Abstract
Effects of quercetin, a kind of flavonoids, on the vasodilating actions were investigated. Among the mechanisms for quercetin-induced vasodilatation in rat aorta, the involvement with the Ca2+ activated K+ (KCa) channel was examined. Pretreatment with NE (5 µM) or KCl (60 mM) was carried out and then, the modulation by quercetin of the constriction was examined using rat aorta ring strips (3 mm) at 36.5℃. Quercetin (0.1 to 100 µM) relaxed the NE-induced vasoconstrictions in a concentration-dependent manner. NO synthesis (NOS) inhibitor, NG-monomethyl-L-arginine acetate (L-NMMA), at 100 µM reduced the quercetin (100 µM)-induced vasodilatation from 97.8±3.7% (n=10) to 78.0±11.6% (n=5, p<0.05). Another NOS inhibitor, L-NG-nitro arginine methyl ester (L-NAME), at 100 µM also had the similar effect. In the presence of both 100 µM L-NMMA and 10 µM indomethacin, the quercetin-induced vasodilatation was further attenuated by 100 µM tetraethylammonium (TEA, a KCa channel inhibitor). Also TEA decreased the quercetin-induced vasodilatation in endothelium-denuded rat aorta. Used other KCa channel inhibitors, the quercetin-induced vasodilatation was attenuated by 0.3 µM apamin (a SK channel inhibitor), but not by 30 nM charybdotoxin (a BK and IK channel inhibitor). Quercetin caused a concentration-dependent vasodilatation, due to the endothelium-dependent and -independent actions. Also quercetin contributes to the vasodilatation selectively with SK channel on smooth muscle.
Keywords: Quercetin, Vasodilatation, KCa channels, PK-C, Endothelium
INTRODUCTION
Quercetin is a polyphenolic flavonoid existing in a wide variety of plants and foods (Hertog et al., 1993a). It has been reported that flavonoids reduce the incidence of cardiovascular diseases and carcinogenesis (Hertog et al., 1993b). Flavonoid is a vasodilator (Duarte et al., 1993) and a scavenger for free radicals (Murota and Terao, 2003). Thus, flavonoids migtht excert cardiovascular protective actions through their various pharmacological effects. Quercetin has also been reported to possess various pharmacological actions: modulation of epoxyeicosanoic acids synthesis, prevention of platelet aggregation (Formica and Regelson, 1995) as well as vasodilatation (Duarte et al., 1993). Therefore, quercetin may play major role for the pharmacological actions of dietary polyphenols or polyphenol-rich herbal medicine. For example, Ginkgo biloba extract, a flavonoid-rich herbal medicine, contains quercetin and ehibits a lot of pharmacological effects (Sticher, 1993; Satoh and Nishida, 2004). In our previous report, Ginkgo biloba extract and quercetin cause the vasodilating actions (Nishida and Satoh, 2004). Therefore, quercetin would be a key for the pharmacological effects induced by Ginkgo biloba extract.
In some reports for the vasodilating mechanisms, quercetin possesses protein kinase C (PK-C) inhibition (Duarte et al., 1993; Murota and Terao, 2003), tyrosine kinase inhibition (Catalin, 1995), and activation of endothelium-dependent actions (Chen and Pace-Asciak, 1996; Kubota et al., 2001). In addition, flavonoids such as hesperidin, luteoline and 7-hydroxyflavone produce vasodilatation due to the Ca2+ activated K+ (KCa) channel modulation on vascular smooth muscle cells (Calderone et al., 2004). The KCa channels hyperpolarize the membrane. They are classified by their conductances as follows: big conductance KCa (BK) channel (200 pS), intermediate conductance KCa (IK) channel (37 pS), and small conductance KCa (SK) channel (32 pS) (Brayden and Nelson, 1992; Neylon et al., 1999). Most recently, quercetin has been demonstrated to activate BK channel in coronary arteries via production of H2O2 (Congolludo et al., 2007). In other study, however, TEA and glibenclamide (KATP channel inhibitor) have not been reported to affect the quercetin-induced vasodilatation in rat aorta (Perez-Vizcaino, 2002). Thus, the effects of quercetin on KCa channels are not clear yet. Aim of this study is to investigate the involvement of KCa channels in the quercetin-induced vasodilatation in rat aorta.
