Abstract
The PcF protein from Phytophthora cactorum is the first member of the “PcF toxin family” from the plant pathogens Phytophthora spp. It is able to induce withering in tomato and strawberry leaves. The lack of sequence similarity with other proteins hampers the identification of the molecular mechanisms responsible for its toxicity. Here, we show that the six cysteines form a disulphide pattern that is exclusive for PcF and essential for the protein withering activity. The NMR solution structure identifies a novel fold among protein effectors: a helix-loop-helix motif. The presence of a negatively charged surface suggests that it might act as a site of electrostatic interaction. Interestingly, a good fold match with Ole e 6, a plant protein with allergenic activity, highlighted the spatial superimposition of a stretch of identical residues. This finding suggests a possible biological activity based on molecular mimicry.
Keywords: NMR solution structure, backbone dynamics, PcF toxin family, oomycete plant pathogen, extracellular effector
Introduction
Phytophthora plant pathogens are fungus-like oomycetes causing destructive diseases in many cultivated crops, thus with relevant impact on staple food production. These pathogens manipulate the host plant metabolism using an array of secreted protein effectors.1 As part of a long-term project, while studying secreted effector proteins from P. cactorum, we isolated a novel phytotoxic protein, that we named PcF (Phytophthora cactorum-Fragaria protein).2 In its mature form, PcF is a 52-residues acidic protein presenting three disulphide bonds.3 Purified PcF triggers withering symptoms on strawberry and tomato leaves, that is, the localized cell-death phenotype known as the “hypersensitive response.”4,5 The only known PcF homologues are predicted proteins: SCR74 and SCR91 encoded by polymorphic genes from P. infestans,6,7 and the products of 19 and 4 genes from P. sojae and P. ramorum, respectively.8 These candidate apoplastic effectors have been grouped into the “PcF toxin family” (Pfam PF09461): none of them has been characterized yet.
This work reports the NMR solution structure of the lead member of the PcF family.
Results
Assignment of disulphide bonds
Combining chemical derivatization with HPLC separation,9 the three disulphide bonds of PcF were unambiguously identified as Cys6-Cys40, Cys11-Cys44, and Cys26-Cys39 (Supporting Information). Although the cysteines pattern of PcF is also found in the SCR74 and SCR91 putative proteins, the disulphide bonds pattern is an exclusive prerogative of PcF (Fig. 1 bottom). Integrity of the disulphide connectivities is essential for the withering activity (Supporting Information).
Figure 1.

Solution structure of PcF. (A) Backbone superposition of the trace of the 20 lower energy NMR-derived models, selected from 100 calculated structures. (B) Color-coded electrostatic protein surface: (red) negative electrostatic potential; (blue) positive electrostatic potential; (white) neutral electrostatic potential. The right-hand view is rotated by 180° around the long axis of the molecule. (C) Ribbon representation of the protein in the same orientation used for the surface map. (Bottom): PcF disulphide bonds pattern.
Solution structure
Using NOE-derived distance restraints, 20 models were selected on the basis of their lower energy, of systematic violations less than 0.5 Å for residual NOE and less than 5° for dihedral angles. PcF is an all-α-protein presenting a helix-loop-helix motif, Figure 1(A,C). The structural statistics indicate good stereochemical and nonbonded interaction properties, Table I. The two helices α1 (Ala18-Asp28) and α2 (Asp35-Gln43) are connected by a six-residue loop, Gln29-Asp34, and their axes make an interhelical angle of ∼140° [Fig. 1(A)]. The N- and C-terminus stretches Tyr-4-Pro3 and Gly45-Ala52, respectively, are unstructured and spatially disordered. Conversely, the region Leu4-Glu17, though lacking secondary structure elements, appears spatially well defined [Fig. 1(A) and Table I]. This feature is due to the Cys6-Cys40 and Cys11-Cys44 bonds that anchor that region to the α2 helix and to C-terminus, respectively. The Cys26-Cys39 bond, instead, connects the two helices. The RMSDs in the region Leu4-Cys44 (Table I) indicate only a satisfactory structures convergence primarily because of a reduced definition of the two termini and of the interhelical loop. The protein presents a largely negative surface opposing a mainly neutral one that is crossed by a positively charged strip [Fig. 1(B)].
Table I.
