Abstract
Recent studies have suggested the existence of separate transduction mechanisms and sensory pathways for histamine and nonhistaminergic types of itch. We studied whether histamine and an agonist of the protease-activated receptor (PAR)-2, associated with nonhistaminergic itch, excite murine dorsal horn neurons. Single units were recorded in superficial lumbar dorsal horn of adult ICR mice anesthetized with pentobarbital. Unit activity was searched using a small intradermal hindpaw injection of histamine or the PAR-2 agonist SLIGRL-NH2. Isolated units were subsequently challenged with intradermal histamine followed by SLIGRL-NH2 (each 50 μg/1 μl) or reverse order, followed by mechanical, thermal, and algogenic stimuli. Forty-three units were classified as wide dynamic range (62%), nociceptive specific (22%), or mechano insensitive (16%). Twenty units gave prolonged (mean, 10 min) discharges to intradermal injection of histamine; 76% responded to subsequent SLIGRL-NH2, often more briefly. Units additionally responded to noxious heat (63%), cooling (43%), topical mustard oil (53%), and intradermal capsaicin (67%). Twenty-two other units gave prolonged (mean, 5 min) responses to initial intradermal injection of SLIGRL-NH2; 85% responded to subsequent intradermal histamine. They also responded to noxious heat (75%), mustard oil (93%), capsaicin (63%), and one to cooling. Most superficial dorsal horn neurons were excited by both histamine and the PAR-2 agonist, suggesting overlapping pathways for histamine- and non–histamine-mediated itch. Because the large majority of pruritogen-responsive neurons also responded to noxious stimuli, itch may be signaled at least partly by a population code.
INTRODUCTION
Chronic itch frequently accompanies dermatologic conditions such as atopic dermatitis and psoriasis, as well as kidney and liver disease, and in general is poorly controlled by antihistamines or other treatments (Carstens 2009; Ikoma et al. 2006; Twycross et al. 2003). Although histamine is the prototypical mediator of acute itch in humans, recent evidence has suggested that proteases may represent an important, nonhistaminergic mechanism involved in itch. It was reported >50 yr ago that spicules from seed pods of the tropical bean plant, cowhage, elicit itch when inserted into human skin (Shelley and Arthur 1955). Recent reinvestigations of cowhage showed that the chemical contained within the spicules, originally named mucunain, acts at protease-activated receptor (PAR) subtypes PAR-2 and PAR-4 (Reddy et al. 2008). PAR-2, in particular, has recently been implicated in itch (Steinhoff et al. 2003) and inflammation (Cottrell et al. 2003; Dai et al. 2004). Intradermal injection of PAR-2 agonists elicits scratching behavior in mice (Akiyama et al. 2009b; Shimada and LaMotte 2008; Shimada et al. 2006; Tsujii et al. 2008; Ui et al. 2006). In humans, itch elicited by cowhage is not accompanied by a flare reaction and is not affected by antihistamine treatment, in contrast to histamine-evoked itch (Johanek et al. 2007). Recent electrophysiological studies supported the notion that itch elicited by histamine versus cowhage may involve separate neural pathways. Histamine excites mechanically insensitive C-fibers over a time course matching that of itch sensation (Schmelz et al. 1997), and such histamine-sensitive fibers are often unresponsive to cowhage (Namer et al. 2008). Conversely, cowhage excites mechanically sensitive C-fiber nociceptors, some of which are insensitive to histamine (Johanek et al. 2008; Namer et al. 2008). Moreover, subpopulations of primate spinothalamic tract (STT) neurons responded to either intradermal injection of histamine or application of cowhage spicules within the neuronal receptive field but not to both stimuli (Davidson et al. 2007). These data suggest separate neural pathways for itch elicited by histamine versus proteases.
We recently reported that intradermal hindpaw injection of a PAR-2 agonist, SLIGRL-NH2, excites neurons in the superficial dorsal horn of mice over a prolonged time course that is consistent with a role in itch (Akiyama et al. 2009a). Furthermore, neurons activated by intradermal histamine versus SLIGRL-NH2, as assessed by Fos expression, seemed to have spatially segregated distributions in lamina IIi and lamina I and IIo, respectively (Nakano et al. 2008). In this study, we investigated whether separate populations of neurons in the mouse superficial dorsal horn are activated by intradermal histamine compared with SLIGRL-NH2.
METHODS
Experiments were performed using adult male ICR mice (Harlan, Oxnard, CA; 34–58 g) under a protocol approved by the UC Davis Animal Care and Use Committee.
