Abstract
Angiotensin II (Ang II) plays an important role in vascular hypertension. The role of the chemokine CCL5 on Ang II-induced activities in vascular smooth muscle cells (VSMCs) has not been studied. In this study, we elucidated the effect of CCL5 on Ang II-induced 12-lipoxygenase (LO) expression and cell proliferation in spontaneously hypertensive rats (SHR) VSMCs. CCL5 decreased Ang II-induced 12-LO mRNA expression and protein production, and it increased Ang II type 2 (AT2) receptor expression in SHR VSMCs. The inhibitory effect of CCL5 on Ang II-induced 12-LO mRNA expression was mediated through the AT2 receptor. Although treatment of CCL5 alone induced SHR VSMCs proliferation, CCL5 inhibited Ang II-induced VSMCs proliferation and PD123,319, an AT2 receptor antagonist, blocked the inhibitory effect of CCL5 on Ang II-induced VSMCs proliferation. Phosphorylation of p38 was detected in VSMCs treated with Ang II or CCL5 alone. But, decrease of p38 phosphorylation was detected in VSMCs treated with Ang II and CCL5 simultaneously (Ang II/CCL5) and PD123,319 increased p38 phosphorylation in VSMCs treated with Ang II/CCL5. Therefore, these results suggest that the inhibitory effect of CCL5 on Ang II-induced VSMCs proliferation is mediated by the AT2 receptor via p38 inactivation, and CCL5 may play a beneficial role in Ang II-induced vascular hypertension.
Keywords: Angiotensin II, Angiotensin II type 2 receptor, Cell proliferation, Chemokine CCL5, 12-lipoxygenase
INTRODUCTION
Angiotensin II (Ang II) plays a major role as a potent vasoconstrictor and blood pressure regulator, and it has also been shown to act as a potential mediator of inflammation (Han et al., 1999; Kashiwagi et al., 2002; Zheng Y et al., 2005). The 12-lipoxygenase (LO) pathway of arachidonic acid metabolism has been linked to cell growth and the pathology of hypertension (Preston et al., 2006). Ang II is a potent positive regulator of 12-LO expression and proliferation in porcine and human vascular smooth muscle cells (VSMCs) (Natarajan et al., 1993; Kim et al., 1995). Increased levels of 12-LO induced by cytokines in porcine VSMCs and elevated activity of 12-LO in the plasma of spontaneously hypertensive rats (SHR) have been reported (Natarajan et al., 1997; Sasaki et al., 1997).
The inflammatory chemokine CCL5 (regulated upon activation, normally T-cell expressed, and presumably secreted; RANTES) is a potent chemoattractant for memory T lymphocytes and monocytes/macrophages, and its production has been described in various types of cells, including human aortic VSMCs (Schall et al., 1990; Jordan et al., 1997). CCL5 plays a functional role in acute and chronic inflammatory responses in atherosclerosis, renal disease progression and vascular wall remodeling in pulmonary arterial hypertension (Wolf et al., 1997; Zoja et al., 1998; Dorfmuller et al., 2002; Kashiwagi et al., 2002). Although the over production of CCL5 is associated with diverse disease progressions, we observed lower expression of CCL5 in SHR VSMCs than in normotensive Wistar-Kyoto rats (WKY) VSMCs. CCL5 has been known to have down-regulatory effect on LPS-induced cytokines expression in human peripheral blood monocytes (Shahrara et al., 2006), and have a possible neuroprotective role in the brains of individual with Alzheimer's disease (Tripathy et al., 2008). We therefore hypothesized that although CCL5 acts as an inflammatory mediator in various disease processes, it may also downregulate Ang II-induced hypertensive activities.
Therefore, we examined the mechanism of action of CCL5 in Ang II-induced hypertensive activities, focusing on 12-LO expression in SHR VSMCs and on VSMCs proliferation.
METHODS
Reagent
Trizol reagent for total RNA isolation was purchased from Invitrogen (Carlsbad, CA, USA). Dulbecco's phosphate-buffered saline (PBS), Dulbecco's modified Eagle's medium (DMEM), penicillin-streptomycin and fetal bovine serum (FBS) were purchased from Gibco/BRL (Life Technologies, Gaithersburg, MD, USA). Recombinant human CCL5 was purchased from R&D systems (Minneapolis, MN, USA). Ang II was obtained from Calbiochem (San Diego, CA, USA). Losartan was obtained from MSD (Delaware, MD, USA). PD123,319 was obtained from Sigma Chemical Co. (St Louis, MO, USA). [3H]-thymidine was purchased from PerkinElmer (Boston, MA, USA). Oligonucleotide primers for real-time PCR for 12-LO, Ang II type 2 (AT2) receptor, ornithine decarboxylase (ODC) and β-actin were synthesized by Bionics (Seoul, Korea). LightCycler FastStart DNA SYBR Green I Mix was obtained from Roche (Mannheim, Germany). The p-38 MAP kinase, phospho-p38 MAP kinase, and 12-LO antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The AT2 receptor antibody was purchased from Abcam (Cambridge, UK). The γ-tubulin antibody was obtained from Sigma Chemical Co. (St. Louis, MO, USA). All other reagents were pure-grade commercial preparations.
Experimental animals
Specific pathogen-free male inbred SHR or WKY, 12~16 weeks of age, were purchased from Japan SLC Inc. (Shizuka). All experimental animals received autoclaved food and bedding to minimize exposure to viral or microbial pathogens. The rats were cared for in accordance with the Guide for the Care and Use of Experimental Animals of Yeungnam Medical Center.
VSMCs preparation
VSMCs were obtained using an explant method from the thoracic aortas of 12-week-old male SHR and WKY as described by Kim et al. (2008). VSMCs were cultured in Dulbecco's modified Eagle's medium (DMEM) that was supplemented with 10% FBS and penicillin-streptomycin. Cells were detached with 0.25% trypsin/EDTA and seeded into 75-cm2 tissue culture flasks at a density of 105 cells per ml. All experiments were conducted between cell passages 3 to 7. Prior to stimulation, 95%-confluent VSMCs were serum-starved overnight by incubating in DMEM with 0.1% FBS. Cell cultures were incubated in a humidified incubator at 37℃ and 5% CO2 in the presence or absence of stimuli for the indicated times.