METHODS
All experiments were carried out according to the guidelines laid down by the Nara Medical University Animal Welfare Committee, and also under the terms of the Declaration of Helsinki.
Wistar male rats (4~10 weeks old) were anesthetized with ether, and euthanized by exsanguination. The thoracic aorta was quickly removed, and the isolated aorta was cut into 3-mm rings in length. The rings were suspended between two triangular-shaped stainless steel stirrups in a jacketed organ chamber filled with 20 ml modified Krebs-Henseleit solution. The modified Krebs-Henseleit solution was, in mM: 118 NaCl, 4.6 KCl, 1.2 MgSO4, 1.2 KH2PO4, 11.1 glucose, 27.2 NaHCO3, 0.03 ethylene glycol- O,O'-bis (2-aminoethyl)-N,N,N',N'-tetraacetic acid (EGTA), and 1.8 CaCl2. The chamber solution was kept at 36.5℃ and oxygenated with 95% O2 and 5% CO2. The lower stirrup was anchored and the upper stirrup was attached to a force-displacement transducer (TB-652T; Nihon Kohden, Tokyo, Japan) to record the isometric force. All rings were stretched to generate a resting tension of 1.2 g.
After 40 min of resting, addition of 5 µM norepinephrine (NE) or setting the concentration of KCl to 60 mM in the bath was performed to induce vasoconstriction. After the contractile response became steady, quercetin was cumulatively administrated into the bath solution. The effects of quercetin were measured 6~10 min after the responses became steady. The relaxation response was analyzed as a percentage decrease from the maximal contraction induced by NE. Pretreatment with the inhibitors was carried out for 40-min before NE was administrated.
The drugs used were quercetin (Tocris Biosci., Northpoint, UK), NG-monomethyl-L-arginine acetate (L-NMMA), L-NG-nitro arginine methyl ester (L-NAME), charybdotoxin, apamin (Sigma Chemical Co. St. Louis, MO, U.S.A.), indomethacin and tetraethylammonium (TEA) (Nacalai Tesque Inc., Kyoto Japan). All values are represented as means±S.E.M. The differences of data in mean values were analyzed by Student's t-test and analysis of variance (ANOVA), and a p value of less than 0.05 was considered significant.
RESULTS
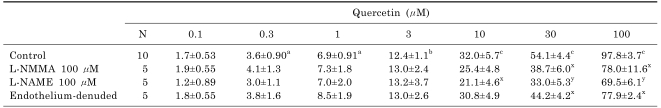
The aorta ring strip of rat exhibited a strong contraction induced by initial application of 5 µM NE. Subsequent applications of quercetin (0.1 to 100 µM) were performed. The responses were concentration-dependent. Quercetin caused significant vasodilatation at concentrations higher than 0.3 µM; by 97.8±3.7% (n=10, p<0.001) at 100 µM (Table 1).
Table 1.
Modulation of the quercetin-induced vasodilatation
Values (%) represent mean±S.E.M. a and x: p<0.05, b and y: p<0.01, c: p<0.001. Symbols of a, b, and c mean significant difference in comparison between effect of quercetin itself at each concentration and the maximal contraction induced by NE. Symbols of x and y mean significant difference as compared with control (quercetin alone).
Prior administration of L-NMMA (100 µM), an NO synthesis (NOS) inhibitor, significantly inhibited the quercetin-induced vasodilatation (Fig. 1). At 100 µM quercetin, the vasodilatation was attenuated from 97.8±3.7% (n=10) to 78.0±11.6 (n=5, p<0.05). Another NOS inhibitor, L-NAME had the similar effects (Table 1). This is enforced by our experiments using the aorta removed endothelium. Also, administration of both L-NMMA (100 µM) and indomethacin (10 µM) attenuated the quercetin-induced vasodilatation more than that with L-NMMA alone, but not significantly.