The methods were the same as reported in our recent study (Akiyama et al. 2009a) and are only briefly summarized here. Anesthesia was induced with pentobarbital sodium (60 mg/kg, ip) and maintained by constant intravenous infusion. The lumbosacral spinal cord was exposed by laminectomy, and a tungsten microelectrode (FHC, Bowdoin, ME) recorded extracellular single-unit activity. A chemical search strategy (Jinks and Carstens 2000, 2002) was used to isolate units in the superficial dorsal horn. In this study, either histamine (Sigma-Aldrich, St. Louis MO) or SLIGRL-NH2 (Quality Controlled Biochemicals, Hopkinton, MA, and GenScript, Piscataway, NJ) (both 50 μg/μl in saline) was used. Using a 30.5-gauge needle connected to a Hamilton microsyringe, a small volume (∼0.25 μl, ∼12 μg of histamine or SLIGRL-NH2) was microinjected in the plantar skin intradermal and the recording electrode positioned to isolate an action potential in the superficial dorsal horn (<300 μm from surface) that had ongoing activity. If no unit was isolated, the procedure was repeated ≥10 min later at a different site on the plantar surface or on the opposite side. We then waited ≥10 min until firing decreased to a steady low level. The same chemical used to isolate the unit was then reinjected in a volume of 1 μl. Responsive units (i.e., >30% increased above baseline) were studied further. The chemically evoked response was recorded for ≥26 min, after which a second 30.5-gauge needle containing the other chemical was inserted intradermally. Experiments were counterbalanced with histamine tested first followed by SLIGRL-NH2 in ∼50%, with the reverse order in the other 50%. The second chemical was injected, and unit activity was recorded for another 20–30 min. After this, we usually tested the unit responsiveness to light brushing with a cotton wisp, followed by pinching using forceps. Units were classified as wide dynamic range (WDR) if they responded at higher firing rate to pinch than light touch (and also noxious heat, if tested). They were classified as nociceptive specific (NS) if they responded to pinch (and noxious heat if tested) but not light touch. Units insensitive to touch, pinch, and heat stimuli were classified as mechanically insensitive. Some units were tested for responsiveness to noxious heat (48–56°C, 10 s) and cooling (down to 0° over 60 s) delivered by a computer-controlled Peltier thermode (NTE-2A, Physitemp, Clifton, NJ). After this, we tested responses to topical application of mustard oil (Sigma; 75% in mineral oil, 2 μl) followed by intradermal capsaicin (Sigma; 3.3 mM/1 μl); in a few experiments, the order of chemical application was reversed and/or they were applied before mechanical and thermal stimuli. Only one unit was studied per hindpaw in a given animal.
Action potentials were recorded to a computer and counted using Chart software (AD Instruments, Colorado Springs, CO). Unit activity was usually quantified as number of action potentials per minutes and displayed in peristimulus time histogram (PSTH) format with 1-s bins. Group responses at 1-min intervals after a given stimulus were compared with activity 1 min before the stimulus by paired t-test or repeated-measures ANOVA (SPSS 9.0, SPSS, Chicago, IL), with P < 0.05 set as significant.
At the end of the experiment, an electrolytic lesion was made through the microelectrode. The spinal cord was fixed in 10% buffered formalin, and 50-μm sections were cut and mounted on slides for microscopic verification of the lesion site.
RESULTS
A total of 43 units (62% WDR type, 22% NS, 16% mechanically insensitive) were isolated. All histologically localized recording sites were in the superficial dorsal horn with most (26/28) in lamina I (Figs. 1, A–C, insets; 2, A and B, insets; and 3, B and C, insets).
Fig. 1.
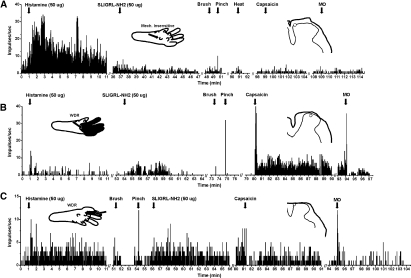
Examples of units isolated using histamine search strategy. A: peristimulus-time histogram (PSTH; bins 1 s) of a unit response to histamine (left PSTH). This mechanically insensitive unit responded weakly, if at all, to subsequent intradermal injection of SLIGRL-NH2 or to topical application of mustard oil (MO) or intradermal capsaicin. Inset: left: drawing of left hindpaw with arrow indicating intradermal injection site. Right: drawing of histological section showing recording site (open circle) in lamina I of superficial dorsal horn. B: a different wide dynamic range (WDR) lamina I unit that responded weakly to histamine and more strongly to SLIGRL-NH2 (format as in A). This unit also responded robustly to capsaicin and weakly to mustard oil. Top left inset: drawing of left hindpaw receptive field (gray) and injection site (arrow). C: a different WDR lamina I unit that responded equally to intradermal histamine and SLIGRL-NH2. This unit also responded phasically to capsaicin and mustard oil (format as in A and B).
Fig. 2.
