activates a cytoplasmic Src family tyrosine kinase (Durbec et al., 1996; Jing et al., 1996; Treanor et al., 1996). Therefore, it is expected that increased GFRα1 expression induced by Ape1/Ref-1 would facilitate GDNF signaling through Ret. To determine whether this is the case in Neuro2a cells stably transfected with Ape1/Ref-1, we performed Ret coimmunoprecipitation experiments using Neuro2a/Ape1 cells stimulated with GDNF. Visualization of immune complexes was accomplished by SDS-PAGE followed by probing with specific antibodies against GFRα1 and c-Src. There was an increase in detectable associations between Ret and GFRα1, and between Ret and c-Src, in the GDNF-stimulated Neuro2a/Ape1cells, but not in the vector/Neuro2a cells (Fig. 2A), suggesting that Ape1/Ref-1 induces the GDNF-mediated association of GFRα1 and Ret in Neuro2a cells.
Fig. 2.
The effect of Ape1/Ref-1 on GDNF/GFRα1 signaling in Neuro2a cells. (A) Vector/Neuro2a (vector) and Neuro2a/Ape1 (Ape1) cells were treated with or without 30 ng/ml GDNF for 10 min. The cells were then lysed and subjected to immunoprecipitation (IP) with anti-Ret antibodies and analyzed for GFRα1, c-Src, phospho-Ret (pRet), and Ret by immunoblotting. (B) Vector/Neuro2a and Neuro2a/Ape1 cells were incubated with or without 30 ng/ml GDNF. At the indicated times, cell extracts were prepared and examined for activated phospho-Ret by immunoblotting.
We next examined whether the Ape1/Ref-1-mediated increase in GFRα1 is indeed involved in downstream GDNF signaling by investigating the phosphorylation status of Ret. After Neuro2a/Ape1 and vector/Neuro2a cells were stimulated with GDNF, cell lysates were prepared and immunoprecipitated with anti-Ret antibodies for Western blotting using anti-phospho-Ret (Tyr905) antibodies. As shown in Fig. 2A, GDNF-triggered Ret tyrosine phosphorylation increased significantly in Neuro2a/Ape1 cells. In contrast, Ret tyrosine phosphorylation in response to GDNF was not detected in vector/Neuro2a cells. GDNF-induced Ret phosphorylation was further characterized by examining the time course of GDNF action in this pathway using Neuro2a/Ape1 cells. Time course analysis indicated that the stimulation of Neuro2a/Ape1cells with GDNF caused a rapid and transient increase in Ret phosphorylation; in contrast, no obvious increase in phosphorylation was observed in vector/Neuro2a cells (Fig. 2B). These results suggest that the Ape1/Ref-1-mediated increase in GFRα1 expression results in the stimulation of Ret phosphorylation in response to GDNF.
Ape1/ Ref-1 induces GDNF-mediated Akt and PLCγ-1 activation in Neuro2a cells
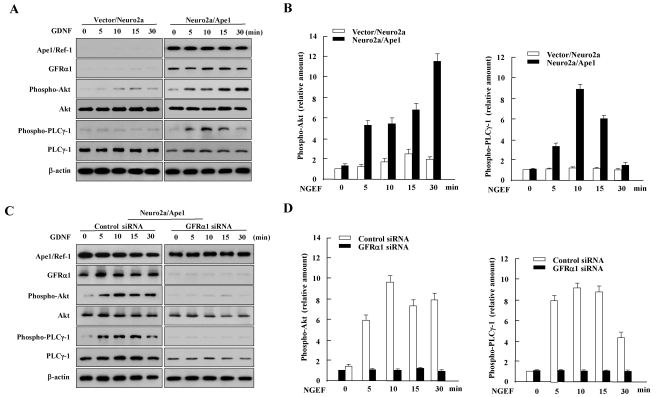
To determine whether pathways downstream of Ret phosphorylation were activated by GFRα1/GDNF, we examined the ability of GDNF to mediate Ret signaling events, such as Akt and PLCγ1 phosphorylation, in Neuro2a/Ape1 cells. GDNF stimulates the PI3K/Akt pathway via activation of Ret tyrosine kinase in several contexts (Pong et al., 1998; Besset et al., 2000). Thus, we examined whether GDNF induced Akt activation by investigating the phosphorylation status of Akt in total lysates prepared from Neuro2a/Ape1 and vector/Neuro2a cells. As shown in Fig. 3A, Akt was phosphorylated in GDNF-stimulated, Ape1/Ref-1-expressing Neuro2a cells. Akt phosphorylation was detected 5 min after GDNF treatment; the signal lasted for up to 30 min after stimulation with GDNF and then gradually decreased with time. The levels of non-phosphorylated Akt were unaffected by treatment with GDNF. In contrast, the vector/Neuro2a cells did not show Akt phosphorylation in response to GDNF. We also saw an increase in the tyrosine phosphorylation of PLCγ-1, which is another signaling target of Ret (Borrello et al., 1996). PLCγ-1 phosphorylation was detected at 5~30 min in GDNF-treated Neuro2a/Ape1 cells. No obvious increase in PLCγ-1 phosphorylation was observed after GDNF stimulation in vector/Neuro2a cells (Fig. 3A).