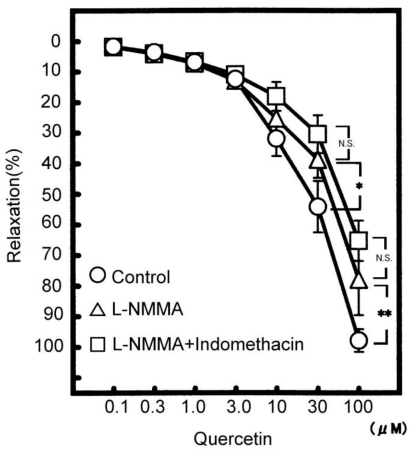
Fig. 1.
Concentration-dependent vasodilatation by cumulative administrations of quercetin. Symbols used are control (open circles, n=10), pretreatment with L-NMMA (triangles, n=5), L-NMMA and indomethacin (squares, n=5). Values (%) are represented as mean±S.E.M. *p<0.05, **p<0.01, with respect to control value.
To investigate whether quercetin produces the relaxation involved with Ca2+ activated K+ channel (KCa channel), the pretreatment with TEA (a KCa channel inhibitor) was carried out in the presence of indomethacin and L-NMMA. The L-NMMA and indomethacin-resistant relaxation induced by quercetin (100 µM) was significantly reduced by 100 µM TEA to 41.1±11.5% (n=5, p<0.01), as shown in Fig. 2. In high K+ (30 mM) solution, furthermore, TEA also attenuated the L-NMMA and indomethacin-resistant relaxation to 43.8±10.2% (n=5, p<0.05). These results indicate that quercetin modulates the KCa channel.
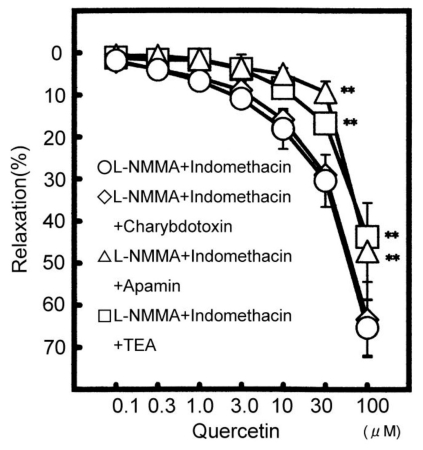
Fig. 2.
Modulation of L-NMMA and indomethacin resistant relaxation at 100 µM quercetin. The vasodilatation in the presence of L-NMMA and indomedhacin (n=5) means as control value in this graph. The vasodilatations by L-NMMA and indomethacin plus TEA (n=5), by L-NMMA and indomethacin plus apamin (n=5), and by L-NMMA and indomethacin plus charybdotoxin (n=5) were compared with that of L-NMMA and indomedhacin. Values (%) are represented as mean±S.E.M. **p<0.01, with respect to control value.
We examined which type of KCa channels quercetin affects. Apamin (0.3 µM), a SK channel inhibitor, strongly decreased the L-NMMA and indomethacin-resistant relaxation induced by 30 µM quercetin from 30.4±6.2% to 9.4±2.7% (n=5, p<0.05) and from 65.2±6.6% to 47.1±11.4% (n=5, p<0.05) by 100 µM quercetin. But charybdotoxin (30 nM), a BK and IK channel inhibitor, had less or no effect (Fig. 2).
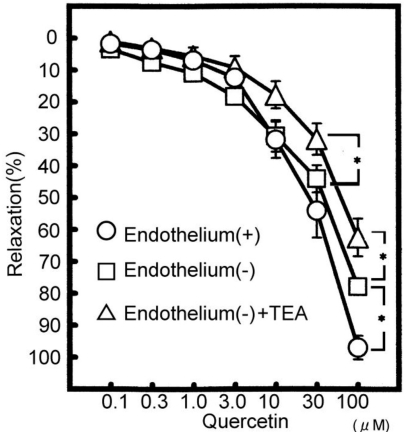
In addition, to clarify which KCa channel on smooth muscle or endothelium quercetin modulates, the experiments using endothelium-denuded aorta were carried out. Under the conditions, TEA significantly decreased the quercetin-induced relaxation from 77.9±2.4% to 62.5±5.9% (n=6, p<0.05) (Fig. 3). Therefore, these results indicate that quercetin affects the KCa channel on smooth muscle cells.