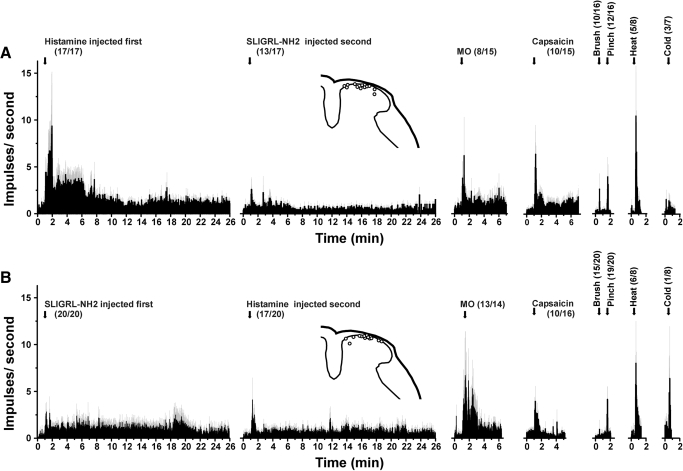
Averaged responses of units isolated using histamine (A) or SLIGRL-NH2 search strategy (B). A: histamine search. Averaged PSTHs (bins: 1 s) show, from left to right, unit responses to histamine, SLIGRL-NH2, MO, capsaicin, brush, pinch, noxious heat, and cooling. Error bars (gray): SE. Numbers in parentheses give the number of responders in relation to the total number tested. B: SLIGRL-NH2 search. Format as in A for 20 different units tested with SLIGRL-NH2 1st.
Fig. 3.
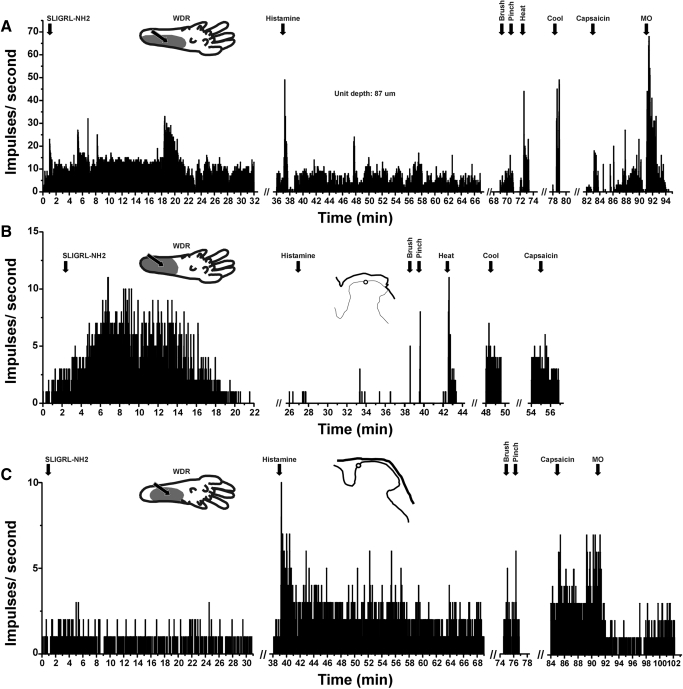
Examples of units isolated using SLIGRL-NH2 search strategy. A: PSTH shows robust response to SLIGRL-NH2, followed by a somewhat weaker response to histamine. This WDR unit responded to graded mechanical stimuli, heating and cooling, weakly to intradermal capsaicin, and robustly to topical MO. Inset: drawing of left hindpaw receptive field (gray) and injection site (arrow). B: different WDR lamina I unit responded robustly to SLIGRL-NH2 and noxious heat but not to histamine or cooling and only weakly to capsaicin. C: different WDR lamina I units that responded weakly, if at all, to SLIGRL-NH2 but more to histamine injected in right hindpaw (top left inset). It responded weakly to capsaicin and MO.
Histamine Search
Of 21 units isolated using the histamine search strategy, 20 (95%) exhibited a >30% increase in firing to intradermal injection of histamine. Figures 1, A–C (left-hand PSTHs), show examples of individual responses to histamine. Figure 2A shows the averaged histamine-evoked response of 17 units that were subsequently tested with other chemicals. The averaged response/60 s was significantly greater than baseline firing out to 13 min after injection.
Seventeen of the histamine-responsive units were subsequently tested with intradermal injection of the PAR-2 agonist SLIGRL-NH2, and 13 (76%) responded. Figure 1, B and C, shows examples of unit responses to SLIGRL-NH2 following histamine, whereas the unit in Fig. 1A responded to histamine but not to SLIGRL-NH2. Mean responses of all 17 units to SLIGRL-NH2 after histamine are shown in Fig. 2A. The mean response to SLIGRL-NH2 was significantly greater than baseline during the first minute after injection (P < 0.001, regardless of whether the 4 unresponsive units were included or not).