Fig. 3.
Ape1/Ref-1 increases Akt and PLCγ-1 phosphorylation in response to GDNF through GFRα1. (A) GDNF-induced Akt and PLCγ-1 phosphorylation in Ape1/Ref-1-expressing Neuro2a cells. Vector/Neuro2a and Neuro2a/Ape1 cells were incubated with or without 30 ng/ml GDNF for the indicated times and total cell extracts were prepared for immunoblotting as indicated. (B) The amounts of phospho-Akt and Phospho-PLCγ-1 were quantified by densitometry and corrected for the amount of Akt and PLCγ-1 in the corresponding lysate, rerspectively. Levels of phospho-Akt and phospho- PLCγ-1 are expressed relative to its level in non-NGEF-treated cells (0 min) transfected with control vector. The data shown are the means±S.D. from three separate experiments. (C) Neuro2a/Ape1 cells were transfected with control or GFRα1 siRNA. At 48 h after transfection, the cells were incubated with or without 30 ng/ml GDNF for the indicated times. Total cell lysates were prepared for immunoblotting as indicated. (D) The amounts of phospho-Akt and Phospho-PLCγ-1 were quantified by densitometry and corrected for the amount of Akt and PLCγ-1 in the corresponding lysate, respectively. Levels of phospho-Akt are expressed relative to its level in non-NGEF-treated cells (0 min) transfected with control siRNA. The data shown are the means±S.D. from three separate experiments.
To determine if GFRα1 contributes to Ape1/Ref-1-induced Akt and PLCγ-1 phosphorylation in response to GDNF, siRNA in the form of 21-base pair RNA duplexes targeted against GFRα1 was used to inhibit its expression. Neuro2a/Ape1 and vector/Neuro2a cells were transfected with the control or GFRα1-specific siRNA. Western blot analysis revealed that GFRα1 expression in cells transfected with GFRα1 siRNA decreased by more than 90% compared to control siRNA-transfected cells (Fig. 3B). The GDNF-induced phosphorylation of Akt and PLCγ-1 following transfection with GFRα1 siRNA was then examined. As shown in Fig. 3B, Ape1/Ref-1-infected cells treated with the GFRα1 siRNA showed the attenuation of GDNF-induced Akt and PLCγ-1 phosphorylation, suggesting that Ape1/Ref-1-induced GFRα1 expression triggered GDNF-mediated Akt and PLCγ-1 phosphorylation in Neuro2a cells.
