Abstract
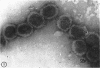
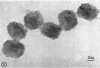
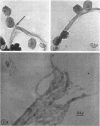
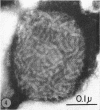
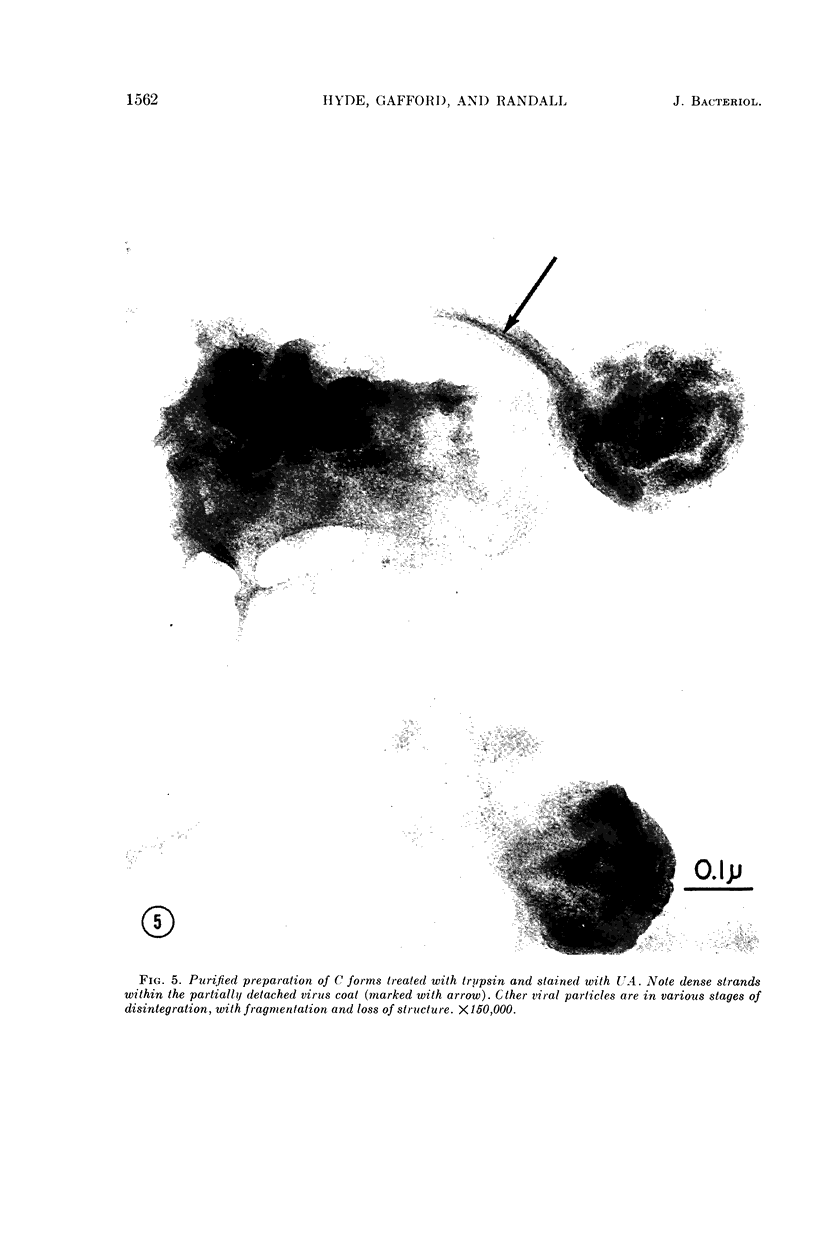
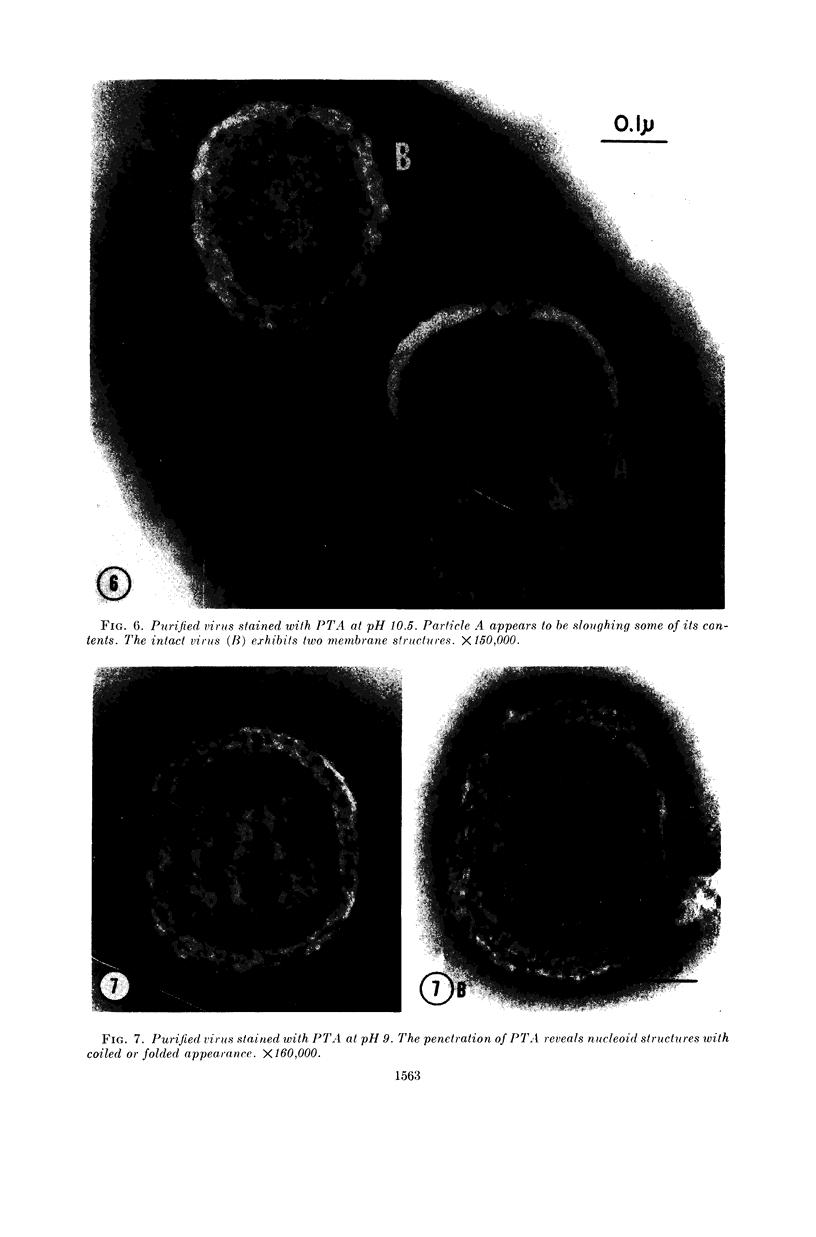
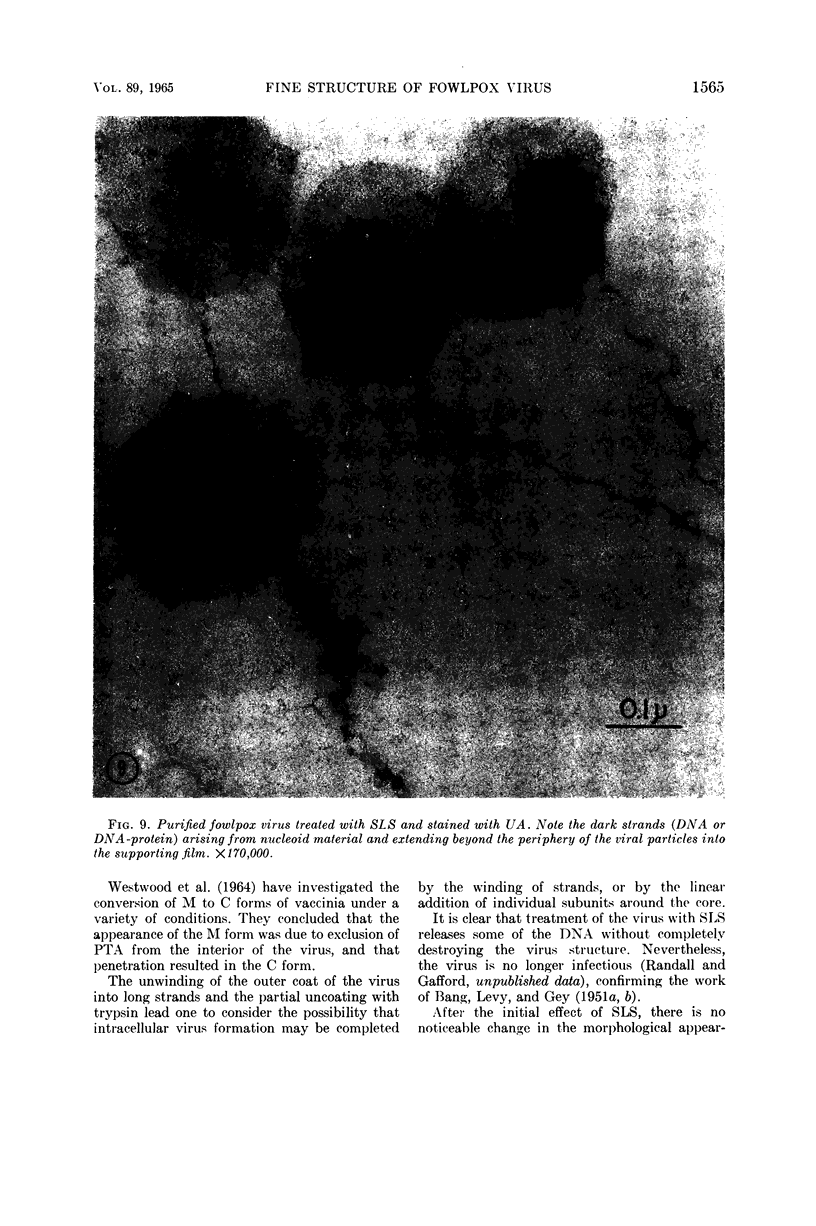
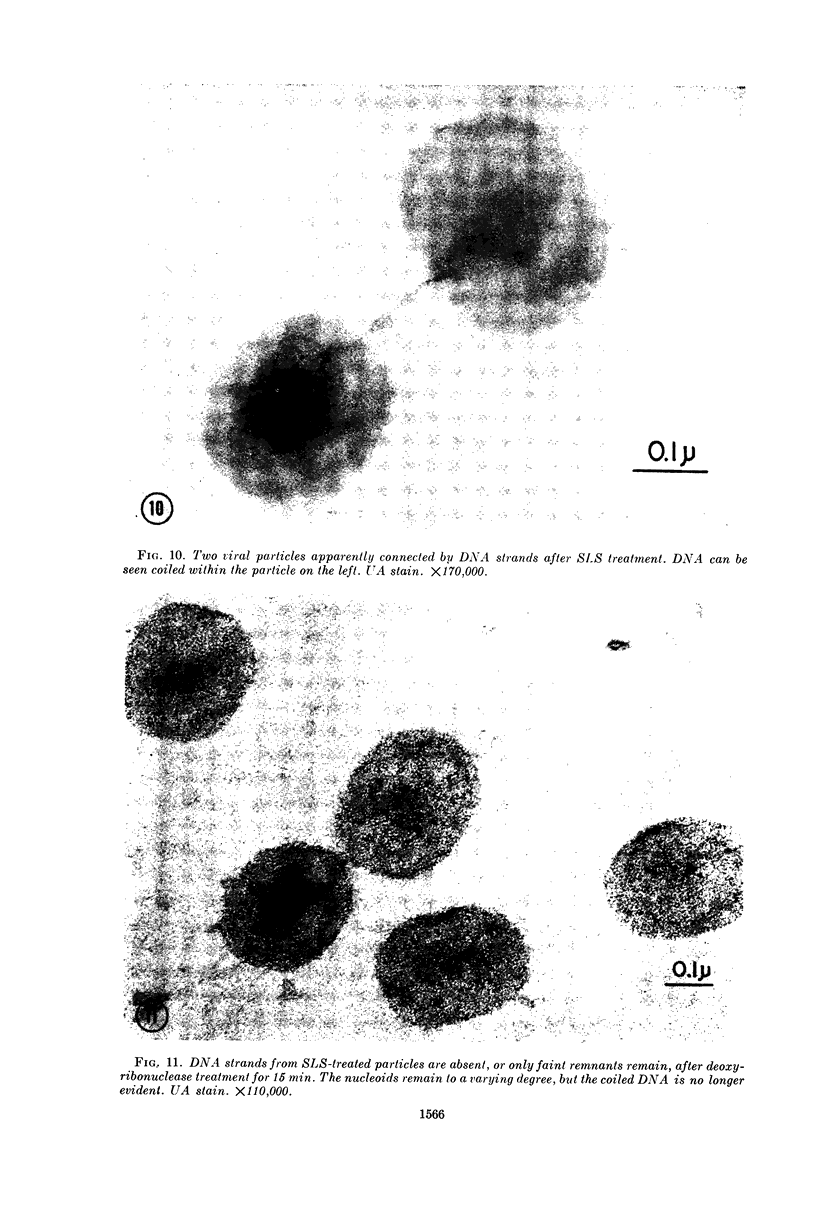
Hyde, James M. (University of Mississippi School of Medicine, Jackson), Lanelle G. Gafford, and Charles C. Randall. Fine structure of the coat and nucleoid material of fowlpox virus. J. Bacteriol. 89:1557–1569. 1965.—Several morphological forms characteristic of the poxvirus group were demonstrated for fowlpox virus with neutral phosphotungstic acid (PTA). Viral particles (purified from viral inclusion bodies) stained with uranyl acetate (UA) and shadowed with platinum were shown to have an external knobby surface not evident with PTA. The external coat of freshly purified viral particles seemed intact, but as the preparation aged, it appeared to unwind, resulting in twisted “rope-like” structures. This process was facilitated by use of 1% trypsin, and three dense fibrils were identified with UA within the partially detached viral coat. Studies with alkaline PTA (pH 9) were interpreted as revealing a complex nucleoid, but solutions above this pH damaged the particles. The morphology of the nucleoid was better depicted in ultrathin sections of whole virus which, when stained with UA, revealed dense coiled threads. Treatment of virus with sodium lauryl sulfate exposed an underlying coat consisting of small subunits approximately 40 A in diameter. Of great interest was the demonstration that the detergent removed strands of deoxyribonucleic acid (DNA) from the virus without destroying the contour of the particle. The origin of the strands was definitely the fine uranophilic, coiled threads of the nucleoid, which probably represent the DNA molecule(s). That the extracted material was largely DNA was proved by digestion with deoxyribonuclease and resistance to ribonuclease and trypsin. These studies illustrate how a variety of electron microscopic techniques may be utilized alone or in combination to reveal hitherto undescribed fine structure of viral particles.
Full text
PDF

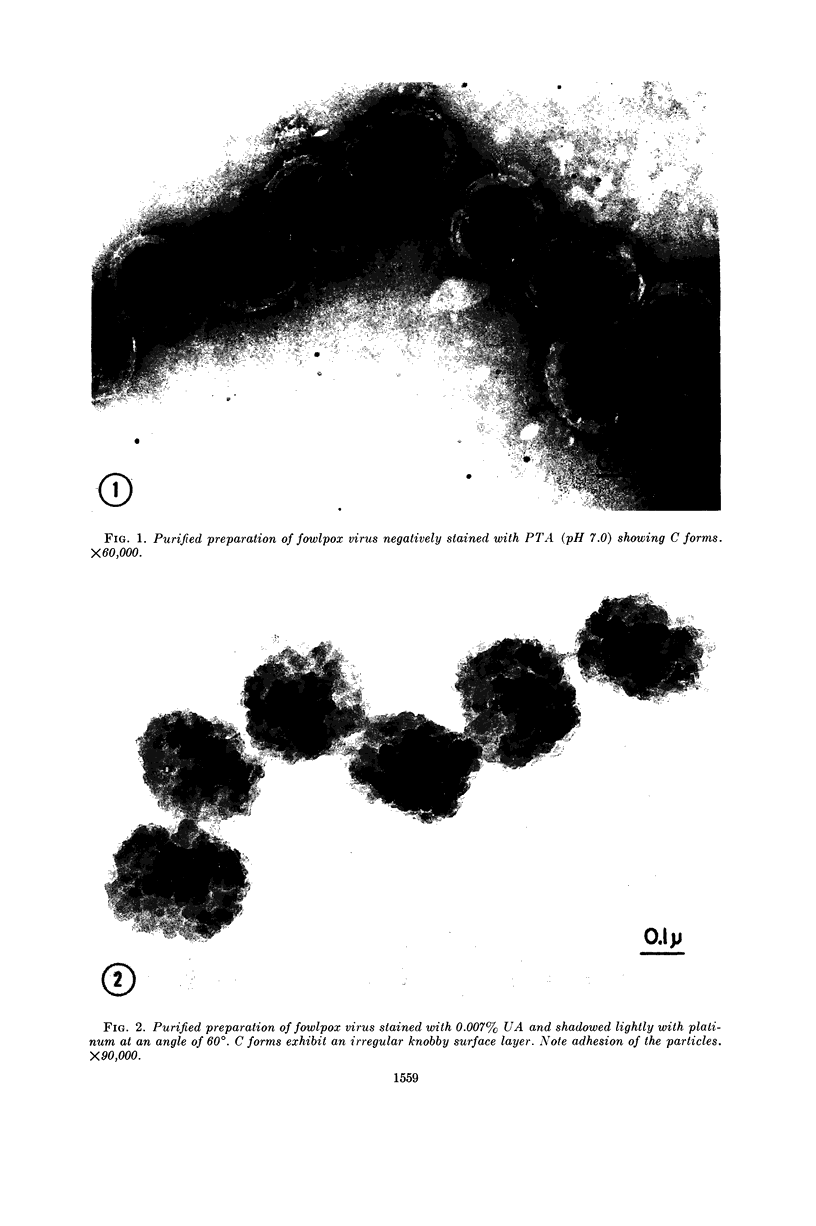
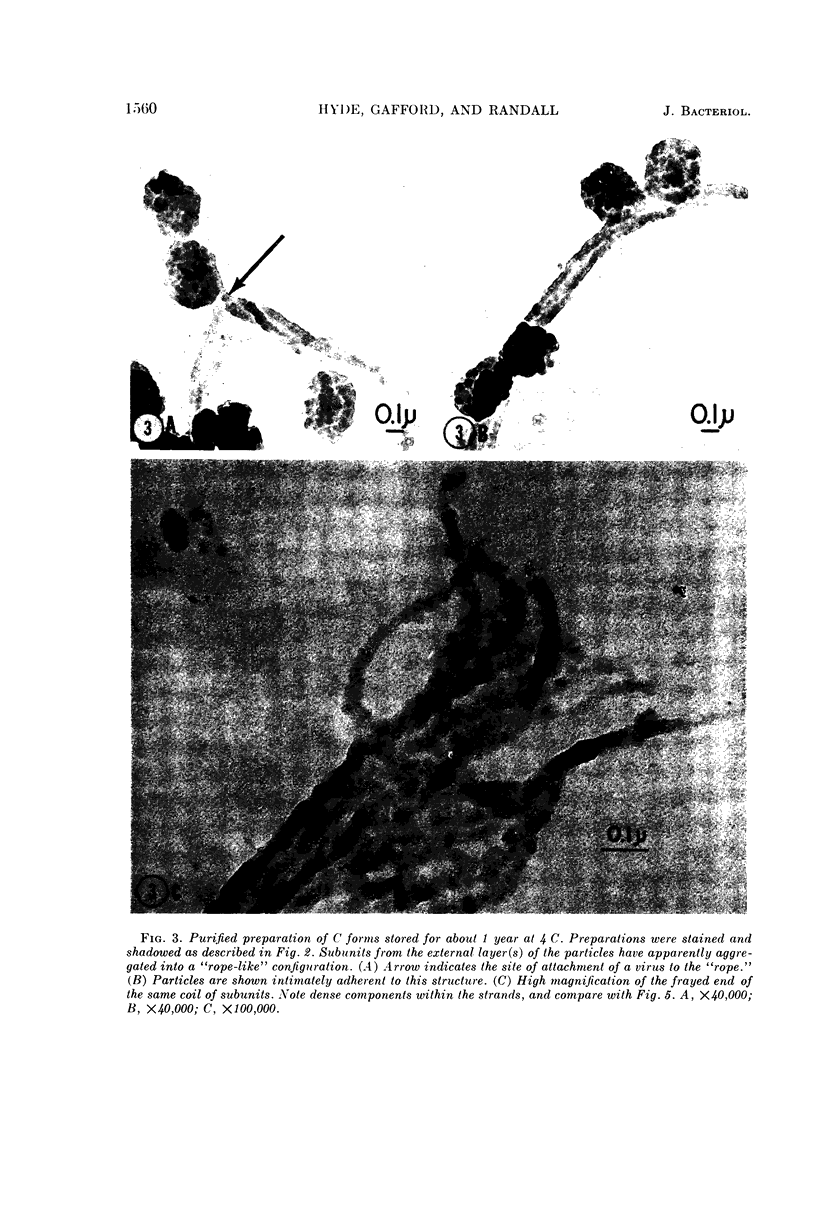




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARHELGER R. B., DARLINGTON R. W., GAFFORD L. G., RANDALL C. C. An electron microscopic study of fowlpox infection in chick scalps. Lab Invest. 1962 Oct;11:814–825. [PubMed] [Google Scholar]
- BANG F. B., LEVY E., GEY G. O. Some observations on host-cell-virus relationships in fowl pox. I. Growth in tissue culture. II. The inclusion produced by the virus on the chick chorio-allantoic membrane. J Immunol. 1951 Mar;66(3):329–345. [PubMed] [Google Scholar]
- BERNHARD W., TOURNIER P. Ultrastructural cytochemistry applied to the study of virus infection. Cold Spring Harb Symp Quant Biol. 1962;27:67–82. doi: 10.1101/sqb.1962.027.001.010. [DOI] [PubMed] [Google Scholar]