Table 1 (top half) shows the incidence of responses of 17 functionally classified units identified by the histamine search to SLIGRL-NH2 and additional stimuli. For units responsive to a given stimulus (Table 1, +), the number in parentheses indicates the relative magnitude of the response. The majority of units additionally responded to topical mustard oil (53%), intradermal capsaicin (67%), and noxious heat (63%), and a minority to cooling (43%). Figure 1B shows an example of a unit's response to capsaicin, and Fig. 1, B and C, shows brief responses to mustard oil. The unit shown in Fig. 1A responded robustly to histamine with little or no response to other stimuli, a rare example of a histamine-selective neuron. Figure 2A shows averaged responses of all tested units to mustard oil, capsaicin, and thermal and mechanical stimuli. The mean response to mustard oil was significantly greater than baseline (P < 0.05) during the first minute after application (including or excluding the 6 unresponsive units). Similarly, the mean response to capsaicin was significantly greater than baseline during the first minute after injection. In a previous study using identical methods (Akiyama et al. 2009a), we reported that intradermal injections of vehicle (saline) had no significant effect on neuronal firing relative to preinjection baseline and for this reason we presently did not routinely test vehicle.
Table 1.
Incidence of responses to tested stumuli
| Unit Type | Histamine | PAR-2 Agonist | MO | Cap | Heat | Cold | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine search | ||||||||||||
| WDR | + (1.5) | + (6.7) | + (↑) | − | + (↑) | − | ||||||
| WDR | + (2.7) | + (2.5) | + (153) | + (64.3) | + (↑) | + (13.8) | ||||||
| WDR | + (2.2) | + (2.2) | − | + (2.7) | + (↑) | − | ||||||
| WDR | + (5.6) | + (2) | − | + (9.4) | + (21) | + (1.4) | ||||||
| WDR | + (4.3) | + (1.4) | + (↑) | + (↑) | − | − | ||||||
| WDR | + (21.9) | + (14.8) | + (20.8) | + (10.8) | ||||||||
| WDR | + (4.5) | + (2.7) | + (5.4) | − | ||||||||
| WDR | + (4.2) | − | − | + (1.3) | + (8) | + (3) | ||||||
| WDR | + (8.2) | + (12) | − | |||||||||
| NS | + (162) | + (1.4) | + (8) | + (1.7) | ||||||||
| NS | + (31.5) | + (16.3) | + (6.3) | + (26) | ||||||||
| NS | + (6) | − | + (↑) | − | ||||||||
| MI | + (106) | + (↑) | − | + (↑) | ||||||||
| MI | + (4) | − | − | − | ||||||||
| MI | + (11.7) | + (3.9) | − | − | − | |||||||
| MI | + (340) | − | + (471) | − | − | |||||||
| MI | + (1.4) | + (↑) | ||||||||||
| Totals | 17/17 | 13/17 | 8/15 | 10/15 | 5/8 | 3/7 | ||||||
| PAR-2 agonist search | ||||||||||||
| WDR | + (3.2) | + (3.4) | + (16.6) | + (4.5) | + (15.7) | + (↑) | ||||||
| WDR | − | + (2) | + (↑) | − | + (3.3) | − | ||||||
| WDR | + (57) | + (6) | + (↑) | + (25.7) | + (↑) | − | ||||||
| WDR | + (32.5) | + (32.5) | + (29.4) | + (29.4) | − | |||||||
| WDR | + (137) | + (43) | + (4.4) | + (8.7) | ||||||||
| WDR | + (67) | + (12.2) | + (9) | + (3.1) | ||||||||
| WDR | + (1.8) | + (4.4) | + (2.3) | + (2.3) | ||||||||
| WDR | + (2.6) | + (5) | + (↑) | − | − | − | ||||||
| WDR | + (4) | + (3.5) | − | + (9.1) | − | |||||||
| WDR | + (12.3) | + (1.7) | − | + (1.6) | ||||||||
| WDR | + (4) | + (5) | ||||||||||
| WDR | + (1.5) | + (15) | ||||||||||
| WDR | − | + (↑) | ||||||||||
| WDR | + (↑) | + (7) | ||||||||||
| NS | + (2) | + (3.3) | + (7) | + (1.8) | ||||||||
| NS | + (6) | + (45) | + (↑) | + (↑) | − | − | ||||||
| NS | + (2.1) | + (↑) | + (↑) | − | + (↑) | − | ||||||
| NS | + (↑) | + (1.8) | + (↑) | + (↑) | ||||||||
| NS | − | + (↑) | + (23.5) | − | ||||||||
| MI | + (↑) | + (23) | + (2.4) | + (↑) | ||||||||
| Totals | 17/20 | 20/20 | 13/14 | 10/16 | 6/8 | 1/8 | ||||||
Numbers in parantheses indicate response magnitude (response during stimulus divided by baseline). 1.3 = 30% increase firing rate ↑, firing increased above a baseline level of zero; +, responsive (>30% above baseline);-: unresponsive (<30% above baseline); open, not tested; WDR, wide dynamic range; NS, nociceptive-specific, MI, mechanically insensitive.
