Abstract
It is hypothesized that a number of environmental factors affect animals' behavior. Without controlling these variables, it is very hard for researchers to get not only reliable, but replicable data from various behavioral experiments testing animals' cognitive as well as emotional functions. For example, laboratory mice which had restricted environment showed different synaptic potentiation properties with wild mice (Zhao MG et al., 2009). While performing behavioral experiments, however, it is sometimes inevitable that the researcher changes the animals' environments, as by switching the cages in which experimental animals are housed and separating animals raised together into small experimental groups. In this study, we investigated the effect of environmental changes on mice's emotional behaviors by socially isolating them or reducing the size of their cage. We found that social isolation selectively increases the animals' levels of anxiety, while leaving depression-like behaviors unchanged. On the other hand, alteration of the housing dimensions affected neither their anxiety levels nor their depression-like behaviors. These results suggest that environmental variables may have a prominent impact on experimental animals' emotional behaviors and possibly their psychological states, leading to bias in the behavioral data produced from experiments.
Keywords: Anxiety level, Social isolation, Housing environment, Experimental animals, Open field test, Tail suspension test, Forced swim test
INTRODUCTION
Environmental conditions that experimental animals are exposed to have been thought to affect various aspects of their behavior (Deacon, 2006). Previous studies have reported that enriched environments can improve experimental animals' cognitive functions, such as learning and memory, whereas either acute or chronic exposure to stressful events impairs their cognitive functions, causing abnormal behaviors. Therefore, housing experimental animals in well-controlled and stable conditions is one of the indispensable prerequisites for beginning any behavioral experiment, because environmental factors may alter both cognitive and emotional brain functions in the animals.
To get reliable and replicable result from behavioral experiments, researchers always try to keep animals' housing environment stable and controlled. Stable housing conditions are achieved by raising animals in constant circadian rhythm, proper temperature and humidity, uniform cage, with consistent cohabitants as possible.
As an experiment goes on, however, experimenters sometimes cannot help changing the animals' external environments. For example, researchers may sort animals that have been raised together into small experimental groups for different treatments during the experimental period. Such sorting is likely to result in the alteration of social interactions; the animals lose old cohabitants or get new cohabitants. These changes may cause stress. In addition, a variety of housing conditions, such as bedding, circadian cycle, temperature, and the size of the cage may be changed during an experiment. The cages housing experimental animals are altered during cage-cleaning and replacement of the soiled bedding with fresh. The size of the cage is often altered during an experiment as well. Even though these environmental changes seem mild, and harmless to the animals, it is not certain whether these kinds of changes affect animals' cognitive and emotional functions.
Until now, a vast number of studies have focused on the effects of relatively strong, stressful environmental changes, such as postnatal maternal separation and swim stress, on animals' cognitive and emotional behaviors (Malberg and Duman, 2003; Hattori et al., 2007; Kirby et al., 2007; Millstein and Holmes, 2007). Nevertheless, there have been few studies on whether and how somewhat mild environmental alterations in housing conditions affect experimental animals' behaviors.
Here, we examined the effects of mild changes in housing environments on experimental animals' behavior, using the mouse as a model system. Social isolation selectively elevated the animals' anxiety levels without affecting depression-like behaviors. In the case of changes in cage dimensions, both anxiety level and depression-like behavior were unaffected, demonstrating the specific impact of social isolation on emotional behavior.
METHODS
Animals and rearing environment
The mice used in this study were the C57BL/6J (B6) (ORIENT). The temperatures for rearing the mice and for the behavioral experiments were the same, 22~24℃. The environmental light levels were controlled, bright, 9 a.m. to 9 p.m., dark, 9 p.m. to 9 a.m.
Open field test and analysis
The open field test (OF) was carried out in an acrylic cube (40×40×40 cm). The starting position within the acrylic cubic was the same for all mice. Subjects' open field exposure time was 15 minutes. We measured the time, in seconds, that each subject spent in the central, 20×20 cm area of the open field.
For this test, the quantitative analysis was carried out by means of a computer program (EthoVision).