Preparation of total RNA, reverse transcriptase-polymerase chain reaction (RT-PCR) and real-time polymerase chain reaction (real-time PCR)
Total RNA was extracted using Trizol reagent according to the manufacturer's instructions. The quantity of total RNA obtained was determined by measuring the optical density (OD) at 260 and 280 nm.
One µg of total RNA per sample was reverse transcribed using Moloney murine leukemia virus reverse transcriptase (Perkin Elmer, Norwalk, CT, USA) and oligo dT priming according to the manufacturer's instruction, at 42℃ for 15 minutes. Amplification with specific primers was performed in a Gene Amp PCR system 9600 (Perkin Elmer) for 35 cycles with a 30 s/94℃ denaturation, 30 s/62℃ annealing, 1 min/72℃ extension profile in the case of CCL5; for 30 cycles with a 20 s/95℃ denaturation, 30 s/60℃ annealing, 30 s/72℃ extension profile in the case of β-actin. Amplification of mRNA for the housekeeping gene β-actin was used as an internal quality standard. Amplified products were electrophoresed on 1.5~2% agarose gel stained with 0.5 µg/ml ethidium bromide. The primer sequences were as follows: β-actin (101 bp) sense, 5'-tactgccctggctcctagca-3', antisense, 5'-tggacagtgaggccaggatag-3'; CCL5 (110 bp) sense, 5'-cgtgaaggagtatttttacaccagc-3', antisense, 5'-cttgaacccacttcttctctggg-3'.
Real-time PCR for the amplification of the 12-LO, ODC, AT2 receptor was performed using the LightCycler (Roche, Germany). RNA was reverse transcribed to cDNA from 1 µg of total RNA and then subjected to real-time PCR. PCR was performed in triplicate. The total PCR volume was 20 µl and contained the LightCycler FastStart DNA SYBR Green I mix (Roche, Germany), primer and 2 µl of cDNA. Prior to PCR amplification, the mixture was incubated at 95℃ for 10 min, and the amplification step consisted of 45 cycles of denaturation (10 s at 95℃), annealing (5 s at the primer-appropriate temperature), and extension (10 s at 72℃) with fluorescence detection at 72℃ after each cycle. After the final cycle, melting point analyses of all samples were performed over the range of 65 to 95℃ with continuous fluorescence detection. β-actin expression levels were used for sample normalization. Results for each gene are expressed as the relative expression level compared with β-actin. The primers used for PCR are as follows: 12-LO (312 bp) sense, 5'-tggggcaactggaagg-3', antisense, 5'-agagcgcttcagcaccat-3'; AT2 receptor (65 bp) sense, 5'-ccgtgaccaagtctttgaagatg-3', antisense, 5'-agggaagccagcaaatgatg-3'; ODC (125 bp) sense, 5'-agccaggcagatactacgtc-3', antisense, 5'-catcaaagtttgctcgtttg-3'; β-actin (101 bp) sense, 5'-tactgccctggctcctagca-3', antisense, 5'-tggacagtgaggccaggatag-3'. The levels of 12-LO, AT2 receptor and ODC mRNA were determined by comparing experimental levels to standard curves and are expressed as relative fold expressions.
Enzyme-linked immunosorbent assay (ELISA) for CCL5 production
The CCL5 protein levels in cell media were measured with an ELISA kit that was obtained from eBioscience (San Diego, CA, USA). All procedures were performed in accordance with the manufacturer's instructions.
Western blotting
Total lysates were prepared in PRO-PREP buffer (iNtRON, Seoul, Korea). Protein concentrations were determined by a Bradford assay (Bio-Rad, CA, USA) using bovine serum albumin as a standard. Twenty microgram protein samples were separated on 10% SDS-polyacrylamide gels and then transferred to nitrocellulose membranes. The membranes were soaked in 5% nonfat dried milk in TBST (10 mmol/l Tris/HCl pH 7.5, 150 mmol NaCl and 0.05% Tween-20) for 1 h and then incubated for 16~18 h with primary antibodies against 12-LO, AT2 receptor, p38 MAP kinase, phospho-p38 MAP kinase and γ-tubulin at 4℃. Membranes were washed three times with TBST for 10 min and then incubated with horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. The membranes were rinsed three times with TBST for 10 min and antigen-antibody complexes were detected using the enhanced chemiluminescence detection system (LAS-3000; Fujifilm, Tokyo).
Small interfering RNA (siRNA)
To confirm whether the AT2 receptor pathway contributes to the inhibitory effect of CCL5 on Ang II-induced 12-LO expression and VSMCs proliferation, AT2 receptor expression was silenced using a small interfering RNA (siRNA). VSMCs were plated on 24-well plates and grown to 90% confluence. VSMCs were then transfected with AT2 receptor siRNA oligomers (50 nmol/l) using lipofectamine 2000 in accordance with the manufacturer's instructions. After 24 h of incubation, VSMCs were placed in growth medium for 24 h before the experiments. Cells were then cultured in the presence or absence of stimuli for 4 h. Sense and antisense oligonucleotides corresponding to the rat AT2 receptor siRNA sequence (sense, 5'-uacauuugcgaaguucaucuucggc-3'; antisense, 5'-gccgaagaugaacuucgcaaaugua-3') were purchased from Invitrogen (Carlsbad, CA, USA).
VSMCs proliferation
VSMCs were plated in 24-well plates for 24 h and then exposed to the stimulant. [3H]-thymidine (1 µCi/ml) (PerkinElmer precisely, Boston, MA, USA) was added to the plates during the last 24 h of incubation. The cells were subsequently washed three times with cold PBS. [3H]-thymidine-labeled cells were collected with 0.1% SDS, and radioactivity was counted using a Packard scintillation counter (Packard Instrument Company, Meriden, USA).
Statistical analysis
Results are expressed as means±SD from at least three or four independent experiments. For comparisons between multiple groups, statistical significance was determined by the Mann-Whitney test using SPSS v. 12.0.
RESULTS
CCL5 inhibits Ang II-induced 12-LO expression
SHR is the most widely studied animal model of essential hypertension. In SHR, enhanced responsiveness to Ang II in VSMCs had been reported (Resink et al., 1989) and Ang II appears to play one of the most important roles in the increase in vascular resistance (Touyz et al., 1999).