Par-2 Agonist Search
All 22 of the units isolated by the PAR-2 agonist search responded to subsequent intradermal injection of the PAR-2 agonist SLIGRL-NH2. Figure 3 shows three examples. Twenty SLIGRL-NH2-responsive units were subsequently tested with histamine, and their averaged responses are shown in Fig. 2B. The mean response to SLIGRL-NH2 was significantly greater than baseline out to 5 min after injection.
Seventeen of the 20 SLIGRL-NH-2 responsive units (85%) responded to subsequent injection of histamine. Figure 3A shows an example in which the response to histamine was somewhat smaller than to SLIGRL-NH2. The unit shown in Fig. 3B responded robustly to SLIGRL-NH2 but not at all to histamine, whereas the unit shown in Fig. 3C responded weakly to SLIGRL-NH2 and more to histamine. Figure 2B shows averaged responses to histamine after SLIGRL-NH2. Firing averaged over 1-min intervals was significantly greater than baseline at minutes 1 and 3–8 after histamine.
The bottom half of Table 1 lists the incidence and relative magnitude of responses of 20 functionally identified units isolated by the SLIGRL-NH2 search to additional stimuli. The large majority (93%) responded to mustard oil. An example is shown in Fig. 3A, and Fig. 2B shows the averaged response to mustard oil, which was significantly greater than baseline at 2 and 3 min after application. Sixty-three percent of units also responded to capsaicin (Fig. 3; Table 1). The averaged response to capsaicin (Fig. 2B) was significantly greater than baseline during the first minute after injection. Seventy-five percent of the units responded to noxious heat and one responded to cooling (Table 1; Fig. 2B).
Cross-tachyphylaxis
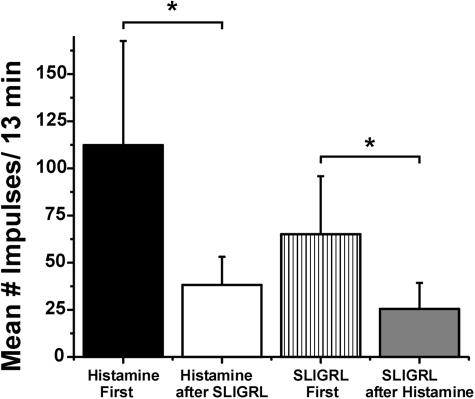
It is apparent from Fig. 2 that the averaged responses to the initial injection of histamine (Fig. 2A) and SLIGRL-NH2 (Fig. 2B) were greater compared with when each chemical was tested second, indicative of cross-tachyphylaxis. Figure 4 plots averaged responses to histamine and SLIGRL-NH2 when tested first or second. Responses were counted over a 13-min postinjection period that corresponds to the duration of significantly elevated firing after the initial histamine injection. The mean response to histamine after SLIGRL-NH2 was significantly (P < 0.001) smaller compared with the mean response to histamine tested first. Similarly, the mean response to SLIGRL-NH2 after histamine was significantly smaller compared with the mean response to the initial injection of SLIGRL-NH2 (P < 0.01, unpaired t-test), regardless of whether four units unresponsive to SLIGRL-NH2 after histamine were excluded from analysis or not.
Fig. 4.
Evidence for cross-tachyphylaxis between histamine and SLIGRL-NH2. Bar graphs plot mean responses over a 13-min postinjection period for histamine tested 1st (black bar) or 2nd after SLIGRL-NH2 (open bar) and for SLIGRL-NH2 tested 1st (hatched bar) or 2nd (gray bar). Error bars: SE. *Significantly different (P < 0.01; unpaired t-test).
DISCUSSION
Using a search strategy designed to isolate chemo-responsive spinal neurons, we identified populations of neurons in the superficial dorsal horn that responded to intradermal injections of the pruritogens histamine and SLIGRL-NH2, a PAR-2 agonist. The pruritogen-evoked responses had a time course on the order of 5 to >10 min, suggesting their involvement in signaling itch. The presently recorded neuronal responses to SLIGRL-NH2 were smaller compared with those of our recent study using identical methods (Akiyama et al. 2009a), a difference for which we have no good explanation beyond the sizable variance observed in individual neuronal responses to SLIGRL-NH2. Although a small number of pruritogen-responsive units did not respond to mustard oil or capsaicin, the vast majority responded to one or both of these algesic agents, as well as thermal stimuli, largely confirming previous studies (Akiyama et al. 2009a; Davidson et al. 2007; Jinks and Carstens 2002; Simone et al. 2004).
Most pruritogen-responsive units were of the WDR type, with some classified as NS and fewer as mechanoinsensitive (Table 1). It should be noted, however, that histamine or SLIGRL-NH2 was always tested before unit characterization, and these chemical stimuli may have altered the unit mechanosensitivity, which could result in misclassification. This chemical search strategy made it impossible to characterize unit mechanosensitivity before chemical testing but did avoid any potential effects that the mechanical and thermal stimuli might have had on unit responses to pruritogens.