- BUETTNER D., GIESE H., MUELLER G., PETERS D. DIE FEINSTRUKTUR REIFER ELEMENTARKOERPER DES ECTHYMA CONTAGIOSUM UND DER STOMATITIS PAPULOSA. Arch Gesamte Virusforsch. 1964 Jun 17;14:657–673. [PubMed] [Google Scholar]
- CASPAR D. L., DULBECCO R., KLUG A., LWOFF A., STOKER M. G., TOURNIER P., WILDY P. Proposals. Cold Spring Harb Symp Quant Biol. 1962;27:49–50. doi: 10.1101/sqb.1962.027.001.007. [DOI] [PubMed] [Google Scholar]
- DALES S. An electron microscope study of the early association between two mammalian viruses and their hosts. J Cell Biol. 1962 May;13:303–322. doi: 10.1083/jcb.13.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALES S. The uptake and development of vaccinia virus in strain L cells followed with labeled viral deoxyribonucleic acid. J Cell Biol. 1963 Jul;18:51–72. doi: 10.1083/jcb.18.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGINGTON J., HORNE R. W. Morphological studies of orf and vaccinia viruses. Virology. 1962 Mar;16:248–260. doi: 10.1016/0042-6822(62)90245-3. [DOI] [PubMed] [Google Scholar]