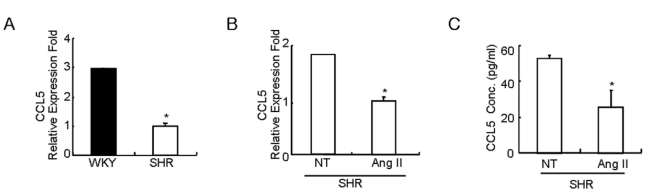
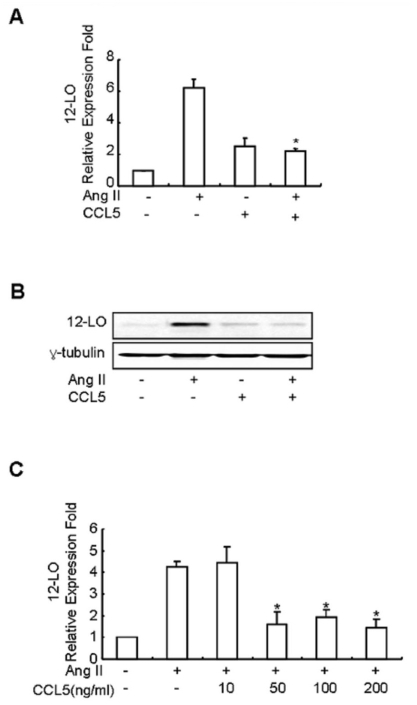
First, we compared the constitutive expression of CCL5 mRNA in SHR VSMCs with the expression in WKY VSMCs. Expression of CCL5 mRNA was found to be lower in SHR VSMCs relative to WKY VSMCs (Fig. 1A). In addition, Ang II inhibited the expression of CCL5 mRNA and protein prodction in SHR VSMCs (Fig. 1B, C). We next examined the direct effect of CCL5 on Ang II-induced 12-LO mRNA expression in SHR VSMCs. SHR VSMCs were untreated or treated with Ang II (0.1 µmol/l), CCL5 (100 ng/ml) or Ang II and CCL5 simultaneously (AngII/CCL5) for 2 h. CCL5 itself increased the expression of 12-LO mRNA, although the expression level was lower than that induced by Ang II. However, CCL5 decreased the expression of Ang II-induced 12-LO mRNA to the level of CCL5-induced 12-LO expression (Fig. 2A). Downregulation of Ang II-induced 12-LO protein by CCL5 was also detected (Fig. 2B). We also observed the dose response of Ang II-induced 12-LO expression to CCL5. CCL5 at 10 ng/ml had no inhibitory effect on Ang II-induced 12-LO expression, but doses of CCL5 ranging from 50 ng/ml to 200 ng/ml were shown to have a similar, remarkable inhibitory effect on Ang II-induced 12-LO expression (Fig. 2C).
Fig. 1.
Costitutive expression of CCL5 mRNA in SHR VSMCs is lower than the expression in WKY VSMCs, and Ang II inhibits CCL5 mRNA expression and protein production in SHR VSMCs. (A) After total RNAs were isolated from SHR or WKY VSMCs, real-time PCR was performed. Bars represent means±SD from three independent experiments. *p<0.05 vs. WKY VSMCs. (B, C) SHR VSMCs were untreated or treated with Ang II (0.1 µmol/l). After total RNAs and cell supernatants were isolated, real time PCR (B) and ELISA (C) were performed. Bars represent means±SD from three independent experiments. *p<0.05 vs. untreated VSMCs.
Fig. 2.
CCL5 inhibits Ang II-induced 12-LO mRNA expression in SHR VSMCs. (A) SHR VSMCs were untreated or treated with Ang II (0.1 µmol/l) and/or CCL5 (100 ng/ml) for 2 h. After total RNAs were isolated, real-time PCR was performed. Bars represent means±SD from three independent experiments. *p<0.05 vs. VSMCs treated with Ang II. (B) SHR VSMCs were untreated or treated with Ang II (0.1 µmol/l) and/or CCL5 (100 ng/ml) for 2 h. Cell lysates were prepared and separated on 10% SDS-polyacrylamide gels and then immunoblotted with 12-LO antibody. The data shown are representative of three independent experiments. (C) Dose response of Ang II-induced 12-LO mRNA expression in SHR VSMCs to CCL5. SHR VSMCs were untreated (NT) or treated with 0, 10, 50, 100, or 200 ng/ml of CCL5 for 2 h. After total RNAs were isolated, real-time PCR was performed. Bars represent means±SD from three independent experiments.
Action mechanism of CCL5 on Ang II-induced 12-LO expression in SHR VSMC
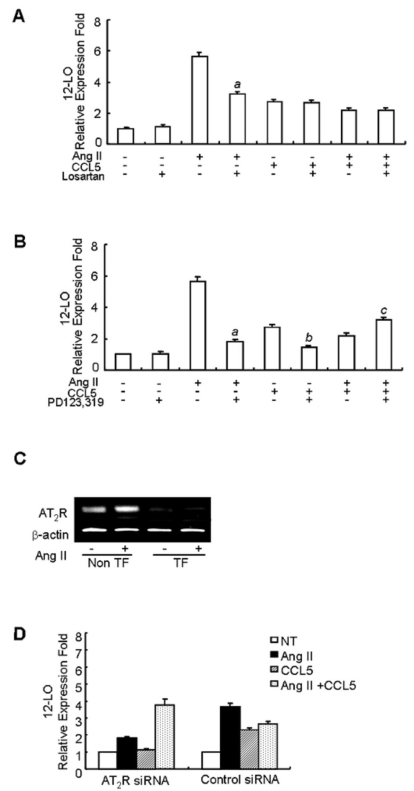
We examined whether the inhibitory effect of CCL5 on Ang II-induced 12-LO expression is mediated by the AT1 or AT2 receptor. SHR VSMCs were untreated or treated with Ang II (0.1 µmol/l) and/or CCL5 (100 ng/ml) in the presence or absence of an antagonist of the AT1 receptor, losartan (10 µmol/l), or an antagonist of the AT2 receptor, PD123,319 (10 µmol/l) for 2 h, and the total RNAs were analyzed by real-time PCR. The expression of Ang II-induced 12-LO mRNA was inhibited by both losartan and PD123, 319 (Fig. 3A, B). However, Losartan had no effect on CCL5- or Ang II/CCL5-induced 12-LO mRNA expression (Fig. 3A). PD123,319 decreased the expression of CCL5-induced 12-LO mRNA and inhibitory effect of CCL5 on Ang II-induced 12-LO expression (Fig. 3B). To confirm these results, real-time PCR was performed on samples treated with AT2 receptor-directed siRNA. First, successful transfection of AT2 receptor siRNA into SHR VSMCs were observed by RT-PCR. Ang II-induced expression of the AT2 receptor was not detected in VSMCs transfected with AT2 receptor siRNA (Fig. 3C). In VSMCs transfected with AT2 receptor siRNA, Ang II decreased 12-LO mRNA expression remarkably, CCL5 decreased 12-LO expression to the basal level, but Ang II/CCL5 did not inhibit 12-LO mRNA expression (Fig. 3D).