We presently selected histamine and the PAR-2 agonist, SLIGRL-NH2, because both of these agents elicit dose-related hindlimb scratching behavior when injected intradermal in ICR mice (Akiyama et al. 2009b; Shimada et al. 2006; Tsujii et al. 2008; Ui et al. 2006). Although these experiments tested effects of histamine and SLIGRL-NH2 injected into the hindpaw, we believe that this approach is relevant to itch. Hindpaw injections of 5-HT in mice elicited biting and licking behavior directed to the injection site in a manner that was significantly attenuated by the μ-opiate antagonist naltrexone (Hagiwara et al. 1999), consistent with itch. It is uncommon to observe quadrupedal animals scratch at a distal extremity with a contralateral limb, suggesting instead that gnawing, biting, or licking is used to relieve itch.
Although the large majority of units responded to both pruritogens, it was noteworthy that responses to histamine were smaller when it was tested after SLIGRL-NH2 compared with when histamine was tested first. Similarly, SLIGRL-NH2–evoked responses were weaker posthistamine compared with when SLIGRL-NH2 was injected first. These data support cross-tachyphylaxis between these two pruritogens. Curiously, histamine and SLIGRL-NH2 injected into the nape of the neck did not exhibit cross-tachyphylaxis for scratching behavior (Akiyama et al. 2009b). Furthermore, superficial dorsal horn neuronal responses to repeated injection of SLIGRL-NH2 exhibited significant tachyphylaxis (Akiyama et al. 2009a), whereas scratching behavior did not (Akiyama et al. 2009b). These discrepancies suggest that pruritogen-evoked responses of superficial dorsal horn neurons do not translate perfectly to behavior. However, there are numerous differences between the neurophysiological and behavioral approaches that may affect direct comparisons: 1) use of general anesthesia in neurophysiological experiments, 2) use of different body regions (hindpaw vs. nape of neck), 3) use of a chemical search stimulus in neurophysiological experiments may initiate tachyphylaxis that would reduce neural responses to subsequent chemical stimuli, and 4) use of smaller injection volumes in neurophysiological (1 μl) versus behavioral (10 μl) experiments. More direct comparisons between neural activity and scratching behavior may be achieved in future studies using identical stimuli and stimulus locations.
The specificity theory holds that itch and pain are signaled by separate neural pathways selectively responsive to pruritic or algesic stimuli, respectively. This concept is supported by observations that pain suppresses itch, that opioids inhibit pain while often inducing itch, and that intraneural (Schmidt et al. 1993) or localized microstimulation of the skin surface (Tuckett 1982) can elicit itch that does not transform to pain at high stimulus frequencies. However, recent findings suggest that the notion of an itch-specific pathway may be an oversimplification. Spicules from the seed pods of cowhage, a tropical bean plant, elicit itch when inserted into the skin (Johanek et al. 2007; Shelley and Arthur 1955). Intradermal insertion of a single cowhage spicule, or a heat-inactivated spicule soaked with histamine or capsaicin, elicits a complex sensation that is dominated by itch but is usually accompanied by nociceptive stinging/pricking and/or burning qualities (LaMotte et al. 2009). Mechanically insensitive primary C-fiber afferents respond to histamine over a time course matching concomitant itch sensation (Namer et al. 2008; Schmelz et al. 1997); however, many of these fibers are also excited by capsaicin. Cowhage excites mainly mechanically sensitive C-fiber nociceptors (Johanek et al. 2008; Namer et al. 2008), which respond to noxious heat and in some cases also to capsaicin (Johanek et al. 2008). The large majority of spinal neurons that responds to pruritogens, such as 5-HT in rats (Jinks and Carstens 2002) or histamine in primates (Davidson et al. 2007; Simone et al. 2004), also responds to capsaicin and noxious thermal stimuli. In cats, a small subpopulation of lamina I spinothalmic tract neurons responded to cutaneous iontophoretic application of histamine, and 50% of those tested also responded to mustard oil (Andrew and Craig 2001). These data from mouse superficial dorsal horn neurons are consistent with these observations. Taken together, the data suggest that pruritogens excite nociceptive afferents that connect with spinal neurons to signal both itch and nociceptive sensory qualities simultaneously.