Structural Restraints and Statistics for the 20 NMR-Derived Structures Ensemble of PcF
| Restraints for structure calculation | ||
| NOE restraints | Total | 481 |
| Intraresidue | 253 | |
| Sequential | 143 | |
| Medium range | 47 | |
| Long range | 63 | |
| Dihedral angles | φ | 35 |
| χ1 | 9 | |
| Statistics for structure calculation | ||
| RMSD from ideal geometry | Bond lengths (Å) | 0.0025 ± 0.0005 |
| Bond angles (°) | 1.02 ± 0.02 | |
| RMSD pairwise | Backbone (N, Cα, C) (Å) (aa 4–44) | 1.30 ± 0.22 |
| Heavy atoms (Å) (aa 4–44) | 2.33 ± 0.31 | |
| Backbone (N, Cα, C) (Å) (aa 18–43) | 1.28 ± 0.25 | |
| Heavy atoms (Å) (aa 18–43) | 2.43 ± 0.38 | |
| Backbone (N, Cα, C) (Å) (aa 4–17) | 0.94 ± 0.22 | |
| Heavy atoms (Å) (aa 4–17) | 1.86 ± 0.36 | |
| Backbone (N, Cα, C) (Å) (aa 18–28) | 0.55 ± 0.16 | |
| Heavy atoms (Å) (aa 18–28) | 1.56 ± 0.31 | |
| Backbone (N, Cα, C) (Å) (aa 35–43) | 0.81 ± 0.33 | |
| Heavy atoms (Å) (aa 35–43) | 1.96 ± 0.45 | |
| PROCHECK-NMR parameters | Most favoured region (%) | 69.8 |
| Additional allowed (%) | 27.9 | |
| Generously allowed (%) | 2.3 | |
| Disallowed (%) | 0.0 | |
| Number of bad contacts (%) | 2.7 | |
Backbone dynamics
The PcF rotational diffusion tensor indicates an average rotational correlation time 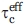 of 4.07 ± 0.18 ns, and a ratio of the rotational diffusion coefficients Dll/D⊥ of 1.99, consistent with a monomeric cylindrical status. The helices have an average S2 of 0.92, indicative of restricted backbone motion. The interhelical loop shows S2 averaging at ∼0.80 indicating a higher flexibility than the helices. Thus, the not ideal structural definition of this region [Fig. 1(A) and Table I] likely results from a combination of its flexibility and solvent exposure that leads to a reduced number of measurable NOEs. The Leu4-Glu17 region presents S2 from 0.6 to 0.9, suggesting that it undergoes restricted coordinated fluctuations. R2 values above average, for residues clustered before helix α1, and located in the loop-α2 helix junction, suggest slow (μs-ms timescale) conformational exchange. Finally, both N-terminus (Glu1-Leu4) and C-terminus (Ser46-Ala52) exhibit a pronounced structural flexibility (low or even negative 1H-15N-NOE and small R2 values). A reduced spectral density mapping analysis confirms this description (Supporting Information).
of 4.07 ± 0.18 ns, and a ratio of the rotational diffusion coefficients Dll/D⊥ of 1.99, consistent with a monomeric cylindrical status. The helices have an average S2 of 0.92, indicative of restricted backbone motion. The interhelical loop shows S2 averaging at ∼0.80 indicating a higher flexibility than the helices. Thus, the not ideal structural definition of this region [Fig. 1(A) and Table I] likely results from a combination of its flexibility and solvent exposure that leads to a reduced number of measurable NOEs. The Leu4-Glu17 region presents S2 from 0.6 to 0.9, suggesting that it undergoes restricted coordinated fluctuations. R2 values above average, for residues clustered before helix α1, and located in the loop-α2 helix junction, suggest slow (μs-ms timescale) conformational exchange. Finally, both N-terminus (Glu1-Leu4) and C-terminus (Ser46-Ala52) exhibit a pronounced structural flexibility (low or even negative 1H-15N-NOE and small R2 values). A reduced spectral density mapping analysis confirms this description (Supporting Information).