Fig. 3.
Modulation of quercetin (100 µM)-induced vasodilatation (n=10) in the removal of endothelium (n=5) and the removal of endothelium plus TEA (n=5). Values (%) are represented as mean±S.E.M. *p<0.05, with respect to control value.
DISCUSSION
The present experiments in rat aorta strips showed that quercetin caused a concentration-dependent vasodilatation. The vasodilatation was modified (1) by L-NMMA or L-NAME, (2) by removal of endothelium, (3) by both L-NMMA and indomethacin, (4) by both L-NMMA and indomethacin plus TEA, (5) also by apamin but not by charybdotoxin, and (6) by TEA in endothelium-denuded aorta.
A lot of the mechanisms for the vasodilatation induced by quercetin have been shown. However, the mechanisms are still conflicting. In some reports, quercetin has less endothelium-dependent mechanism (Perez-Vizcaino, 2002) or possesses only weak endothelium-dependency (at lower concentrations) (Fusi et al., 2003). In this study, however, quercetin exhibited the remarkable endothelium-dependent actions. NOS inhibitors and removal of endothelium abolished or attenuated the quercetin-induced vasodilatation in rat aorta. Our findings are also supported by other previous reports (Kubota et al., 2001; Ajay et al., 2003). Thus, the vasodilatation by quercetin in rat aorta is considered to be due to NO secretion from endothelium (EDRF). Although the difference is unable to explain now, there might be some conditions to disguise the quercetin's endothelium-dependent mechanisms. In addition, the pretreatment with both indomethacin and L-NMMA reduced the relaxation more strongly than the pretreatment with L-NMMA alone (but not significantly). Thus, it appears possible that the quercetin-induced relaxation is also partly responsible for PGI2 secretion from endothelium, consistent with a report of Ajay et al. (2003).
Quercetin also dilated the KCl-induced vasoconstriction (Duarte et al., 1993). In our laboratory, quercetin dilated the KCl-induced vasoconstriction and the quercetin-induced vasodilatation was inhibited by nicardipine in rat aorta (Nishida and Satoh, 2004; Satoh and Nishida, 2004). These results indicate that quercetin also causes the vasodilatation via its Ca2+ channel inhibitory action. Satoh (2005) has recently reported that quercetin is an inhibitor of Ca2+ channels of cardiomyocytes by means of patch-clamp experiments. On the other hand, quercetin has been shown to be a stimulator of Ca2+ channel (Fusi et al., 2003). From our results, however, it is possible that quercetin inhibits the L-type Ca2+ channel mediated through second messengers such as PK-A, PK-G and PK-C (Satoh and Sperelakis, 1991; 1995; Satoh, 1996).
PK-C phosphrylates tyrosin kinase and vasodilator-stimulated phosphoprotein (VASP) as a substrate of cGMP-dependent protein kinase (cGMP-PK) (Catalin et al., 1995; Moussazadeh and Haimovich, 1998; Wentworth et al., 2006). But genistein (tyrosine kinase inhibitor) in this study failed to affect the quercetin-induced constriction. The activation of MLCK was abolished by PK-C (Hagiwara et al., 1988; Murthy et al., 2000). Furthermore, quercetin inhibits the phosphorylation of mitogen-activated protein kinases (MAPKs); extracellular signal-regulated kinase (ERK) 1/2, p38 MAPK, and c-jun amino-terminal kinase (JNK) in cultured aortic cells and phosphatidylinositol 3-kinase (PI3-kinase)/protein kinase B (Akt), leading to protection of proliferation and inflammation (Shin et al., 2004; Granado-Serrano et al., 2008; Lin et al., 2008; Kwon et al., 2009). Thus, quercetin produces the beneficial effects mediated through many cellular signaling pathways.