- NOYES W. F. Further studies on the structure of vaccinia virus. Virology. 1962 Dec;18:511–516. doi: 10.1016/0042-6822(62)90051-x. [DOI] [PubMed] [Google Scholar]
- PALADE G. E. A study of fixation for electron microscopy. J Exp Med. 1952 Mar;95(3):285–298. doi: 10.1084/jem.95.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERS D., MUELLER G., BUETTNER D. THE FINE STRUCTURE OF PARAVACCINIA VIRUSES. Virology. 1964 Aug;23:609–611. doi: 10.1016/0042-6822(64)90246-6. [DOI] [PubMed] [Google Scholar]
- PETERS D., MUELLER G. THE FINE STRUCTURE OF THE DNA-CONTAINING CORE OF VACCINIA VIRUS. Virology. 1963 Oct;21:267–269. doi: 10.1016/0042-6822(63)90267-8. [DOI] [PubMed] [Google Scholar]
- RANDALL C. C., GAFFORD L. G., DARLINGTON R. W. Bases of the nucleic acid of fowlpox virus and host deoxyribonucleic acid. J Bacteriol. 1962 May;83:1037–1041. doi: 10.1128/jb.83.5.1037-1041.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANDALL C. C., GAFFORD L. G., DARLINGTON R. W., HYDE J. COMPOSITION OF FOWLPOX VIRUS AND INCLUSION MATRIX. J Bacteriol. 