Fig. 3.
The inhibitory effect of CCL5 on Ang II-induced 12-LO mRNA expression is mediated through the AT2 receptor in SHR VSMCs. (A, B) SHR VSMCs were untreated or treated with Ang II (0.1 µmol/l) and/or CCL5 in the presence or absence of losartan (AT1 receptor antagonist, 10 µmol/l, A) or PD123,319 (AT2 receptor antagonist, 10 µmol/l, B) for 2 h and total RNAs were analyzed by real-time PCR. Bars represent means±SD from three independent experiments. ap <0.05 vs. VSMCs treated with Ang II. bp <0.05 vs. VSMCs treated with CCL5, cp <0.05 vs. VSMCs treated with Ang II and CCL5 simultaneously (Ang II/CCL5). (C, D) SHR VSMCs were plated on 24-well plates, grown to 90% confluence and then transfected with AT2 receptor siRNA oligomers (50 nmol/l). VSMCs were then untreated or treated with AngII (0.1 µmol/l) for 2 h for RT-PCR (C) and VSMCs were untreated or treated with AngII (0.1 µmol/l) and/or CCL5 (100 ng/ml) for 2 h, and total RNAs were analyzed by real-time PCR (D). Non TF: non-transfected VSMCs, TF: transfected VSMCs. Bars represent means±SEM from two independent experiments. The data are representative of two independent experiments.
CCL5 increases the expression of the AT2 receptor in SHR VSMCs
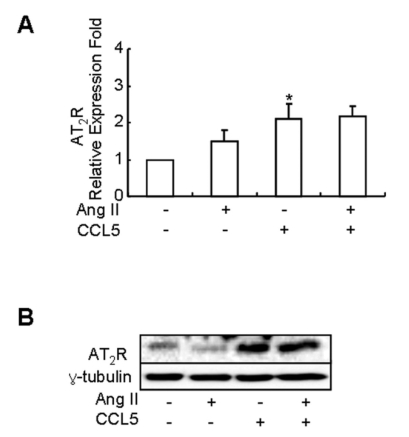
It is widely accepted that Ang II exerts its effects on hypertension-related genes in cells through Ang II subtype receptors (Wolf et al., 2002). Most pathophysiological actions of Ang II are mediated through the AT1 receptor, and the AT2 receptor has been reported to antagonize the action of the AT1 receptor (Horiuchi et al., 1999; Gallinat et al., 2000). And, PD123,319 decreased the inhibitory effect of CCL5 on Ang II-induced 12-LO mRNA expression. Thus, we examined the effect of CCL5 on AT2 receptor mRNA expression in SHR VSMCs treated with Ang II. SHR VSMCs were untreated or treated with Ang II (0.1 µmol/l), CCL5 (100 ng/ml) or AngII/CCL5 for 2 h. CCL5 induced the expression of AT2 receptor mRNA, although the expression of AT2 receptor mRNA was not remarkable. Ang II/CCL5 also induced AT2 receptor mRNA expression (Fig. 4A). As further confirmation of these results, we performed western blotting to detect protein production. an increase in AT2 receptor protein was detected in cells treated with CCL5 or AngII/CCL5 (Fig. 4B).
Fig. 4.
CCL5 increases the mRNA expression and production of AT2 receptor in SHR VSMCs. SHR VSMCs were untreated (NT) or treated with CCL5 (100 ng/ml) and or Ang II (0.1 µmol/l) for 2 h. Then, total RNAs for real time PCR (A) and cell lysates for protein detection (B) were isolated. Cell lysates were separated on 10% SDS-polyacrylamide gels and then immunoblotted with AT2 receptor antibody. Bars represent means±SD from four independent experiments *p<0.05 vs. untreated SHR VSMCs. The data shown are representative of three independent experiments.
Inhibitory mechanism of CCL5 on Ang II-induced SHR VSMCs proliferation
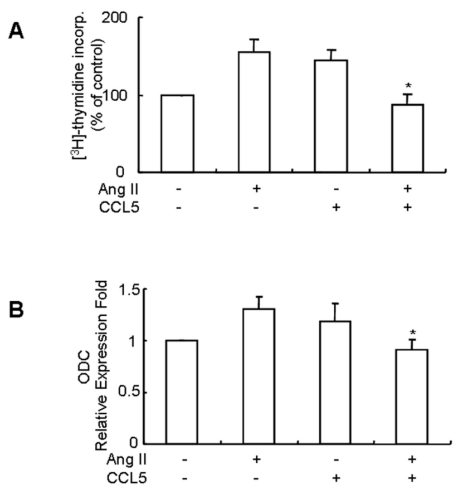
VSMCs proliferation is one of major events in the development of hypertension, and Ang II induces rat and human VSMCs proliferation (Mabrouk et al., 2001), therefore, we examined the effect of CCL5 on Ang II-induced VSMCs proliferation. SHR VSMCs were untreated or treated with Ang II and/or CCL5 for 24 h. Both Ang II and CCL5-independently induced the proliferation of SHR VSMCs. However, simultaneous treatment with Ang II and CCL5 (Ang II/CCL5) inhibited the proliferation of VSMCs (Fig. 5A). Ornithine decarboxylase (ODC) is the first and rate-controlling enzyme in the synthesis of polyamines, which are essential for normal cell growth (Wei et al., 2008). Therefore, an increase in ODC expression is associated with an increase in cell proliferation. As shown in Fig. 5B, treatment with Ang II or CCL5 for 2 h increased ODC mRNA expression. However, Ang II/CCL5 treatment did not increase ODC mRNA expression. The expression pattern of ODC mRNA correlated with SHR VSMCs proliferation.