It has recently been shown that flavonoids-induced vasodilatation is involved with potassium channels (Calderone et al., 2004). Some types of potassium channels are expressed in vascular smooth muscle cells and cause the vasodilating actions by hyperpolarizing the membrane. The KCa channels are classified by their conductances as follows: BK channel, IK channel, and SK channel (Brayden and Nelson, 1992; Neylon et al., 1999). In the present experiments, three types of KCa channel inhibitors were used. TEA is sensitive to all KCa channels (Neylon et al., 1999), apamin to SK channels (Garcia-Pascual et al., 1995; Murphy and Brayden, 1995), and charybdotoxin to BK and IK channels (Carl et al., 1995; Vogalis et al., 1998; Mitamura et al., 2002). Apamin and TEA attenuated the quercetin-induced vasodilatation in the presence of L-NMMA and indomethacin, but charybdotoxin failed to affect it. Thus, quercetin would selectively possess a sensitivity to SK channel (but less to BK and IK channels) among the KCa channels. In this study, furthermore, TEA attenuated the quercetin-induced vasodilatation of endothelium-denuded rat aorta. Therefore, these results indicate that quercetin regulates the SK channels on smooth muscles.
Most recently, plant polyphenols have been reported to induce endothelium-derived hyperpolarizing factor (EDHF)-type relaxation (Ndiaye et al., 2003). The vasodilatation induced by EDHF has recently been considered to be resistant to both inhibitors of NO synthase and cyclooxygenase (Chen and Suzuki, 1990; Fukao et al., 1997; Félétou and Vanhoutte, 2002). Furthermore, the EDHF-induced relaxation is attenuated by high K+ or TEA (Campbell et al., 1996; Martinez-Orgado et al., 1999). In our laboratory, the quercetin-induced vasodilatation had less or no involvement in EDHF in rat aorta and mesenteric artery (unpublished data). Therefore, it is concluded that quercetin possesses no effect on EDHF.
In conclusion, quercetin caused a concentration-dependent vasodilatation. Quercetin's effects are due to endothelium-dependent actions mediated through the NO (EDRF) and partly PGI2 syntheses, and also to endothelium-independent actions mediated through the Ca2+ channel and PK-C inhibitions (Nishida and Satoh, 2004; Satoh and Nishida, 2004). Moreover, quercetin modulates the KCa channels on smooth muscles with the selectivity to SK channel. Thus, quercetin possesses multiple mechanisms. Further studies are needed to elucidate the detailed mechanism about the quercetin-induced vasodilatation.
ACKNOWLEDGEMENTS
This work was in part supported by the grants from Tokiwa Phytochemical Co.
ABBREVIATIONS
- Akt
phosphatidylinositol 3-kinase (PI3-kinase)/protein kinase B
- BK
big conductance KCa channel
- cGMP-PK
cGMP-dependent protein kinase
- EDHF
endothelium-derived hyperpolarizing factor
- EDRF
endothelium-derived releasing factor
- EGTA
ethylene glycol-O,O'-bis (2-aminoethyl)-N,N,N',N'-tetraacetic acid
- ERK
extracellular signal-regulated kinase
- IK
intermediate conductance KCa channel (37 pS)
- JNK
c-jun amino-terminal kinase
- KCa
Ca2+ activated K+
- L-NMMA
NG-monomethyl-L-arginine acetate
- L-NAME
L-NG-nitro arginine methyl ester
- MAPKs
mitogen-activated protein kinases
- NE
norepinephrine
- NOS
NO synthesis
- PK-C
protein kinase C
- SK
small conductance KCa channel
- TEA
tetraethylammonium
- VASP
vasodilator-stimulated phosphoprotein
References
- 1.Ajay M, Gilani AH, Mustafa MR. Effects of flavonoids on vascular smooth muscle of the isolated rat thoracic aorta. Life Sci. 2003;74:603–612. doi: 10.1016/j.lfs.2003.06.039. [DOI] [PubMed] [Google Scholar]