GDNF enhances cellular proliferation in Ape1/ Ref-1-expressing Neuro2a cells
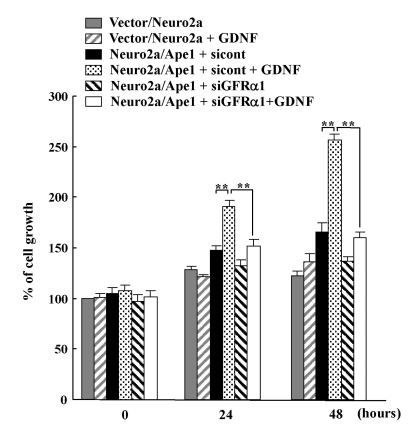
The GDNF/GFRα system regulates cell survival and proliferation (Airaksinen and Saarma, 2002; Sariola and Saarma, 2003). Therefore, in this study, we examined the effect of GDNF on the proliferation of Ape1/Ref-1-expressing Neuro2a cells. Neuro2a/Ape1 and vector/Neuro2a cells were either left untreated or incubated with GDNF, and the number of cells was counted after a period of one to two days. As shown in Fig. 4, the Neuro2a/Ape1 cells treated with GDNF showed a more rapid increase in the number of cells on days 1 and 2 than did the vector/Neuro2a cells treated with GDNF. These results suggest that the Ape1/Ref-1-mediated increase in GFRα1 expression results in the stimulation of neuronal cell proliferation in response to GDNF. To determine whether GFRα1 is indeed required for GDNF-induced proliferation in Ape1/Ref-1-expressing Neuro2a cells, Neuro2a/Ape1 cells were transfected with control or GFRα1 siRNA and the number of cells was counted. As shown in Fig. 4, the transfection of GFRα1-specific siRNA significantly reduced the level of cellular proliferation in response to GDNF compared to transfection with control siRNA. These results suggest that Ape1/Ref-1-mediated GFRα1 expression is involved in neuronal proliferation in response to GDNF.
Fig. 4.
Ape1/Ref-1 expression increases neuronal proliferation in response to GDNF. Vector/Neuro2a and Neuro2a/Ape1 cells were transfected with control (sicont) or GFRα1-siRNA (siGFRα1). At 24 h after transfection, the cells were incubated with or without 30 ng/ml GDNF for up to 48 h. The number of cells was then determined by counting every 24 h after GDNF treatment. Each value is the mean±S.D. from three separate experiments. **p<0.01.
DISCUSSION
In the present study, we investigated the relevance of Ape1/Ref-1 and GDNF/GFRα signaling in neuronal cell proliferation. We found that Ape1/Ref-1 expression in Neuro2a cells caused the clustering of GFRα1 immunoreactivity in lipid rafts in response to GDNF. In addition, Ret, Akt, and PLCγ-1, downstream targets of the GDNF/GFRα pathway, were activated by GDNF in Ape1/Ref-1-expressing Neuro2a cells, and the Ape1/Ref-1-mediated increase in GFRα1 contributed to Neuro2a cellular proliferation in response to GDNF. These data suggest that Ape1/Ref-1 is involved in neuronal survival and proliferation via GDNF/GFRα signaling.
The GDNF was originally characterized as a potent neurotropic factor specific for the survival and differentiation of the midbrain dopaminergic neurons (Lin et al., 1993). Subsequently, the biological effects of GDNF on the uterine branching in kidney morphogenesis, spermatogenesis, and survival as well as the differentiation of several other neuronal populations have considerably extended the range of activities of this polypeptide (Moore et al., 1996). Currently, four GFRα proteins, GFRα1, 2, 3, and 4 have been identified. GFRα1 mainly binds GNDF, and GFRα2, 3, and 4 bind neurturin (NTN), artemin (ART), and persephin (PSP), respectively, which are the GDNF family of growth factors (Ernsberger, 2008). The GDNF protein signals through a multi-component receptor complex, which consists of a glycosyl-phosphatidylinositol (GPI) binding subunit, which is known as the GDNF family receptor α (GFR α), and the transmembrane receptor tyrosine kinase (Ret) (Paratcha and Ledda, 2008).