Fig. 5.
CCL5 induces SHR VSMCs proliferation, but simultaneous treatment of Ang II and CCL5 inhibits SHR VSMCs proliferation. (A) SHR VSMCs were treated with AngII (0.1 µmol/l) and/or CCL5 (100 ng/ml) for 24 h in medium containing [3H]-thymidine (1 µCi/ml). [3H]-thymidine incorporation is shown on the Y-axis. Bars represent means±SD from three independent experiments. *p<0.05 vs. VSMCs treated with Ang II. (B) SHR VSMCs were untreated (NT) or treated with Ang II (0.1 µmol/l) and/or CCL5 (100 ng/ml) for 2 h. Total RNAs were isolated and real-time PCR for ODC mRNA expression was performed. Bars represent means±SD from three independent experiments. *p<0.05 vs. VSMCs treated with Ang II.
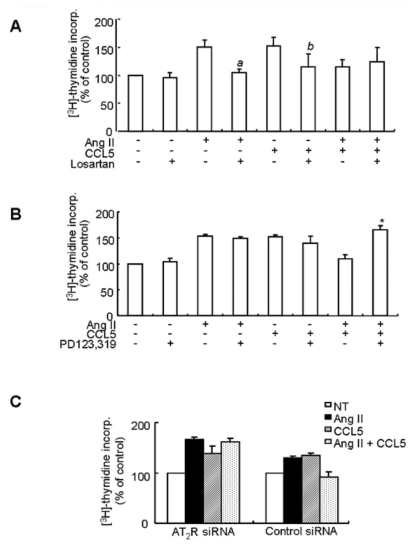
To determine whether the inhibitory mechanism of CCL5 on Ang II-induced VSMCs proliferation is related to Ang II subtype receptor, SHR VSMCs were treated with Ang II and/or CCL5 in the presence or absence of losartan or PD123,319. Ang II- and CCL5-induced VSMCs proliferation were both inhibited by losartan, but losartan did not affect the inhibitory effect of Ang II/CCL5 on VSMCs proliferation (Fig. 6A). PD123,319 did not affect VSMCs proliferation induced by Ang II or CCL5 but rather blocked the inhibitory action of Ang II/CCL5 on VSMCs proliferation (Fig. 6B). In VSMCs transfected with AT2 receptor siRNA, VSMCs treated with Ang II/CCL5 proliferated to almost the same level as VSMCs treated with Ang II (Fig. 6C). Therefore, the inhibitory effect of Ang II/CCL5 on VSMCs proliferation is related to AT2 receptor activation.
Fig. 6.
The inhibitory effect of CCL5 on Ang II-induced VSMCs proliferation is mediated by the AT2 receptor. (A, B) SHR VSMCs were treated with Ang II (0.1 µmol/l) and/or CCL5 (100 ng/ml) in the presence or absence of losartan (AT1 receptor antagonist, 10 µmol/l, A) or PD123,319 (AT2 receptor antagonist, 10 µmol/l, B) for 24 h in medium containing [3H]-thymidine (1 µCi/ml). [3H]-thymidine incorporation is shown on the Y-axis. Bars represent means±SD from three independent experiments. ap <0.05 vs. VSMCs treated with Ang II. bp <0.05 vs. VSMCs treated with CCL5. *p<0.05 vs. VSMCs treated with Ang II/CCL5. (C) SHR VSMCs were plated on 24-well plates, grown to 90% confluence and then transfected with AT2 receptor siRNA oligomers (50 nmol/l). VSMCs were then untreated or treated Ang II (0.1 µmol/l) and/or CCL5 (100 ng/ml) for 24 h in medium containing [3H]-thymidine (1 µCi/ml). [3H]-thymidine incorporation is shown on the Y-axis. Bars represent means±SD from three independent experiments.
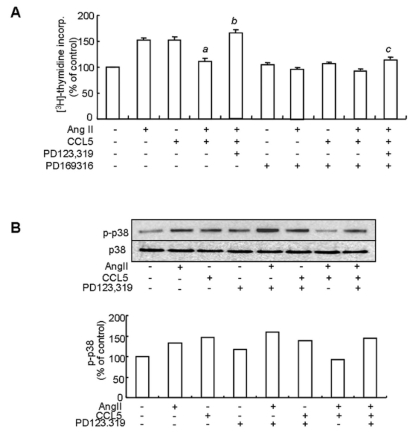
Ang II-induced VSMCs proliferation is mediated via the MAP kinase pathway (Mabrouk et al., 2001). To clarify the relationship between the AT2 receptor and MAP kinase activation with respect to the inhibitory effect of CCL5 on Ang II-induced VSMCs proliferation, we examined phosphorylation of p38 MAP kinase in VSMCs treated with Ang II and/or CCL5 in the presence or absence of PD123,319. Prior this experiment, we observed that a p38 inhibitor, PD169316 blocked VSMCs proliferation in cells treated with Ang II and/or CCL5 (Fig. 7A). Phosphorylation of p38 was detected in VSMCs treated with Ang II or CCL5 alone. However, phosphorylation of p38 was decreased in VSMCs treated with AngII/CCL5. Nevertheless, PD123,319 increased p38 phosphorylation in VSMCs treated with Ang II/CCL5 (Fig. 7B). Therefore, the AT2 receptor mediates the inhibitory action of Ang II/CCL5 on VSMCs proliferation via p38 inactivation.
Fig. 7.
The inhibitory effect of Ang II/CCL5 on VSMCs proliferation is mediated by the AT2 receptor via p38 inactivation. (A) SHR VSMCs were untreated (NT) or pretreated with PD169316 for 30 min. Then, cells were treated with Ang II (0.1 µmol/l) and/or CCL5 (100 ng/ml) in the presence or absence of PD123,319 (10 µmol/l) for 24 h in medium containing [3H]-thymidine (1 µCi/ml). [3H]-thymidine incorporation is shown on the Y-axis. Bars represent means±SD from three independent experiments. ap <0.05 vs. VSMCs treated with Ang II. bp <0.05 vs. VSMCs treated with Ang II/CCL5. cp <0.05 vs. VSMCs treated with PD123,319 and Ang II/CCL5. (B) SHR VSMCs were untreated or treated with Ang II (0.1 µmol/l) and/or CCL5 (100 ng/ml) in the presence or absence of PD123,319 (10 µmol/l) for 2 h. Cell lysates were separated on 10% SDS-polyacrylamide gels and then immunoblotted with phospho-p38 or p38 antibody. Data shown are representative of three independent experiments.