Tail suspension test, forces swim test, and analysis
The tail suspension test (TST) and forced swim test (FST) were carried out using the same acrylic cube used in the OF. During the 6-minute TST, the mice were suspended by their tails from a steel bar. The distance between floor and tail was about 30 cm.
The FST was performed 1 day after the TST, to reduce the stress caused by the TST. The cylinder used in FST was transparent, and it had a diameter of 14 cm and height of 25 cm. The entire duration of the FST was 6 minutes.
In the TST, when the mice hung suspended from the bar with no active escape response, this was counted as immobility time. For the FST, we counted as immobility time when the mice just floated on the water, showing no active escape response. We performed all such counting blind. Also, we excluded all TST data from those cases where the mice fell from the bar because of their strong escape movements.
RESULTS
Procedure of behavioral experiments
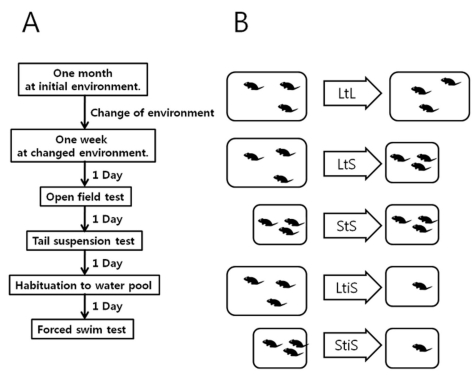
All the behavioral experiments were conducted as illustrated in Fig. 1A. The mice were reared in an initial environment for one month. After that month, the mice were exposed to one of two types of environmental changes. One was social isolation, in which a single mouse was separated from the original colony and housed alone. The other was a change in cage dimensions, where the mice were transferred to a smaller cage. After giving the subjects one week of habituation in their new environments, we performed behavioral experiments to examine whether the animals' behaviors were affected by these changes.
Fig. 1.
Experimental design. (A) Procedure of behavior experiment. (B) Experimental groups. LtL, large to large cage; LtS, large to small cage; StS, small to small cage; LtiS, large to small cage, isolated; StiS, small to small cage, isolated.
On the first day of experimentation, we exposed the mice to the open field and observed their locomotive behavior (OF test). One day after the OF test, we performed the tail suspension test (TST) to observe the depression-like behavior in each group. After a day period to recover from the TST, each mouse was placed in a columnar plastic container filled with water to become habituated to this environment. On the following day, we carried out the forced swim test (FST). The experimental groups are shown in Fig. 1B.
Effect of environmental changes on anxiety related behavior
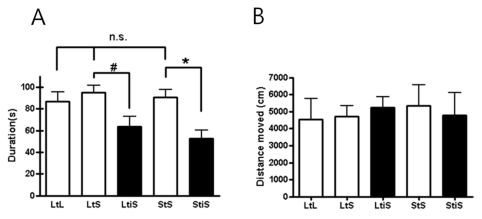
To examine anxiety levels in the mice, we conducted open field tests (Prut and Belzung, 2003). After placing the mice in the open field, we measured their movements into the field's center area. First, to check the effect of cage size on anxiety level, we compared the levels of movement into the center region among the LtL, LtS, and StS. We could not observe any notable difference in central movements among these groups (Fig 2A, p=0.7537, one-way ANOVA test), indicating that a reduction in cage dimensions had nothing to do with anxiety levels. In addition, the size of cage area itself didn't affect anxiety levels of the mice. These results suggest that both the size of cage and the change in cage dimensions have no effect on anxiety-related behavior in mice (Fig 2A, p=0.7537, one-way ANOVA).
Fig. 2.
(A) The result of the open field (OF) test indicates that social isolation induced increasing anxiety levels (LtS vs. LtiS; LtS, 94.73±10.37 s, n=6; LtiS, 63.37±14.15 s, n=6; unpaired t-test, #p= 0.1041; StS vs. StiS; StS, 90.70±10.55 s, n=9; StiS, 52.53±11.03 s, n=6; unpaired t-test, *p=0.0317). (B) Not all mice showed different locomotion activity levels (one-way ANOVA, p=0.5529).