Structural homology evaluation and K+ channel inhibitory test
The PcF fold has been used to query the whole PDB in search for functionally characterized structural homologues. Based on fold superposition, charge similarity, extracellular location, and cysteines relative abundance, a few small all-α-proteins were retained. They included three scorpion toxins acting as K+-channel inhibitors (OmTx1, OmTx3,10 and κ-hefutoxin11) and Ole e 6 from Olea europaea, a pollen protein of unknown biochemical function in plant but acting as allergen in humans.12 The proteins, with respect to PcF, display very similar orientation of the two α-helices, in all cases kept in place by multiple disulphide bonds, whereas their interhelical loop appears shorter and poorly superimposed (see Fig. 2). Fold alignments also revealed the spatial superposition of the stretch CEExC, at the end of helix α2 (OmTxs), and KECxD, at the end of helix α1 (Ole e 6).
Figure 2.
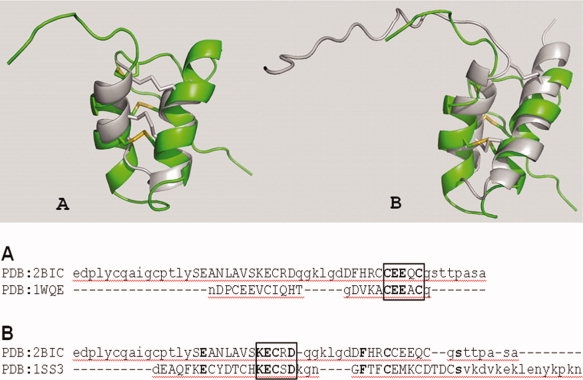
PcF structural alignments. (Top) Cartoon representation of the fold alignment of PcF (PDB 2BIC) with: panel A, a representative K+-channel blocker scorpion toxin (OmTx3, PDB 1WQE) and panel B, the pollen allergen Ole e 6 from Olea europea (PDB 1SS3). Molecular rendering was performed with PyMol program. All structures are in right-left orientation starting from N-terminus. PcF is highlighted in green, whereas the other proteins are in gray. Disulphide bonds are in yellow. (Bottom) Structure-based sequence alignment was performed using SSM.13 α-helical regions are in capital letters and identical residues are in bold. Boxed: the structurally superimposed conserved residues.
As K+ channels have been recently characterized in plant cells and it has been shown that they are influenced by protein effectors,14 we tested the possibility that the basis of PcF activity might reside in its ability to inhibit plant K+-channels. The results indicated that up to 1 mM PcF does not affect any of the selected plant channels (the inward-rectifying KAT1, the weakly rectifying AKT2/3, and the outward-rectifying GORK). In addition, no other effects on oocyte viability were observed.
Discussion
There is a widespread uncertainty on the molecular mechanisms of plant-pathogen interactions. In fact, also for PcF, though its selectivity for tomato and strawberry strongly suggests a receptor-based mechanism of action, no gene-for-gene model has been described, yet. Nonetheless, the PcF negatively charged face seems fit for the interaction with a positively charged ligand, an event that might contribute to receptor-mediated recognition and therefore could justify the observed host-plant selectivity.2 Moreover, the protein tightly bound structure appears appropriate to tolerate the rather harsh plant apoplast where it is delivered during plant infection.
PcF's all-alpha organization is atypical among known fungal effectors, usually exhibiting a β-structured fold,15,16 and not even resembles the mainly α-structured elicitins from Phytophthora spp.17 However, fold alignment with Ole e 6 highlighted a possible structural determinant, that is, the KECxD homologous stretch that, noteworthy, turns out to be present both in plants, as in the predicted protein NtP-CysR, the tobacco homologue of Ole e 6,12 and in plant pathogens, as in the PcF toxin subfamily SCR91.6 Indeed, in the absence of other sequence homologies, this finding suggests that PcF might mimic the structural signature of a plant signaling protein. A similar structural mimicry in pathogenesis is novel for oomycete effectors but has been already described: the anthrax protein BclA18 and a number of effectors from both plant and human pathogens.19,20
Materials and Methods
NMR studies and structure calculation
15N-labeled PcF was expressed by yeast culturing as reported in Ref.3. 15N-PcF was dissolved at 0.7 mM in 10 mM sodium phosphate, pH 5.0, 10% D2O. Data were recorded at 25°C, using a Varian Inova spectrometer operating at 600 MHz for the 1H. Backbone and aliphatic side-chain signals were assigned by analysis of 2D 1H-15N HSQC, 3D-TOCSY-HSQC, 3D-NOESY-HSQC, 2D-TOCSY, and 2D-NOESY spectra. A total of 35φ backbone torsion angles were obtained using the 3JNHHα coupling constants, derived from analysis of a 3D HNHA spectrum.21 Stereospecific assignments of β-methylene resonances and χ1 torsion angles were determined from the relative intensities of intraresidue HN-Hβ NOEs in a 50 ms 15N-edited 3D NOESY-HSQC spectrum in conjunction with the relative size of 3Jαβ coupling constants estimated from a 30 ms 15N-edited 3D TOCSY-HSQC spectrum and the 3JHNHβ coupling from 3D HNHB.22
Structures were computed using the simulated annealing method.23 The final energy minimization was performed with full consistent valence force field to a maximum derivative of 1 cal/Å. A total of 100 simulated annealing structures were calculated, and the 20 lower-energy structures were selected to represent the protein solution structure. The structures were analyzed with PROCHECK-NMR and MOLMOL.24,25 Atomic coordinates and chemical shifts have been deposited in the PDB under accession number 2BIC and in the BMRB (www.bmrb.wisc.edu) under accession number 6520, respectively.