DISCUSSION
Ang II increases CCL5 expression in rat glomerular endothelial cells and the renal cortex (Wolf et al., 1997; Kashiwagi et al., 2002), but we observed that Ang II suppresses CCL5 expression in SHR VSMC. Moreover, we detected lower expression of CCL5 in SHR VSMCs than in WKY VSMCs. We therefore hypothesized that CCL5 may downregulate Ang II-induced hypertensive activities in SHR VSMCs, in contrast to the upregulation of Ang II-induced hypertension by chemokine CCL2 and CXCL8 (Chen et al., 1998; Buemi et al., 2004; Ishibashi et al., 2004; Kim et al., 2008). As previously shown, Ang II increases 12-LO expression (Natarajan et al., 1993; Kim et al., 1995). The activities of 12-LO and the 12-LO metabolite 12(S)-hydroxyeicosatetraenoic acid (12(S)-HETE) are increased in SHR, and many studies have demonstrated a role for 12-LO and 12(S)-HETE in the pathogenesis of experimental and Ang II-induced hypertension (Sasaki et al., 1997; González-Núnez et al., 2001) and in cytokine-induced VSMCs migration and proliferation (Natarajan et al., 1997). In the present study, we demonstrated the inhibitory action of CCL5 on Ang II-induced 12-LO mRNA expression and production via AT2 receptor in SHR VSMCs. Although CCL5 itself increases 12-LO mRNA expression, and PD123,319, an AT2 receptor antagonist, inhibited CCL5-induced 12-LO mRNA expression, the inhibitory effect of CCL5 on Ang II-induced 12-LO expression was also mediated through the AT2 receptor. Reduction of the AT2 receptor by siRNA prior to the addition of Ang II/CCL5 abrogated the inhibitory effect of CCL5 on Ang II-induced 12-LO expression, and PD123,319, an AT2 receptor antagonist, increased 12-LO mRNA expression.
Although a positive role of the AT2 receptors in Ang II-induced CCL5 expression has been demonstrated in rat glomerular endothelial cells and the rat renal cortex (Wolf et al., 1997; Kashiwagi et al., 2002), the direct effect of CCL5 on AT2 receptor expression has not been studied until now. In this study, CCL5 and Ang II/CCL5 increased AT2 receptor mRNA expression significantly (p<0.05), and the production of the AT2 receptor induced by CCL5 or Ang II/CCL5 was clearly detected by western blot in SHR VSMCs. We also examined the effect of CCL5 on AT1 receptor mRNA expression and production in SHR VSMCs. But, the increase of mRNA expression or production of AT1 receptor was not detected in cells treated with CCL5 or AngII/CCL5 (Data not shown).
Ang II has two subtype receptors, the AT1 receptor and the AT2 receptor, and the density of the AT2 receptor is lower than that of the AT1 receptor in VSMCs (Mabrouk et al., 2001). The AT1 receptor mediates the major stimulatory actions of Ang II, including vasoconstriction, cell proliferation, aldosterone secretion and sodium retention (Wolf et al., 1996). In contrast, the AT2 receptor has been reported to antagonize the vascular actions of AT1 receptors. Many studies suggest that AT2 receptors have growth inhibitory and proapoptotic effect on VSMCs (Horiuchi et al., 1999; Gallinat et al., 2000; Ruiz-Ortega et al., 2000). However, several studies have reported growth and proinflammatory actions of AT2 receptors in VSMCs (Saward et al., 1996; Mabrouk et al., 2001; Wolf et al., 2002). These discrepancies may be related to different ages, different experimental rat models of hypertension or different experimental conditions. In this study, CCL5 itself induced the SHR VSMCs proliferation and it was mediated by AT1 receptors. But, inhibitory effect of CCL5 on Ang II-induced SHR VSMCs proliferation was related to AT2 receptors. Mabrouk et al. (2001) reported that Ang II stimulates VSMCs proliferation via both of AT1 and AT2 receptors in SHR and via AT1 receptor only in WKY. In contrast, we could not observe inhibitory effect of PD123,319 on Ang II-induced SHR VSMCs poroliferation. Ang II-induced SHR VSMCs proliferation was mediated by AT1 receptors. This discrepancy may be related to different ages and different vascular tissues of SHR. However, the exact role of AT1 and AT2 receptors in the regulation of VSMCs proliferation in vascular hypertension requires further investigation.
The downregulatory effect of CCL5 on Ang II-induced VSMCs proliferation was related to the AT2 receptor via p38 inactivation. The growth inhibitory effect of the AT2 receptor is mediated by protein tyrosine phosphatase activation, which inhibits activation of MAP kinases (Bedecs et al., 1997; Horiuchi et al., 1999). Ang II stimulates the activation of MAP kinases, including ERK1/2, p38 and JNK in VSMCs (Touyz et al., 1999; Mabrouk et al., 2001; Lee et al., 2007). ERK1/2 and p38 activation play important roles in Ang II-induced VSMCs proliferation (Wilkie et al., 1997; Viedt et al., 2000; Zhao et al., 2002; Lee et al., 2007). Zhao et al. (2002) reported that simultaneous upregulation of c-Jun and c-Fos proteins is crucial for VSMCs proliferation, and p38 MAP kinase have additive effects on both the induction of c-Jun expression and VSMCs proliferation. In this CCL5 study, inhibition of p38 phosphorylation blocked VSMCs proliferation regardless of the kind of stimulants used, and phosphorylation of p38 in response to Ang II or CCL5 was detected in SHR VSMCs (Fig. 7). However, simultaneous stimulation with Ang II and CCL5 inhibited p38 phosphorylation and blocked VSMCs proliferation in SHR VSMCs. And, although PD123,319 itself did not significantly affect VSMCs proliferation or p38 activation in SHR VSMCs treated with Ang II or CCL5 alone. PD123,319 increased p38 phosphorylation and VSMCs proliferation in SHR VSMCs treated with Ang II/CCL5. Taken together, it seems likely that the downregulatory effect of CCL5 on Ang II-induced VSMCs proliferation is mediated by the AT2 receptor via p38 inactivation.