As noted in the Introduction, current data suggest that histamine and cowhage may activate largely separate populations of primary afferent C-fibers (Johanek et al. 2008; Namer et al. 2008) and STT neurons (Davidson et al. 2007). These data are partially consistent with this, in that a fraction of pruritogen-responsive dorsal horn units responded to either histamine or the PAR-2 agonist, but not both. However, the large majority of units isolated using either the histamine or PAR-2 agonist search strategies responded to both chemicals (76 and 85%, respectively). This suggests that, in mouse, there is considerable overlap in neural pathways signaling histaminergic and nonhistaminergic types of itch, in contrast to primates in which there seems to be greater segregation of these pathways. We speculate that this is most likely explained by a species difference. However, there were also notable methodological differences between this study and that of that of Davidson et al. (2007): 1) use of antidromic stimulation versus chemical micronjection to isolate neurons and 2) use of cowhage spicules versus intradermal injection of PAR-2 agonist via 30.5-gauge needles. In particular, insertion of cowhage spicules very likely results in delivery of a much smaller volume of chemical to a more superficial location in the epidermis compared with microinjections using intradermal needles. This might explain why some histamine-sensitive STT neurons did not respond to cowhage. Furthermore, the pruritogen in cowhage spicules, called mucunain, acts at both PAR-2 and PAR-4 (Reddy et al. 2008). Thus the cowhage spicules used by Davidson et al. (2007) are not the same as the PAR-2 agonist used presently. Conceivably, our chemical search strategy might have introduced a bias toward isolating broadly tuned chemonociceptive neurons compared with more narrowly tuned subpopulations of primate STT neurons identified by antidromic stimulation. Thus both methodological and species differences may account for the lack of segregation in histamine- and non–histamine-mediated itch transmission observed presently in the mouse.
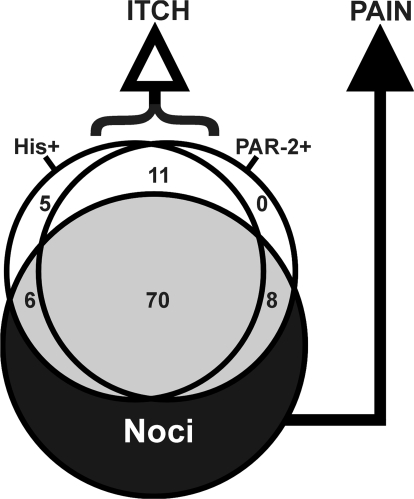
Figure 5 presents a schematic of itch- and pain-signaling central neuronal populations. The overlapping circles represent populations of central neurons responsive to histamine, the PAR-2 agonist, and to noxious stimuli (noci = nociceptive neurons, i.e., WDR or NS). Of units responsive to histamine and/or the PAR-2 agonist (gray shading in Fig. 5), the vast majority also responded to noxious stimuli. Presumably, these neurons signal pain, although they may also signal itch, consistent with the ability of punctuate pruritic stimuli to simultaneously elicit both itch and nociceptive sensory qualities. A smaller subpopulation of neurons responded to histamine, the PAR-2 agonist, or both, but were unresponsive to noxious stimuli. It is presumed that such pruritogen-selective neurons signal itch sensation.
Fig. 5.
Schematic Venn diagram showing overlapping populations of central neurons responsive to histamine (His+), PAR-2 agonist (PAR−2+), or noxious stimuli (Noci). Numbers: percentage of neurons within each overlap region. White: mechanically insensitive units. Gray: WDR and nociceptive-specific (NS) units that responded to histamine and/or PAR-2 agonist. Black: WDR and NS units unresponsive to histamine or PAR-2 agonist.
GRANTS
This work was supported by National Institute of Health Grants DE-013685 and AR-057194.
REFERENCES
- Akiyama T, Merrill AW, Iodi Carstens M, Carstens E. Activation of superficial dorsal horn neurons in the mouse by a PAR-2 agonist and 5-HT: potential role in itch. J Neurosci 29: 6691–6699, 2009a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Merrill AW, Zanotto K, Iodi Carstens M, Carstens E. Scratching behavior and Fos expression in superficial dorsal horn elicited by protease-activated receptor agonists and other itch mediators in mice. J Pharm Exp Ther 329: 945–951, 2009b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew D, Craig AD. Spinothalamic lamina I neurons selectively sensitive to histamine: a central neural pathway for itch. Nat Neurosci 4: 72–77, 2001 [DOI] [PubMed] [Google Scholar]
- Carstens E. Neurobiology of itch and pain: scratching for answers. In: Current Topics in Pain: 12th World Congress on Pain, edited by Castro-Lopes J. Seattle, WA: IASP Press, 2009. p. 73–93 [Google Scholar]
- Cottrell GS, Amadesi S, Schmidlin F, Bunnett N. Protease-activated receptor 2: activation, signalling and function. Biochem Soc Trans 31: 1191–1197, 2003 [DOI] [PubMed] [Google Scholar]