The GDNF/GFRα signaling pathway promotes the survival of various neurons, including peripheral autonomic and sensory neurons, as well as central motor and dopamine neurons (Airaksinen et al., 1999). Moreover, in various animal models of Parkinson's disease, GDNF can prevent the neurotoxin- induced death of dopamine neurons and promote functional recovery (Tomac et al., 1995; Gash et al., 1996). The ability of GDNF to rescue dopaminergic neurons supports the idea that GDNF can ameliorate the degeneration of dopaminergic neurons in patients with Parkinson's disease. Therefore, GDNF has been suggested as a therapeutic candidate for the treatment of Parkinson's disease (Kirik et al., 2004; Hong et al., 2008). GDNF is also a good candidate molecule for the possible treatment of motor neuron-related diseases, such as amyotrophic lateral sclerosis or acute neuronal trauma (Saarma and Sariola, 1999; Klein et al., 2005). Recently, several lines of evidence have suggested that dysfunction in Ape1/Ref-1 may contribute to the development of neurodegenerative disease. For example, alterations in Ape1/Ref-1 expression and mutations in the Ape1/Ref-1 gene have been found in patients with a variety of neurodegenerative diseases (Kisby et al., 1997; Olkowski, 1998; Tan et al., 1998). Furthermore, preventing the loss of Ape1/Ref-1 by protein synthesis rescued neurons from experimentally induced cell death (Chiarini et al., 2000), whereas overepxression of Ape1/Ref-1 protects neuronal cells against oxidative stress (Vasko et al., 2005; Jiang et al., 2008). Our results indicate that an Ape1/Ref-1-mediated increase in GFRα1 contributes to GDNF-induced GFRα1 localization to lipid rafts and mediates both proximal (i.e., receptor-complex formation and Ret phosphorylation) and distal Ret signaling events (i.e., Akt and PLC-γ1 phosphorylation). It was also demonstrated that Ape1/Ref-1 expression led to enhanced neuronal proliferation, and that Ape1/Ref-1-induced GFRα1 expression is required for these effects. Therefore, it is possible that Ape1/Ref-1-induced GFRα expression is involved in the regulation of neuronal function, including GDNF-induced neuronal proliferation, which suggests a protective role for the protein against the development of neurodegenerative diseases. These results highlight the potential role of redox factor-1 in neuronal function through GDNF/GFRα signaling.
During development, high level of redox factor-1 expression is present in all somatic tissues (Wilson et al., 1996). The presence of widespread and high level of redox factor-1 expression during development is expected to play an important role in embryogenesis. redox factor-1 null mice exhibits die during the embryonic stage, which results from a developmental defect (Xanthoudakis et al., 1996). The phenotype of embryonic death observed in the redox factor-1-/- mice may be a consequence of defective DNA repair as well as inappropriate gene regulation whose expression is dependent on redox factor-1. This study demonstrated that a defect in redox factor-1 expression by siRNA suppressed GFRα1 expression and the GDNF responsiveness. Mice lacking GDNF (Pichel et al., 1996; Sanchez et al., 1996) and GFRα (Cacalano et al., 1998) all die soon after birth and share a similar phenotype of kidney agenesis and absence of enteric neurons below the stomach, suggesting GNDF/GFRα signaling pathway plays an important role in morphogenesis during embryonic development. Although little is known about why redox factor-1 null mice are embryonic lethal, one may speculate that arising from redox factor-1 functional defect in redox factor-1-null embryos, a failure of GDNF/GFRα signal pathway needed to stimulate morphogenesis may contribute to embryonic death.
ACKNOWLEDGEMENTS
This research was supported by research funds from Chosun University, 2004.