In the present study, we demonstrated that CCL5 down-regulates Ang II-induced 12-LO expression and VSMCs proliferation in SHR VSMCs, and these effects of CCL5 are mediated through AT2 receptors. These results suggest that CCL5 may play a downregulatory role in Ang II-induced hypertension.
ACKNOWLEDGEMENTS
This study was supported by the Korean Science and Engineering Foundation through the Aging-associated Vascular Disease Research Center at Yeungnam University (R13-2005-005-02002-0 (2008)).
ABBREVIATIONS
- SHR
spontaneously hypertensive rats
- VSMCs
vascular smooth muscle cells
- Ang II
angiotensin II
- AT2 receptor
angiotensin II subtype 2 receptor
- 12-LO
12-liopxygenase
References
- 1.Bedecs K, Elbaz N, Sutren M, Masson M, Susini C, Strosberg AD. Angiotensin II type 2 receptors mediate inhibition of mitogen activated protein kinase cascade and functional activation of SHP-1 tyrosine phosphatase. Biochem J. 1997;325:449–454. doi: 10.1042/bj3250449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buemi M, Marino D, Floccari F, Ruello A, Nostro L, Aloisi C. Effect of interleukin 8 and ICAM-1 on calcium-dependent outflow of K+ in erythrocytes from subjects with essential hypertension. Curr Med Res Opin. 2004;20:19–24. doi: 10.1185/030079903125002720. [DOI] [PubMed] [Google Scholar]
- 3.Chen XL, Tummala PE, Olbrych MT, Alexander RW, Medford RM. Angiotensin II induces monocyte chemoattractant protein-1 gene expression in rat vascular smooth muscle cells. Circ Res. 1998;83:952–959. doi: 10.1161/01.res.83.9.952. [DOI] [PubMed] [Google Scholar]
- 4.Dorfmüller P, Zarka V, Durand-Gasselin I, Monti G, Balabanian K, Garcia G. Chemokine RANES in severe pulmonary arterial hypertension. Am J Respir Crit Care Med. 2002;165:534–539. doi: 10.1164/ajrccm.165.4.2012112. [DOI] [PubMed] [Google Scholar]
- 5.Gallinat S, Busche S, Raizada MK, Sumners C. The angiotesin II type 2 receptor: An enigma with multiple variations. Am J Physiol. 2000;278:E357–E374. doi: 10.1152/ajpendo.2000.278.3.E357. [DOI] [PubMed] [Google Scholar]
- 6.González-Núnez D, Claria J, Rivera F, Roch E. Increased levels of 12(S)-HETE in patients with essential hypertension. Hypertension. 2001;37:334–338. doi: 10.1161/01.hyp.37.2.334. [DOI] [PubMed] [Google Scholar]
- 7.Han Y, Runge MS, Brasier AR. Angiotensin II induced interleukin-6 transcription in vascular smooth muscle cells through pleiotropic activation of nuclear factor-kB transcription factor. Circ Res. 1999;84:695–703. doi: 10.1161/01.res.84.6.695. [DOI] [PubMed] [Google Scholar]
- 8.Horiuchi M, Akishita M, Dzau VJ. Recent progress in angiotensin II type 2 receptor research in the cardiovascular system. Hypertension. 1999;33:613–621. doi: 10.1161/01.hyp.33.2.613. [DOI] [PubMed] [Google Scholar]
- 9.Horiuchi M, Lehtonen JY, Daviet L. Signaling mechanism of the AT2 angiotensin II receptor: crosstalk between AT1 and AT2 receptors in cell growth. Trends Endocrinol Metab. 1999;10:391–396. doi: 10.1016/s1043-2760(99)00191-5. [DOI] [PubMed] [Google Scholar]
- 10.Ishibashi M, Hiasa KI, Zhao Q, Inoue S, Ohtani K, Kitamoto S. Critical role of monocyte chemoattractant protein-1 receptor CCR2 on monocyte in hypertension-induced vascular inflammation and remodeling. Circ Res. 2004;94:1203–1210. doi: 10.1161/01.RES.0000126924.23467.A3. [DOI] [PubMed] [Google Scholar]
- 11.Jordan NJ, Watson ML, Williams RJ, Roach AG, Yoshimura T, Westwick J. Chemokine production by human vascular smooth muscle cells: modulation by IL-13. Br J Pharmacol. 1997;122:749–757. doi: 10.1038/sj.bjp.0701433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kashiwagi M, Masutani K, Shinozaki M, Hirakata H. MCP-1 and RANTES are expressed in renal cortex of rats chronically treated with nitric oxide synthase inhibitor. Nephron. 2002;92:165–173. doi: 10.1159/000064454. [DOI] [PubMed] [Google Scholar]
- 13.Kim HY, Kang YJ, Song IH, Choi HC, Kim HS. Upregulation of interleukin-8/CXCL8 in vascular smooth muscle cells from spontaneously hypertensive rats. Hypertens Res. 2008;31:515–523. doi: 10.1291/hypres.31.515. [DOI] [PubMed] [Google Scholar]
- 14.Kim JA, Gu JL, Natarajan R, Rerliner JA, Nadler JL. A leukocyte type 12-lipoxygenase is expressed in human vascular and mononuclear cells. Evidence for upregulation by angiotensin II. Arterioscler Thromb Vasc Bio. 1995;15:942–948. doi: 10.1161/01.atv.15.7.942. [DOI] [PubMed] [Google Scholar]
- 15.Lee HM, Lee CK, Lee SH, Roh HY, Bae YM, Lee KY. p38 mitogen-activated protein kinase contributes to angiotensin II-stimulated migration of rat aortic smooth muscle cells. J Pharmacol Sci. 2007;105:74–81. doi: 10.1254/jphs.fp0070770. [DOI] [PubMed] [Google Scholar]
- 16.Mabrouk ME, Touyz RM, Schiffrin EL. Differential Ang II-induced growth activation pathways in mesenteric artery smooth muscle cells from SHR. Am J Physiol Heart Circ Physiol. 2001;281:H30–H39. doi: 10.1152/ajpheart.2001.281.1.H30. [DOI] [PubMed] [Google Scholar]