- 2.Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992;256:532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- 3.Calderone V, Chericoni S, Martinelli C, Tetai L, Nardi A, Morelli I. Vasorelaxing effects of flavonoids: investigation on the possible involvement of potassium channels. Naunyn Schmiedebergs Arch Pharmacol. 2004;370:290–298. doi: 10.1007/s00210-004-0964-z. [DOI] [PubMed] [Google Scholar]
- 4.Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- 5.Carl A, Bayguinov O, Shuttleworth CW, Ward SM, Sanders KM. Role of Ca2+ activated K+ channels in electrical activity of longituidinal and circular muscle layers of canine colon. Am J Physiol. 1995;268:619–627. doi: 10.1152/ajpcell.1995.268.3.C619. [DOI] [PubMed] [Google Scholar]
- 6.Catalin MF, Eugen B, Gabi H, Sebastian S, Ovidu B, Dimitrie DB. Multiple effects of tyrosine kinase inhibitors on vascular smooth muscle contraction. Eur J Pharmacol. 1995;281:29–35. doi: 10.1016/0014-2999(95)00220-f. [DOI] [PubMed] [Google Scholar]
- 7.Chen CK, Pace-Asciak CR. Vasorelaxing activity of reveratrol and quercetin in isolated rat aorta. Gen Pharmacol. 1996;27:363–366. doi: 10.1016/0306-3623(95)02001-2. [DOI] [PubMed] [Google Scholar]
- 8.Chen GF, Suzuki H. Calcium dependency of the endothelium-dependent hyperpolarization in smooth muscle cells of the rabbit carotid artery. J Physiol(Lond) 1990;421:521–534. doi: 10.1113/jphysiol.1990.sp017959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Congolludo A, Frazziano G, Briones AM, Cobeno L, Moreno L, Lodi F. The dietary flavonoid quercetin activates BKCa currents in coronary arteries via production of H2O2. Role in vasodilatation. Cardiovasc Res. 2007;73:424–431. doi: 10.1016/j.cardiores.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Duarte J, Perez-Vizcaino F, Zarzurelo J, Tamargo J. Vasodilator effects of quercetin in isolated rat vascular smooth muscle. Eur J Pharmacol. 1993;239:1–7. doi: 10.1016/0014-2999(93)90968-n. [DOI] [PubMed] [Google Scholar]
- 11.Duarte J, Perez-Vizcaino F, Utrilla P, Jiménez J, Tamargo J, Zarzuelo A. Vasodilatory effects of flavonoids in rat aortic smooth muscle. Structure-activity relation ship. Gen Pharmacol. 1993;24:857–862. doi: 10.1016/0306-3623(93)90159-u. [DOI] [PubMed] [Google Scholar]
- 12.Félétou M, Vanhoutte PM. The Alternative: EDHF. J Mol Cell Cardiol. 2002;31:15–22. doi: 10.1006/jmcc.1998.0840. [DOI] [PubMed] [Google Scholar]
- 13.Formica JV, Regelson W. Review of the biology of quercetin and related bioflavonoids. Food Chem Toxicol. 1995;33:1061–1080. doi: 10.1016/0278-6915(95)00077-1. [DOI] [PubMed] [Google Scholar]
- 14.Fukao M, Hattori Y, Kanno M, Sakuma I, Kitabatake A. Sources of Ca2+ in relation to generation of acetylcholine-induced endothelium-dependent hyperpolarization in rat mesenteric artery. Br J Pharmacol. 1997;120:1328–1334. doi: 10.1038/sj.bjp.0701027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fusi F, Saponara S, Pessina F, Gorelli B, Sgaragli G. Effects of quercetin and rutin on vascular preparations. A comparison between mechanichal and electrophysiological phenomena. Eur J Nutr. 2003;42:10–17. doi: 10.1007/s00394-003-0395-5. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Pascual A, Labadia A, Jimenez E, Costa G. Endothelium-dependent relaxation to acetylcholine in bovine oviductal arteries: mediation by nitric oxide and changes in apamin-sensitive K+ conductance. Br J Pharmacol. 1995;105:429–435. doi: 10.1111/j.1476-5381.1995.tb15029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Granado-Serrano AB, Angeles Martin M, Bravo L, Gaya L, Ramos S. Time-course regulation of quercetin on cell survival/proliferation pathways in human hepatoma cells. Mol Nutr Food Res. 2008;52:457–464. doi: 10.1002/mnfr.200700203. [DOI] [PubMed] [Google Scholar]