Next, to assess the effect of social isolation on anxiety-related behavior, we examined mice that we isolated, housing each mouse individually, apart from its cohabitants. According to a previous study (Chourbaji et al., 2005), social isolation elevates anxiety levels of experimental animals. When the mice were so isolated, the size of their cage was either reduced from large cage to small cage (LtiS) or preserved (StiS).
The LtiS group did not differ in anxiety level from the LtS group (#p=0.1041, unpaired t-test). Mice in this group experienced social isolation accompanied by a reduction in cage size. In the StiS group, where each mouse was socially isolated without any change in cage size, anxiety levels increased significantly compared to the StS group (*p=0.0317, unpaired t-test). Mice in the StiS group showed significantly decreased movements into the center area of the open field. However, there was no marked difference in the distance moved between the StS and StiS groups (Fig. 2B). This result implies that social isolation might elevate anxiety levels in mice.
Effect of environmental changes on depression-like behavior of mice
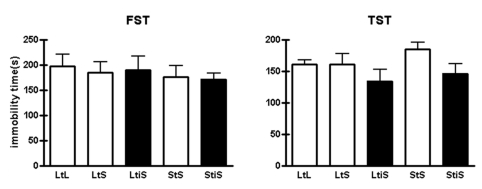
After the OF test, we performed the tail suspension test and forced swim test to determine whether environmental changes affect depression-like behavior in mice. Both of the tests are well-established behavioral paradigms for examining the depression-like behavior of mice or rats (Porsolt et al., 1978; Steru et al., 1985). The more depressed mice are, the less they show active escaping behavior. In these experiments, we could not observe any difference among any of the groups (Fig. 3. TST, p=0.3152; FST, p=0.8973; one-way ANOVA). Therefore, at least in our experimental condition, the environmental changes social isolation and change in cage dimension had no effect on depression-like behavior in mice.
Fig. 3.
In the tail suspension test (TST) and forced swim test (FST), groups didn't show notably different levels of depression-like behavior among groups (TST: LtL, 160.33±7.38 s, n=6; LtS, 160.20±17.32 s, n=5; LtiS, 134.00±18.59 s, n=6; StS, 185.00±9.10 s; StiS, 146.11±15.21 s, n=9; one-way ANOVA, p=0.3152. FST: LtL, 197.50±23.42 s, n=6; LtS, 184.16±21.94 s; LtiS, 189.67±28.00 s, n=6; StS, 176.17±22.65 s, n=6; StiS, 171.33±11.85 s, n=9; one-way ANOVA, p=0.8973).
DISCUSSION
In the present study, we found that social isolation elevated the anxiety levels of mice, whereas a reduction in cage size had no effect on anxiety level. In the other behavioral tests, we found that depression-like behavior was not affected by social isolation or by a reduction in cage dimensions. Thus, it seems that the "downsizing" of a cage where mice are housed is not such a significant modification; it does not affect the animals' emotional behavior as much as social isolation appears to.
Mice in the StiS group showed significantly increased levels of anxiety, while the LtiS group did not show notable differences in anxiety level when compared to LtS group (Fig. 2A). This finding suggests that a reduction in cage size might relieve the negative effects of social isolation. How might this be possible? One possibility is that the reduction of individual living space due to a diminution in cage size may lead to elevated social interactions among the animals. As the size of a cage decreases, the average distance between any two mice in that cage is reduced in parallel, possibly causing the probability of interactions between the animals to increase. This means that the smaller the cage size that a group of mice has, the more social interaction those mice might have. Therefore, social isolation's negative effect on emotional behavior could be greater in the StiS group than in the LtiS group, which would explain why StiS mice showed significantly increased levels of anxiety, while anxiety levels increased just slightly in the LtiS group. If the size of the cage area has an effect on social interaction of mice, then the anxiety level of the LtS group might be lower than that of the LtL group. Nonetheless, there was no notable change in anxiety level. This indicates that, in the absence of social isolation, a reduction in cage size is too mild to influence the anxiety levels of animals, in our experimental conditions. At least, in our experimental system, social isolation seemed to be the only environmental alteration that influenced anxiety level of the animals.