- 17.Natarajan R, Gi JL, Rossi J, Gonzales N, Lanting L, Xu L. Elevated glucose and angiotensin II increase 12-lipoxygenase activity and expression in porcine aortic smooth muscle cells. Proc Natl Acad Sci USA. 1993;90:4947–4951. doi: 10.1073/pnas.90.11.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Natarajan R, Rosdahl J, Gonzales N, Bai W. Regulation of 12-lipoxygenase by cytokine in vascular smooth muscle cells. Hypertension. 1997;30:873–879. doi: 10.1161/01.hyp.30.4.873. [DOI] [PubMed] [Google Scholar]
- 19.Preston IR, Hill NS, Warburton RR, Fanburg BL. Role of 12-lipoxygenase in hypoxia-induced rat pulmonary artery smooth muscle cell proliferation. Am J Lung Cell Mol Physiol. 2006;290:L367–L374. doi: 10.1152/ajplung.00114.2005. [DOI] [PubMed] [Google Scholar]
- 20.Resink TJ, Scott-Burden T, Burgin M, Buhler FR. Enhanced responsiveness to angiotensin II in vascular smooth muscle cells from spontaneously hypertensive rats is not associated with alterations in protein kinase C. Hypertension. 1989;14:293–303. doi: 10.1161/01.hyp.14.3.293. [DOI] [PubMed] [Google Scholar]
- 21.Rouiz-Ortega M, Lorenzo O, Ruperez M, Konig S, Wittig B, Egido J. Angiotensin II activates nuclear transcription factor Kappa B through At1 and At2 in vascular smooth muscle cells: molecular mechanisms. Circ Res. 2000;86:1266–1272. doi: 10.1161/01.res.86.12.1266. [DOI] [PubMed] [Google Scholar]
- 22.Sasaki M, Hori MT, Hino T, Golub MS, Tuck ML. Elevated 12-lipoxygenase activity in the spontaneously hypertensive rat. Am J Hypertens. 1997;10:371–378. [PubMed] [Google Scholar]
- 23.Saward L, Zahradka P. The angiotensin type 2 receptor mediates RNA synthesis in A10 vascular smooth muscle cells. J Mol Cell Cardiol. 1996;28:499–506. doi: 10.1006/jmcc.1996.0046. [DOI] [PubMed] [Google Scholar]
- 24.Schall TJ, Bacon K, Toy KJ, Goeddel DV. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990;347:669–671. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- 25.Shahrara S, Park CC, Temkin V, Jarvis JW, Volin MV, Pope RM. RANTES modulates TLR4-induced cytokine secretion in human peripheral blood monocytes. J Immunol. 2006;177:5077–5087. doi: 10.4049/jimmunol.177.8.5077. [DOI] [PubMed] [Google Scholar]
- 26.Touyz RM, Mabrouk ME, He G, Wu X-H, Schiffrin EL. Mitogen-activated protein/extracellular signal-regulated kinase inhibition attenuates angiotensin II-mediated signaling and contraction in spontaneously hypertensive rat vascular smooth muscle cells. Circ Res. 1999;84:505–515. doi: 10.1161/01.res.84.5.505. [DOI] [PubMed] [Google Scholar]
- 27.Tripathy D, Thirumangalakudi L, Grammas P. RANTES upregulation in the Alzheimer's disease brain: a possible neuroprotective role. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2008.03.009. doi: 10.1016/j.neurobiol aging 2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Viedt C, Soto U, Krieger-brauer HI, Fei J, Elsing C, Kübler W. Differential activation of mitogen-activated protein kinase in smooth muscle cells by angiotensin II: involvement of p22phox and reactive oxygen species. Arterioscler Thromb Vasc Biol. 2000;20:940–948. doi: 10.1161/01.atv.20.4.940. [DOI] [PubMed] [Google Scholar]
- 29.Wei LH, Yang Y, Wu G, Ignarro LJ. IL-4 and IL-13 upregulate ornithine decarboxylase expression by PI3K and MAP kinase pathways in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2008;294:C1198–C1205. doi: 10.1152/ajpcell.00325.2007. [DOI] [PubMed] [Google Scholar]
- 30.Wilkie N, Ng LL, Boarder MR. Angiotensin II responses of vascular smooth muscle cells from hypertensive rats: enhancedment at the level of p42 and p44 mitogen activated protein kinase. Br J Pharmacol. 1997;122:209–216. doi: 10.1038/sj.bjp.0701366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolf G, Neilson EG. From converting enzyme inhibition to angiotensin II receptor blockade: New insight on angiotensin II receptor subtypes in the kidney. Exp Nephrol. 1996;4:8–19. [PubMed] [Google Scholar]
- 32.Wolf G, Wenzel U, Burns KD, Harris RC, Stahl RA, Thaiss F. Angiotensin II activates nuclear transcription factor-kB through AT1 and AT2 receptors. Kidney Int. 2002;61:1986–1995. doi: 10.1046/j.1523-1755.2002.00365.x. [DOI] [PubMed] [Google Scholar]
- 33.Wolf G, Ziyadeh FN, Thaiss F, Tomaszewski J, Caron RJ, Wenzel U. Angiotensin II stimulates expression of the chemokine RANTES in rat glomerular endothelial cells. J Clin Invest. 1997;100:1047–1058. doi: 10.1172/JCI119615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao M, Liu Y, Bao M, Kato Y, Han J, Eaton JW. Vascular smooth muscle cell proliferation requires both p38 and BMK1 MAP kinases. Arch Biochem Biophys. 2002;400:199–207. doi: 10.1016/S0003-9861(02)00028-0. [DOI] [PubMed] [Google Scholar]
- 35.Zheng Y, Song HJ, Yun SH, Chae YJ, Jia H, Kim CH, Ha TS, Sachinidis A, Ahn HY, Davidge ST. Inhibition of angiotensin ii-induced vascular smooth muscle cell hypertrophy by different catechins. Korean J Physiol Pharmacol. 2005;9:117–123. [Google Scholar]
- 36.Zoja C, Donadelli R, Colleoni S, Figliuzzi M, Bonazzola S, Morigi M. Protein overload stimulates RANTES production by proximal tubular cells depending on NF-kB activation. Kidney Int. 1998;53:1608–1615. doi: 10.1046/j.1523-1755.1998.00905.x. [DOI] [PubMed] [Google Scholar]