- 18.Hagiwara M, Inoue S, Tanaka T, Nunoki K, Ito M, Hidaka H. Differential effects of flavonoids as inhibitors of tyrosine protein kinases and serin/threonine protein kinases. Biochem Pharmacol. 1988;37:2987–2992. doi: 10.1016/0006-2952(88)90286-9. [DOI] [PubMed] [Google Scholar]
- 19.Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhaut D. Dietary antioxidant flavonoids and risk of coronary heart disease: the zutphen elderly study. Lancet. 1993b;342:1007–1011. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- 20.Hertog MG, Hollman PC, Katan MB, Kromhout D. Intake of potentially anticarcinogenic flavonoids and determinants in adults in the Netherlands. Nutr Cancer. 1993a;20:21–29. doi: 10.1080/01635589309514267. [DOI] [PubMed] [Google Scholar]
- 21.Kubota Y, Tanaka T, Umegaki K. Ginkgo biloba extract-induced relaxation of rat aorta is associated with increase in endothelial intracellular calcium level. Life Sci. 2001;69:2327–2336. doi: 10.1016/s0024-3205(01)01303-0. [DOI] [PubMed] [Google Scholar]
- 22.Kwon SH, Nam JI, Kim SH, Kim JH, Yoon JH, Kim KS. Kaempferol and quercetin, essential ingredients in Ginkgo biloba extrat, inhibit interleukin-1β-induced MUC5AC gene expression in human airway epithelial cells. Phytother Res. 2009 doi: 10.1002/ptr.2817. (in press) [DOI] [PubMed] [Google Scholar]
- 23.Lin CW, Hou WC, Shen SC, Juan SH, Ko CH, Wang LM, Chen YC. Quercetin inhibition of tumor invasion via suppressing PKCδ/ERK/AP-1-dependent matrix metalloproteinase-9 activation in breast carcinoma cells. Carcinogenesis. 2008;29:1807–1815. doi: 10.1093/carcin/bgn162. [DOI] [PubMed] [Google Scholar]
- 24.Martinez-Orgado J, Gonzalez R, Alonso MJ, Marin J. Nitric oxide-dependent and -independent mechanisms in the relaxation elicited by acetylcholine in fetal rat aorta. Life Sci. 1999;64:269–277. doi: 10.1016/s0024-3205(98)00562-1. [DOI] [PubMed] [Google Scholar]
- 25.Mitamura M, Boussery K, Horie S, Murayama T, Voorde JV. Vasorelaxing effect of mesaconitine, an alkaloid from aconicum japonicum, on rat small gastric artery: possible involvement of endothelium-derived hyperpolarizing factor. Jpn J Pharmacol. 2002;89:380–387. doi: 10.1254/jjp.89.380. [DOI] [PubMed] [Google Scholar]
- 26.Moussazadeh M, Haimovich B. Protein kinase C-delta activationand tyrosine phophorylation in platelets. FEBS Lett. 1998;438:225–230. doi: 10.1016/s0014-5793(98)01302-7. [DOI] [PubMed] [Google Scholar]
- 27.Murota K, Terao J. Antioxidative flavonoid quercetin; implication of its intestinal absorption and metabolism. Arch Biochem Biophys. 2003;417:12–17. doi: 10.1016/s0003-9861(03)00284-4. [DOI] [PubMed] [Google Scholar]
- 28.Murphy ME, Brayden JE. Apamin-sensitive K+ channels mediate an endothelium-dependent hyperporalization in rabbit mesenteric arteries. J Physiol (Lond) 1995;489:723–724. doi: 10.1113/jphysiol.1995.sp021086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murthy KS, Grider JR, Kuemmerle JF, Makhlouf GM. Sustained muscle contraction induced by agonists, growth factors, and Ca2+ mediated by distinct PKC isozymes. Am J Physiol Gastrointest Liver Physiol. 2000;279:G201–G210. doi: 10.1152/ajpgi.2000.279.1.G201. [DOI] [PubMed] [Google Scholar]