ABBREVIATIONS
- GDNF
glial cell line-derived neurotropic factor
- Ape1
apurinic/apyrimidinic endonuclease 1
- Ref-1
redox factor-1
- ROS
reactive oxygen species
- Ret
receptor tyrosine kinase
References
- 1.Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- 2.Airaksinen MS, Titievsky A, Saarma M. GDNF family neurotrophic factor signaling: four masters, one servant? Mol Cell Neurosci. 1999;13:313–325. doi: 10.1006/mcne.1999.0754. [DOI] [PubMed] [Google Scholar]
- 3.Anderson RG. The caveolae membrane system. Annu Rev Biochem. 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. [DOI] [PubMed] [Google Scholar]
- 4.Besset V, Scott RP, Ibanez CF. Signaling complexes and protein-protein interactions involved in the activation of the Ras and phosphatidylinositol 3-kinase pathways by the c-Ret receptor tyrosine kinase. J Biol Chem. 2000;275:39159–39166. doi: 10.1074/jbc.M006908200. [DOI] [PubMed] [Google Scholar]
- 5.Bobola MS, Finn LS, Ellenbogen RG, Geyer JR, Berger MS, Braga JM, Meade EH, Gross ME, Silber JR. Apurinic/apyrimidinic endonuclease activity is associated with response to radiation and chemotherapy in medulloblastoma and primitive neuroectodermal tumors. Clin Cancer Res. 2005;11:7405–7414. doi: 10.1158/1078-0432.CCR-05-1068. [DOI] [PubMed] [Google Scholar]
- 6.Borrello MG, Alberti L, Arighi E, Bongarzone I, Battistini C, Bardelli A, Pasini B, Piutti C, Rizzetti MG, Mondellini P, Radice MT, Pierotti MA. The full oncogenic activity of Ret/ptc2 depends on tyrosine 539, a docking site for phospholipase Cgamma. Mol Cell Biol. 1996;16:2151–2163. doi: 10.1128/mcb.16.5.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cacalano G, Farinas I, Wang LC, Hagler K, Forgie A, Moore M, Armanini M, Phillips H, Ryan AM, Reichardt LF, Hynes M, Davies A, Rosenthal A. GFRalpha1 is an essential receptor component for GDNF in the developing nervous system and kidney. Neuron. 1998;21:53–62. doi: 10.1016/s0896-6273(00)80514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chattopadhyay R, Das S, Maiti AK, Boldogh I, Xie J, Hazra TK, Kohno K, Mitra S, Bhakat KK. Regulatory role of human AP-endonuclease (APE1/Ref-1) in YB-1-mediated activation of the multidrug resistance gene MDR1. Mol Cell Biol. 2008;28:7066–7080. doi: 10.1128/MCB.00244-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiarini LB, Freitas FG, Petrs-Silva H, Linden R. Evidence that the bifunctional redox factor / AP endonuclease Ref-1 is an anti-apoptotic protein associated with differentiation in the developing retina. Cell Death Differ. 2000;7:272–281. doi: 10.1038/sj.cdd.4400639. [DOI] [PubMed] [Google Scholar]
- 10.Coulpier M, Anders J, Ibanez CF. Coordinated activation of autophosphorylation sites in the RET receptor tyrosine kinase: importance of tyrosine 1062 for GDNF mediated neuronal differentiation and survival. J Biol Chem. 2002;277:1991–1999. doi: 10.1074/jbc.M107992200. [DOI] [PubMed] [Google Scholar]
- 11.Durbec P, Marcos-Gutierrez CV, Kilkenny C, Grigoriou M, Wartiowaara K, Suvanto P, Smith D, Ponder B, Costantini F, Saarma M. GDNF signalling through the Ret receptor tyrosine kinase. Nature. 1996;381:789–793. doi: 10.1038/381789a0. [DOI] [PubMed] [Google Scholar]