- Dai Y, Moriyama T, Higashi T, Togashi K, Kobayashi K, Yamanaka H, Tominaga M, Noguchi K. Proteinase-activated receptor 2-mediated potentiation of transient receptor potential vanilloid subfamily 1 activity reveals a mechanism for proteinase-induced inflammatory pain. J Neurosci 24: 4293–4299, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S, Zhang X, Yoon CH, Khasabov SG, Simone DA, Giesler GJ., Jr The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci 27: 10007–10014, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara K, Nojima H, Kuraishi Y. Serotonin-induced biting of the hind paw is itch-related response in mice. Pain Res 14: 53–59, 1999 [Google Scholar]
- Ikoma A, Steinhoff M, Ständer S, Yosipovitch G, Schmelz M. The neurobiology of itch. Nat Rev Neurosci 7: 535–547, 2006 [DOI] [PubMed] [Google Scholar]
- Jinks SL, Carstens E. Superficial dorsal horn neurons identified by intracutaneous histamine: chemonociceptive responses and modulation by morphine. J Neurophysiol 84: 616–627, 2000 [DOI] [PubMed] [Google Scholar]
- Jinks SL, Carstens E. Responses of superficial dorsal horn neurons to intradermal serotonin and other irritants: comparison with scratching behavior. J Neurophysiol 87: 1280–1289, 2002 [DOI] [PubMed] [Google Scholar]
- Johanek LM, Meyer RA, Friedman RM, Greenquist KW, Shim B, Borzan J, Hartke T, LaMotte RH, Ringkamp M. A role for polymodal C-fiber afferents in nonhistaminergic itch. J Neurosci 28: 7659–7669, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanek LM, Meyer RA, Hartke T, Hobelmann JG, Maine DN, LaMotte RH, Ringkamp M. Psychophysical and physiological evidence for parallel afferent pathways mediating the sensation of itch. J Neurosci 27: 7490–7497, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMotte RH, Shimada SG, Green BG, Zelterman D. Pruritic and nociceptive sensations and dysesthesias from a spicule of cowhage. J Neurophysiol 101: 1430–1443, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T, Andoh T, Lee JB, Kuraishi Y. Different dorsal horn neurons responding to histamine and allergic itch stimuli. Neuroreport 19: 723–726, 2008 [DOI] [PubMed] [Google Scholar]
- Namer B, Carr R, Johanek LM, Schmelz M, Handwerker HO, Ringkamp M. Separate peripheral pathways for pruritus in man. J Neurophysiol 100: 2062–2069, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy VB, Iuga AO, Shimada SG, LaMotte RH, Lerner EA. Cowhage-evoked itch is mediated by a novel cysteine protease: a ligand of protease-activated receptors. J Neurosci 28: 4331–4335, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjörk HE. Specific C-receptors for itch in human skin. J Neurosci 17: 8003–8008, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R, Torebjork E, Jorum E. Pain and itch from intraneural microstimulation. Abstr. 7th World Congr. Pain 143, 1993 [Google Scholar]
- Shelley WB, Arthur RP. Mucunain, the active pruritogenic proteinase of cowhage. Science 122: 469–470, 1955 [DOI] [PubMed] [Google Scholar]
- Shimada SG, Lamotte RH. Behavioral differentiation between itch and pain in mouse. Pain 139: 681–687, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada SG, Shimada KA, Collins JG. Scratching behavior in mice induced by the proteinase-activated receptor-2 agonist, SLIGRL-NH2. Eur J Pharmacol 530: 281–283, 2006 [DOI] [PubMed] [Google Scholar]
- Simone DA, Zhang X, Li J, Zhang JM, Honda CN, LaMotte RH, Giesler GJ., Jr Comparison of responses of primate spinothalamic tract neurons to pruritic and algogenic stimuli. J Neurophysiol 91: 213–222, 2004 [DOI] [PubMed] [Google Scholar]
- Steinhoff M, Neisius U, Ikoma A, Fartasch M, Heyer G, Skov PS, Luger TA, Schmelz M. Proteinase-activated receptor-2 mediates itch: a novel pathway for pruritus in human skin. J Neurosci 23: 6176–6180, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujii K, Andoh T, Lee JB, Kuraishi Y. Activation of proteinase-activated receptors induces itch-associated response through histamine-dependent and -independent pathways in mice. J Pharmacol Sci 108: 385–388, 2008 [DOI] [PubMed] [Google Scholar]
- Tuckett RP. Itch evoked by electrical stimulation of the skin. J Invest Dermatol 79: 368–373, 1982 [DOI] [PubMed] [Google Scholar]
- Twycross R, Greaves MW, Handwerker H, Jones EA, Libretto SE, Szepietowski JC, Zylicz Z. Itch: scratching more than the surface. Q J Med 96: 7–26, 2003 [DOI] [PubMed] [Google Scholar]
- Ui H, Andoh T, Lee JB, Nojima H, Kuraishi Y. Potent pruritogenic action of tryptase mediated by PAR-2 receptor and its involvement in anti-pruritic effect of nafamostat mesilate in mice. Eur J Pharmacol 530: 172–178, 2006 [DOI] [PubMed] [Google Scholar]