- 30.Ndiaye M, Chataigneau T, Andriantsitohaina R, Stoclet JC, Schini-Kerth VB. Red wine polyphenols cause endothelium-dependent EDHF-mediated relaxations in porcine coronary arteries via a redox-sensitive mechanisms. Biochem Biophys Res Commun. 2003;310:371–377. doi: 10.1016/j.bbrc.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 31.Neylon CB, Lang RJ, Fu Y, Bobik A, Reinhart PH. Molecular cloning and characterization of the intermediate-conductance Ca2+-activated K+ channel in vascular smooth muscle: relationship between K (Ca) channel diversity and smooth muscle cell function. Circ Res. 1999;85:33–43. doi: 10.1161/01.res.85.9.e33. [DOI] [PubMed] [Google Scholar]
- 32.Nishida S, Satoh H. Comparative vasodilating actions among terpenoids and flavonoids contained in Ginkgo biloba extract. Clin Chim Acta. 2004;339:129–133. doi: 10.1016/j.cccn.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Perez-Vizcaino F, Ibarra M, Cogolludo AL, Duarte J, Zaragoza-Arnaez F, Moreno L. Endothelium-Independent Vasodilator effects of the flavonoid quercetin and its methylated metabolites in rat conductance and resistance arteries. J Pharmacol Exp Ther. 2002;302:66–72. doi: 10.1124/jpet.302.1.66. [DOI] [PubMed] [Google Scholar]
- 34.Satoh H. Comparative electropharmacological actions of some constituents from Ginkgo biloba extract in guinea pig ventricularcardiomyocytes. Evid Based Complement Altern Med. 2005;1:277–284. doi: 10.1093/ecam/neh044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Satoh H, Nishida S. Electropharmacological actions of Ginkgo biloba extract on vascular smooth and heart muscles. Clin Chim Acta. 2004;342:13–22. doi: 10.1016/j.cccn.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 36.Satoh H, Sperelakis N. Calcium and potassium currents in cultured in rat aortic vascular smooth muscle cell lines. In: Sperelakis N, editor. Ion Channels of Vascular Smooth Muscle Cells and Endothelial Cells. New York: Academic Press; 1991. pp. 55–63. [Google Scholar]
- 37.Satoh H, Sperelakis N. Modulation of L-typeCa2+ current by isoprenaline, carbachol and phorbol ester in cultured rat aorticvascular smooth muscle (A7r5) cells. Gen Pharmacol. 1995;26:369–379. doi: 10.1016/0306-3623(94)00193-q. [DOI] [PubMed] [Google Scholar]
- 38.Satoh H. Modulation of Ca2+-activated K+ current by isoprenaline, carbachol, and phorbol ester in cultured (and fresh) rat aortic vascular smooth muscle cells. Gen Pharmacol. 1996;27:319–324. doi: 10.1016/0306-3623(95)02005-5. [DOI] [PubMed] [Google Scholar]
- 39.Shin CM, Lin H, Liag YC, Lee WS, Bi WF, Juan SH. Concentration-dependent differential effects of quercetin on rat aortic smooth muscle cell. Eur J Pharmacol. 2004;496:41–44. doi: 10.1016/j.ejphar.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 40.Sticher O. Quality of Ginkgo preparations. Planta Med. 1993;59:2–11. doi: 10.1055/s-2006-959593. [DOI] [PubMed] [Google Scholar]
- 41.Vogalis F, Zhang Y, Goyal RK. An intermediate conductance K+ channel in the cell membrane of mouse intestinal smooth musucle. Biochim Biophys Acta. 1998;1371:309–316. doi: 10.1016/s0005-2736(98)00029-7. [DOI] [PubMed] [Google Scholar]
- 42.Wentworth JK, Pula G, Poole AW. Vasodilator-stimulated phosphoprotein (VASP) is phosphorylated on Ser157 by protein kinase C-dependent and -independent mechanisms in thrombin-stimulated human platelets. Biochem J. 2006;393:555–564. doi: 10.1042/BJ20050796. [DOI] [PMC free article] [PubMed] [Google Scholar]