15N relaxation measurements and analysis
T1, T2, and 1H-15N-NOE values were acquired using pulse sequences adapted from standard schemes.26 The T1 and T2 intensities were extracted using nonlinear spectral lineshape modeling and fitted to single exponential using routines within NMRPipe.27 The 15N heteronuclear relaxation parameters were analyzed using the TENSOR2 program.28
Structure comparisons
Cysteine and S-S pairing pattern similarities were evaluated by MOTIF Search (http://motif.genome.jp) and by CysView,29 respectively. Fold comparison was carried out by secondary-structure matching algorithm (SSM),13 using the PcF structured core (residues Leu4-Cys44) as the query.
K+-channels inhibition assay
Assays were performed in Xenopus laevis oocytes expressing three Shaker-like K+ channels from Arabidopsis thaliana, that is, the inward-rectifying KAT1, the weakly rectifying AKT2/3, and the outward-rectifying GORK30 For KAT1 and AKT2/3, experiments were carried out at pH 6.0 to mimic apoplastic conditions,31 whereas pH 7.4 was used for GORK to obtain measurable and stable currents.
Acknowledgments
The CIM (University of Parma, Italy) is acknowledged for the use of its equipments. The authors thank Dr. Daniel L. Purich (University of Florida, Gainesville, USA), Dr. Gale Bozzo (University of Guelph, Canada), and Dr. Nadia Raffaelli (Università Politecnica delle Marche) for critical reading and helpful discussions on the manuscript. L.M is recipient of a Doctoral degree Fellowship funded by Diatech SpA, Jesi, Italy.
Glossary
Abbreviations:
- HSQC
heteronuclear single-quantum coherence
- NOE
nuclear Overhauser effect
- PcF
Phytophthora cactorum-Fragaria protein
- PDB
Protein Data Bank
- RMSD
root mean square deviation
- SCR
secreted cysteine-rich protein.
References
- 1.Erwin DE, Ribeiro OK. Phytophthora disease worldwide. St. Paul, MN: APS Press; 1996. [Google Scholar]
- 2.Orsomando G, Lorenzi M, Raffaelli N, Dalla Rizza M, Mezzetti B, Ruggieri S. Phytotoxic protein PcF, purification, characterization, and cDNA sequencing of a novel hydroxyproline-containing factor secreted by the strawberry pathogen Phytophtora cactorum. J Biol Chem. 2001;276:21578–21584. doi: 10.1074/jbc.M101377200. [DOI] [PubMed] [Google Scholar]
- 3.Orsomando G, Lorenzi M, Ferrari E, De Chiara C, Spisni A, Ruggieri S. PcF protein from Phytophthora cactorum and its recombinant homologue elicit phenylalanine ammonia lyase activation in tomato. Cell Mol Life Sci. 2003;60:1470–1476. doi: 10.1007/s00018-003-3079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vleeshouwers VG, van Dooijeweert W, Govers F, Kamoun S, Colon LT. The hypersensitive response is associated with host and nonhost resistance to Phytophthora infestans. Planta. 2000;210:853–864. doi: 10.1007/s004250050690. [DOI] [PubMed] [Google Scholar]
- 5.Benschop JJ, Mohammed S, O'Flaherty M, Heck AJ, Slijper M, Menke FL. Quantitative phosphoproteomics of early elicitor signaling in Arabidopsis. Mol Cell Proteomics. 2007;6:1198–1214. doi: 10.1074/mcp.M600429-MCP200. [DOI] [PubMed] [Google Scholar]
- 6.Bos JI, Armstrong M, Whisson SC, Torto TA, Ochwo M, Birch PRJ, Kamoun S. Intraspecific comparative genomics to identify avirulence genes from Phytophthora. New Phytol. 2003;159:63–72. doi: 10.1046/j.1469-8137.2003.00801.x. [DOI] [PubMed] [Google Scholar]