1964 Apr;87:939–944. doi: 10.1128/jb.87.4.939-944.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RHIM J. S., SMITH K. O., MELNICK J. L. Complete and coreless forms of reovirus (ECHO 10). Ratio of number of virus particles to infective units in the one-step growth cycle. Virology. 1961 Dec;15:428–435. doi: 10.1016/0042-6822(61)90110-6. [DOI] [PubMed] [Google Scholar]
- SMITH K. O., MELNICK J. L. A method for staining virus particles and identifying their nucleic acid type in the electron microscope. Virology. 1962 Jul;17:480–490. doi: 10.1016/0042-6822(62)90143-5. [DOI] [PubMed] [Google Scholar]
- SZYBALSKI W., ERIKSON R. L., GENTRY G. A., GAFFORD L. G., RANDALL C. C. Unusual properties of fowlpox virus DNA. Virology. 1963 Apr;19:586–589. doi: 10.1016/0042-6822(63)90056-4. [DOI] [PubMed] [Google Scholar]
- WESTWOOD J. C., HARRIS W. J., ZWARTOUW H. T., TITMUSS D. H., APPLEYARD G. STUDIES ON THE STRUCTURE OF VACCINIA VIRUS. J Gen Microbiol. 1964 Jan;34:67–78. doi: 10.1099/00221287-34-1-67. [DOI] [PubMed] [Google Scholar]