The findings of the present study are partly in agreement with results from a previous study (Chourbaji et al., 2005). In our conditions, social isolation elevated anxiety levels without making the mice depressed. However, Chourbaji et al. (2005) found social isolation increased not only anxiety levels but also depression-like behavior. This disagreement can be explained in several ways. First, the earlier researchers did not alter the environmental conditions for breeding. The mice that they used were reared in the same environmental conditions throughout their experiments. In our experiment, however, we changed rearing conditions during the experiment. Second, to evaluate depression-like behavior, we made use of the TST and FST, while Chourbaji et al. (2005) used the learned-helplessness paradigm. Accordingly, different types of behavioral experiments might produce different resolutions, to the extent that only a certain sort of behavioral paradigm can reveal whether animals' cognitive or emotional behaviors are affected by an experimental treatment.
There is also a study reporting behavioral results (Karolewicz and Paul, 2001) contradictory to our result. In this study, group housing of the mice elevated their anxiety levels. Group housing is the opposite of the social isolation used in the present study. This inconsistency demonstrates that not only social isolation but group housing may affect animals' emotional behavior. Therefore, social isolation is not the only such factor that elevates anxiety levels. Rather, it seems that improper levels of social interaction may influence experimental animals' cognitive and emotional behaviors.
To sum up, our study revealed that environmental changes such as social isolation can selectively affect certain types of emotional functions. In our study, subjects' anxiety levels were elevated, while their depression-like behavior was unchanged. Therefore, various factors inherent in housing environments may play a crucial role in shaping animals' emotional behaviors. Also, it is necessary for researchers to pay attention to the environmental conditions to which experimental animals are exposed throughout their experiments in order to test their hypotheses while excluding the non-specific effects caused by certain conditions.
ACKNOWLEDGEMENTS
We thank J.I. Kim, D.J. Jang, and Y.S. Rim for reading this manuscript and offering critical discussion. This work was supported by the Creative Research Initiative Program of the Korean Ministry of Science and Technology (to B.-K.K.).
ABBREVIATIONS
- OF test
open field test
- TST
tail suspension test
- FST
forced swim test
References
- 1.Chourbaji S, Zacher C, Sanchis-Segura C, Spanagel R, Gass P. Social and structural housing conditions influence the development of a depressive-like phenotype in the learned helplessness paradigm in male mice. Behav Brain Res. 2005;164:100–106. doi: 10.1016/j.bbr.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Deacon RM. Housing, husbandry and handling of rodents for behavioral experiments. Nat Protoc. 2006;1:936–946. doi: 10.1038/nprot.2006.120. [DOI] [PubMed] [Google Scholar]
- 3.Hattori S, Hashimoto R, Miyakawa T, Yamanaka H, Maeno H, Wada K, Kunugi H. Enriched environments influence depression-related behavior in adult mice and the survival of newborn cells in their hippocampi. Behav Brain Res. 2007;180:69–76. doi: 10.1016/j.bbr.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 4.Karolewicz B, Paul IA. Group housing of mice increases immobility and antidepressant sensitivity in the forced swim and tail suspension tests. Eur J Pharmacol. 2001;415:197–201. doi: 10.1016/s0014-2999(01)00830-5. [DOI] [PubMed] [Google Scholar]
- 5.Kirbya LG, Panb YZ, Freeman-Danielsa E, Rania S, Nunana JD, Akanwab A, Beckb SG. Cellular effects of swim stress in the dorsal raphe nucleus. Psychoneuroendocrinology. 2007;32:712–723. doi: 10.1016/j.psyneuen.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28:1562–1571. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- 7.Millstein RA, Holmes A. Effects of repeated maternal separation on anxiety- and depression-related phenotypes in different mouse strains. Neurosci Biobehav Rev. 2007;31:3–17. doi: 10.1016/j.neubiorev.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;4:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- 9.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- 10.Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 11.Zhao MG, Toyoda H, Wang YK, Zhuo M. Enhanced synaptic long-term potentiation in the anterior cingulate cortex of adult wild mice as compared with that in laboratory mice. Mol Brain. 2009;2:11. doi: 10.1186/1756-6606-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]