- 12.Edwards M, Rassin DK, Izumi T, Mitra S, Perez-Polo JR. APE/Ref-1 responses to oxidative stress in aged rats. J Neurosci Res. 1998;54:635–638. doi: 10.1002/(SICI)1097-4547(19981201)54:5<635::AID-JNR8>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 13.Ernsberger U. The role of GDNF family ligand signalling in the differentiation of sympathetic and dorsal root ganglion neurons. Cell Tissue Res. 2008;333:353–371. doi: 10.1007/s00441-008-0634-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fantini D, Vascotto C, Deganuto M, Bivi N, Gustincich S, Marcon G, Quadrifoglio F, Damante G, Bhakat KK, Mitra S, Tell G. APE1/Ref-1 regulates PTEN expression mediated by Egr-1. Free Radic Res. 2008;42:20–29. doi: 10.1080/10715760701765616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fishel ML, He Y, Reed AM, Chin-Sinex H, Hutchins GD, Mendonca MS, Kelley MR. Knockdown of the DNA repair and redox signaling protein Ape1/Ref-1 blocks ovarian cancer cell and tumor growth. DNA Repair (Amst) 2008;7:177–186. doi: 10.1016/j.dnarep.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fung H, Demple B. A vital role for Ape1/Ref1 protein in repairing spontaneous DNA damage in human cells. Mol Cell. 2005;17:463–470. doi: 10.1016/j.molcel.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 17.Fung H, Liu P, Demple B. ATF4-dependent oxidative induction of the DNA repair enzyme Ape1 counteracts arsenite cytotoxicity and suppresses arsenite-mediated mutagenesis. Mol Cell Biol. 2007;27:8834–8847. doi: 10.1128/MCB.00974-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gash DM, Zhang Z, Ovadia A, Cass WA, Yi A, Simmerman L, Russell D, Martin D, Lapchak PA, Collins F, Hoffer BJ, Gerhardt GA. Functional recovery in parkinsonian monkeys treated with GDNF. Nature. 1996;380:252–255. doi: 10.1038/380252a0. [DOI] [PubMed] [Google Scholar]
- 19.Hong M, Mukhida K, Mendez I. GDNF therapy for Parkinson's disease. Expert Rev Neurother. 2008;8:1125–1139. doi: 10.1586/14737175.8.7.1125. [DOI] [PubMed] [Google Scholar]
- 20.Iwamori M, Shimomura J, Nagai Y. Specific binding of cholera toxin to rat erythrocytes revealed by analysis with a fluorescence-activated cell sorter. J Biochem. 1985;97:729–735. doi: 10.1093/oxfordjournals.jbchem.a135112. [DOI] [PubMed] [Google Scholar]
- 21.Jiang Y, Guo C, Vasko MR, Kelley MR. Implications of apurinic/apyrimidinic endonuclease in reactive oxygen signaling response after cisplatin treatment of dorsal root ganglion neurons. Cancer Res. 2008;68:6425–6434. doi: 10.1158/0008-5472.CAN-08-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jing S, Wen D, Yu Y, Holst PL, Luo Y, Fang M, Tamir R, Antonio L, Hu Z, Cupples R, Louis JC, Hu S, Altrock BW, Fox GM. GDNF-induced activation of the ret protein tyrosine kinase is mediated by GDNFR-alpha, a novel receptor for GDNF. Cell. 1996;85:1113–1124. doi: 10.1016/s0092-8674(00)81311-2. [DOI] [PubMed] [Google Scholar]
- 23.Kim MH, Kim HB, Acharya S, Sohn HM, Jun JY, Chang IY, You HJ. Ape1/Ref-1 induces glial cell-derived neurotropic factor (GDNF) responsiveness by upregulating GDNF receptor alpha1 expression. Mol Cell Biol. 2009;29:2264–2277. doi: 10.1128/MCB.01484-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirik D, Georgievska B, Bjorklund A. Localized striatal delivery of GDNF as a treatment for Parkinson disease. Nat Neurosci. 2004;7:105–110. doi: 10.1038/nn1175. [DOI] [PubMed] [Google Scholar]
- 25.Kisby GE, Milne J, Sweatt C. Evidence of reduced DNA repair in amyotrophic lateral sclerosis brain tissue. Neuroreport. 1997;8:1337–1340. doi: 10.1097/00001756-199704140-00004. [DOI] [PubMed] [Google Scholar]
- 26.Klein SM, Behrstock S, McHugh J, Hoffmann K, Wallace K, Suzuki M, Aebischer P, Svendsen CN. GDNF delivery using human neural progenitor cells in a rat model of ALS. Hum Gene Ther. 2005;16:509–521. doi: 10.1089/hum.2005.16.509. [DOI] [PubMed] [Google Scholar]
- 27.Larsen E, Meza TJ, Kleppa L, Klungland A. Organ and cell specificity of base excision repair mutants in mice. Mutat Res. 2007;614:56–68. doi: 10.1016/j.mrfmmm.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 28.Lewen A, Sugawara T, Gasche Y, Fujimura M, Chan PH. Oxidative cellular damage and the reduction of APE/Ref-1 expression after experimental traumatic brain injury. Neurobiol Dis. 2001;8:380–390. doi: 10.1006/nbdi.2001.0396. [DOI] [PubMed] [Google Scholar]