- 7.Liu Z, Bos JI, Armstrong M, Whisson SC, da Cunha L, Torto-Alalibo T, Win J, Avrova AO, Wright F, Birch PR, Kamoun S. Patterns of diversifying selection in the phytotoxin-like scr74 gene family of Phytophthora infestans. Mol Biol Evol. 2005;22:659–672. doi: 10.1093/molbev/msi049. [DOI] [PubMed] [Google Scholar]
- 8.Tyler BM, Tripathy S, Zhang X, Dehal P, Jiang RH, Aerts A, Arredondo FD, Baxter L, Bensasson D, Beynon JL, Chapman J, Damasceno CM, Dorrance AE, Dou D, Dickerman AW, Dubchak IL, Garbelotto M, Gijzen M, Gordon SG, Govers F, Grunwald NJ, Huang W, Ivors KL, Jones RW, Kamoun S, Krampis K, Lamour KH, Lee MK, McDonald WH, Medina M, Meijer HJ, Nordberg EK, Maclean DJ, Ospina-Giraldo MD, Morris PF, Phuntumart V, Putnam NH, Rash S, Rose JK, Sakihama Y, Salamov AA, Savidor A, Scheuring CF, Smith BM, Sobral BW, Terry A, Torto-Alalibo TA, Win J, Xu Z, Zhang H, Grigoriev IV, Rokhsar DS, Boore JL. Phytophthora genome sequences uncover evolutionary origins and mechanisms of pathogenesis. Science. 2006;313:1261–1266. doi: 10.1126/science.1128796. [DOI] [PubMed] [Google Scholar]
- 9.Brune DC. Alkylation of cysteine with acrylamide for protein sequence analysis. Anal Biochem. 1992;207:285–290. doi: 10.1016/0003-2697(92)90013-w. [DOI] [PubMed] [Google Scholar]
- 10.Chagot B, Pimentel C, Dai L, Pil J, Tytgat J, Nakajima T, Corzo G, Darbon H, Ferrat G. An unusual fold for potassium channel blockers: NMR structure of three toxins from the scorpion Opisthacanthus madagascariensis. Biochem J. 2005;388:263–271. doi: 10.1042/BJ20041705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srinivasan KN, Sivaraja V, Huys I, Sasaki T, Cheng B, Kumar TK, Sato K, Tytgat J, Yu C, San BC, Ranganathan S, Bowie HJ, Kini RM, Gopalakrishnakone P. kappa-Hefutoxin1, a novel toxin from the scorpion Heterometrus fulvipes with unique structure and function. Importance of the functional diad in potassium channel selectivity. J Biol Chem. 2002;277:30040–30047. doi: 10.1074/jbc.M111258200. [DOI] [PubMed] [Google Scholar]
- 12.Trevino MA, Garcia-Mayoral MF, Barral P, Villalba M, Santoro J, Rico M, Rodriguez R, Bruix M. NMR solution structure of Ole e 6, a major allergen from olive tree pollen. J Biol Chem. 2004;279:39035–39041. doi: 10.1074/jbc.M406045200. [DOI] [PubMed] [Google Scholar]
- 13.Krissinel E, Henrick K. Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr D Biol Crystallogr. 2004;60:2256–2268. doi: 10.1107/S0907444904026460. [DOI] [PubMed] [Google Scholar]
- 14.Lebaudy A, Very AA, Sentenac H. K+ channel activity in plants: genes, regulations and functions. FEBS Lett. 2007;581:2357–2366. doi: 10.1016/j.febslet.2007.03.058. [DOI] [PubMed] [Google Scholar]
- 15.van den Hooven HW, van den Burg HA, Vossen P, Boeren S, de Wit PJ, Vervoort J. Disulfide bond structure of the AVR9 elicitor of the fungal tomato pathogen Cladosporium fulvum: evidence for a cystine knot. Biochemistry. 2001;40:3458–3466. doi: 10.1021/bi0023089. [DOI] [PubMed] [Google Scholar]
- 16.van't Slot KA, van den Burg HA, Kloks CP, Hilbers CW, Knogge W, Papavoine CH. Solution structure of the plant disease resistance-triggering protein NIP1 from the fungus Rhynchosporium secalis shows a novel beta-sheet fold. J Biol Chem. 2003;278:45730–45736. doi: 10.1074/jbc.M308304200. [DOI] [PubMed] [Google Scholar]