- 29.Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 30.Liu H, Colavitti R, Rovira II, Finkel T. Redox-dependent transcriptional regulation. Circ Res. 2005;97:967–974. doi: 10.1161/01.RES.0000188210.72062.10. [DOI] [PubMed] [Google Scholar]
- 31.McNeill DR, Lam W, DeWeese TL, Cheng YC, Wilson DM., 3rd Impairment of APE1 function enhances cellular sensitivity to clinically relevant alkylators and antimetabolites. Mol Cancer Res. 2009;7:897–906. doi: 10.1158/1541-7786.MCR-08-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McNeill DR, Wilson DM., 3rd A dominant-negative form of the major human abasic endonuclease enhances cellular sensitivity to laboratory and clinical DNA-damaging agents. Mol Cancer Res. 2007;5:61–70. doi: 10.1158/1541-7786.MCR-06-0329. [DOI] [PubMed] [Google Scholar]
- 33.Moore MW, Klein RD, Farinas I, Sauer H, Armanini M, Phillips H, Reichardt LF, Ryan AM, Carver-Moore K, Rosenthal A. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996;382:76–79. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- 34.Olkowski ZL. Mutant AP endonuclease in patients with amyotrophic lateral sclerosis. Neuroreport. 1998;9:239–242. doi: 10.1097/00001756-199801260-00012. [DOI] [PubMed] [Google Scholar]
- 35.Ono Y, Matsumoto K, Furuta T, Ohmoto T, Akiyama K, Seki S. Relationship between expression of a major apurinic/apyrimidinic endonuclease (APEX nuclease) and susceptibility to genotoxic agents in human glioma cell lines. J Neurooncol. 1995;25:183–192. doi: 10.1007/BF01053151. [DOI] [PubMed] [Google Scholar]
- 36.Paratcha G, Ledda F. GDNF and GFRalpha: a versatile molecular complex for developing neurons. Trends Neurosci. 2008;31:384–391. doi: 10.1016/j.tins.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 37.Paratcha G, Ledda F, Baars L, Coulpier M, Besset V, Anders J, Scott R, Ibanez CF. Released GFRalpha1 potentiates downstream signaling, neuronal survival, and differentiation via a novel mechanism of recruitment of c-Ret to lipid rafts. Neuron. 2001;29:171–184. doi: 10.1016/s0896-6273(01)00188-x. [DOI] [PubMed] [Google Scholar]
- 38.Pichel JG, Shen L, Sheng HZ, Granholm AC, Drago J, Grinberg A, Lee EJ, Huang SP, Saarma M, Hoffer BJ, Sariola H, Westphal H. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996;382:73–76. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- 39.Pong K, Xu RY, Baron WF, Louis JC, Beck KD. Inhibition of phosphatidylinositol 3-kinase activity blocks cellular differentiation mediated by glial cell line-derived neurotrophic factor in dopaminergic neurons. J Neurochem. 1998;71:1912–1919. doi: 10.1046/j.1471-4159.1998.71051912.x. [DOI] [PubMed] [Google Scholar]
- 40.Rass U, Ahel I, West SC. Defective DNA repair and neurodegenerative disease. Cell. 2007;130:991–1004. doi: 10.1016/j.cell.2007.08.043. [DOI] [PubMed] [Google Scholar]
- 41.Saarma M, Sariola H. Other neurotrophic factors: glial cell line-derived neurotrophic factor (GDNF) Microsc Res Tech. 1999;45:292–302. doi: 10.1002/(SICI)1097-0029(19990515/01)45:4/5<292::AID-JEMT13>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 42.Sakurai M, Nagata T, Abe K, Horinouchi T, Itoyama Y, Tabayashi K. Oxidative damage and reduction of redox factor-1 expression after transient spinal cord ischemia in rabbits. J Vasc Surg. 2003;37:446–452. doi: 10.1067/mva.2003.100. [DOI] [PubMed] [Google Scholar]
- 43.Sanchez MP, Silos-Santiago I, Frisen J, He B, Lira SA, Barbacid M. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996;382:70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- 44.Sariola H, Saarma M. Novel functions and signalling pathways for GDNF. J Cell Sci. 2003;116:3855–3862. doi: 10.1242/jcs.00786. [DOI] [PubMed] [Google Scholar]
- 45.Tan Z, Sun N, Schreiber SS. Immunohistochemical localization of redox factor-1 (Ref-1) in Alzheimers hippocampus. Neuroreport. 1998;9:2749–2752. doi: 10.1097/00001756-199808240-00012. [DOI] [PubMed] [Google Scholar]
- 46.Tansey MG, Baloh RH, Milbrandt J, Johnson EM. GFRalpha-mediated localization of RET to lipid rafts is required for effective downstream signaling, differentiation, and neuronal survival. Neuron. 2000;25:611–623. doi: 10.1016/s0896-6273(00)81064-8. [DOI] [PubMed] [Google Scholar]
- 47.Tomac A, Lindqvist E, Lin LF, Ogren SO, Young D, Hoffer BJ, Olson L. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature. 1995;373:335–339. doi: 10.1038/373335a0. [DOI] [PubMed] [Google Scholar]
- 48.Treanor JJ, Goodman L, de Sauvage F, Stone DM, Poulsen KT, Beck CD, Gray C, Armanini MP, Pollock RA, Hefti F, Phillips HS, Goddard A, Moore MW, Buj-Bello A, Davies AM, Asai N, Takahashi M, Vandlen R, Henderson CE, Rosenthal A. Characterization of a multicomponent receptor for GDNF. Nature. 1996;382:80–83. doi: 10.1038/382080a0. [DOI] [PubMed] [Google Scholar]
- 49.Tsui-Pierchala BA, Encinas M, Milbrandt J, Johnson EM. Lipid rafts in neuronal signaling and function. Trends Neurosci. 2002;25:412–417. doi: 10.1016/s0166-2236(02)02215-4. [DOI] [PubMed] [Google Scholar]
- 50.Vasko MR, Guo C, Kelley MR. The multifunctional DNA repair/redox enzyme Ape1/Ref-1 promotes survival of neurons after oxidative stress. DNA Repair(Amst) 2005;4:367–379. doi: 10.1016/j.dnarep.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 51.Walton M, Lawlor P, Sirimanne E, Williams C, Gluckman P, Dragunow M. Loss of Ref-1 protein expression precedes DNA fragmentation in apoptotic neurons. Brain Res Mol Brain Res. 1997;44:167–170. doi: 10.1016/s0169-328x(96)00291-4. [DOI] [PubMed] [Google Scholar]
- 52.Wang D, Luo M, Kelley MR. Human apurinic endonuclease 1 (APE1) expression and prognostic significance in osteosarcoma: enhanced sensitivity of osteosarcoma to DNA damaging agents using silencing RNA APE1 expression inhibition. Mol Cancer Ther. 2004;3:679–686. [PubMed] [Google Scholar]
- 53.Wilson TM, Rivkees SA, Deutsch WA, Kelley MR. Differential expression of the apurinic / apyrimidinic endonuclease (APE/ref-1) multifunctional DNA base excision repair gene during fetal development and in adult rat brain and testis. Mutat Res. 1996;362:237–248. doi: 10.1016/0921-8777(95)00053-4. [DOI] [PubMed] [Google Scholar]
- 54.Xanthoudakis S, Curran T. Identification and characterization of Ref-1, a nuclear protein that facilitates AP-1 DNA-binding activity. Embo J. 1992;11:653–665. doi: 10.1002/j.1460-2075.1992.tb05097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xanthoudakis S, Smeyne RJ, Wallace JD, Curran T. The redox/DNA repair protein, Ref-1, is essential for early embryonic development in mice. Proc Natl Acad Sci USA. 1996;93:8919–8923. doi: 10.1073/pnas.93.17.8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xiang DB, Chen ZT, Wang D, Li MX, Xie JY, Zhang YS, Qing Y, Li ZP, Xie J. Chimeric adenoviral vector Ad5/F35-mediated APE1 siRNA enhances sensitivity of human colorectal cancer cells to radiotherapy in vitro and in vivo. Cancer Gene Ther. 2008;15:625–635. doi: 10.1038/cgt.2008.30. [DOI] [PubMed] [Google Scholar]
- 57.Yang ZZ, Chen XH, Wang D. Experimental study enhancing the chemosensitivity of multiple myeloma to melphalan by using a tissue-specific APE1-silencing RNA expression vector. Clin Lymphoma Myeloma. 2007;7:296–304. doi: 10.3816/CLM.2007.n.006. [DOI] [PubMed] [Google Scholar]