- 17.Ponchet M, Panabieres F, Milat ML, Mikes V, Montillet JL, Suty L, Triantaphylides C, Tirilly Y, Blein JP. Are elicitins cryptograms in plant-Oomycete communications? Cell Mol Life Sci. 1999;56:1020–1047. doi: 10.1007/s000180050491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rety S, Salamitou S, Garcia-Verdugo I, Hulmes DJ, Le Hegarat F, Chaby R, Lewit-Bentley A. The crystal structure of the Bacillus anthracis spore surface protein BclA shows remarkable similarity to mammalian proteins. J Biol Chem. 2005;280:43073–43078. doi: 10.1074/jbc.M510087200. [DOI] [PubMed] [Google Scholar]
- 19.Abramovitch RB, Anderson JC, Martin GB. Bacterial elicitation and evasion of plant innate immunity. Nat Rev Mol Cell Biol. 2006;7:601–611. doi: 10.1038/nrm1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stebbins CE, Galàn JE. Structural mimicry in bacterial virulence. Nature. 2001;412:701–705. doi: 10.1038/35089000. [DOI] [PubMed] [Google Scholar]
- 21.Vuister GW, Bax A. Quantitative J correlation: a new approach for measuring homonuclear three-bond J(HNHα) coupling constants in 15N-enriched proteins. J Am Chem Soc. 1993;115:7772–7777. [Google Scholar]
- 22.Archer SJ, Ikura M, Torchia DA, Bax A. An alternative 3D NMR technique for correlating backbone 15N with side-chain Hβ resonances in larger proteins. J Magn Reson. 1991;95:636–641. [Google Scholar]
- 23.Nilges M, Clore GM, Gronenborn AM. Determination of three-dimensional structures of proteins from interproton distance data by dynamical simulated annealing from a random array of atoms. Circumventing problems associated with folding. FEBS Lett. 1988;239:129–136. doi: 10.1016/0014-5793(88)80559-3. [DOI] [PubMed] [Google Scholar]
- 24.Laskowski RA, Rullmann JA, MacArthur MW, Kaptein R, Thornton JM. AQUA and PROCHECK-NMR: programs for checking the quality of protein structures solved by NMR. J Biomol NMR. 1996;8:477–486. doi: 10.1007/BF00228148. [DOI] [PubMed] [Google Scholar]
- 25.Koradi R, Billeter M, Wüthrich K. MOLMOL: a program for display and analysis of macromolecular structures. J Mol Graph. 1996;14:51–55. 29–32. doi: 10.1016/0263-7855(96)00009-4. [DOI] [PubMed] [Google Scholar]
- 26.Kay LE, Torchia DA, Bax A. Backbone dynamics of proteins as studied by 15N inverse detected heteronuclear NMR spectroscopy: application to staphylococcal nuclease. Biochemistry. 1989;28:8972–8979. doi: 10.1021/bi00449a003. [DOI] [PubMed] [Google Scholar]
- 27.Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRpipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 28.Dosset P, Hus JC, Blackledge M, Marion D. Efficient analysis of macromolecular rotational diffusion from heteronuclear relaxation data. J Biomol NMR. 2000;16:23–28. doi: 10.1023/a:1008305808620. [DOI] [PubMed] [Google Scholar]
- 29.Lenffer J, Lai P, El Mejaber W, Khan AM, Koh JL, Tan PT, Seah SH, Brusic V. CysView: protein classification based on cysteine pairing patterns. Nucleic Acids Res. 2004;32:W350–W355. doi: 10.1093/nar/gkh475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gambale F, Uozumi N. Properties of shaker-type potassium channels in higher plants. J Membr Biol. 2006;210:1–19. doi: 10.1007/s00232-006-0856-x. [DOI] [PubMed] [Google Scholar]
- 31.Grignon C, Sentenac H. pH and ionic conditions in the apoplast. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:103–128. [Google Scholar]


