Abstract
Recent biochemical and behavioral data suggest right-hemispheric lateralization of amygdala functions in pain. Our previous electrophysiological studies showed pain-related neuroplasticity in the latero-capsular division of the central nucleus of the amygdala (CeLC) in the right brain hemisphere. Here we determined differences in the processing of pain-related signals in right versus left CeLC neurons. Individual CeLC neurons were recorded extracellularly before and after induction of an arthritis pain state in anesthetized rats. Brief innocuous and noxious test stimuli were applied to peripheral tissues ipsi- and contralateral to the recording site. A monoarthritis was induced in the ipsi- or contralateral knee by intraarticular injections of kaolin and carrageenan. Under normal conditions, CeLC neurons in the left amygdala had smaller receptive fields than those in the right, but the magnitude of background and evoked activity was not significantly different. After arthritis induction, neurons in the right, but not left, CeLC developed increased background activity and evoked responses, irrespective of the location of the arthritis (ipsi- or contralateral to the recording site). A protein kinase A (PKA) inhibitor decreased the activity of right CeLC neurons after arthritis induction but had no effect in the left amygdala. Forskolin, however, increased the activity of left and right CeLC neurons under normal conditions. The results show for the first time laterality of pain-related electrophysiological activity changes in individual amygdala neurons. Whereas both left and right amygdala neurons receive nociceptive inputs and can become sensitized in principle, a yet unknown mechanism prevents PKA activation and pain-related changes in the left amygdala.
INTRODUCTION
Hemispheric lateralization in emotional processing is now well documented, but it remains to be determined if brain asymmetries are based on right hemispheric dominance, positive versus negative valence, appetitive approach versus defensive withdrawal, or behavioral activation versus inhibition systems (Atchley et al. 2003; Davidson et al. 2004; Demaree et al. 2005; Stephan et al. 2007). Hemispheric specialization for emotions involves not only the cerebral cortex but also subcortical areas such as the amygdala, a key player in emotion (Adolphs 2002; Davidson 2002; Maren 2005; Pare et al. 2004; Phelps and Ledoux 2005).
Lateralization of amygdala function in emotional processing has been suggested to depend on valence, gender, and other factors such as level of awareness, actuality of experience, and temporal activation dynamics. Predominant activation or involvement of the right amygdala in aversive behavior and negative emotions was found in animal models (Baker and Kim 2004; Coleman-Mesches and McGaugh 1995a,b; Coleman-Mesches et al. 1996; Lalumiere and McGaugh 2005) and in humans (Angrilli et al. 1996; Canli et al. 1998; Funayama et al. 2001; LaBar et al. 1998; Lee et al. 2004; Yoshimura et al. 2008). There is also evidence to suggest the preferential involvement of the right amygdala in emotional responses and emotional memory in men and of the left amygdala in women (see Cahill 2006 for review). The underlying principle of hemispheric lateralization of amygdala function in emotions remains unclear and needs to be determined for different emotions and conditions.
Pain has a strong emotional-affective component. The amygdala plays a critical role in the emotional response to pain and in pain modulation (Carrasquillo and Gereau 2007; Fields 2004; Gauriau and Bernard 2004; Heinricher and McGaraughty 1999; Ikeda et al. 2007; Neugebauer et al. 2004, 2006; Pedersen et al. 2007; Rhudy and Meagher 2001). Our previous studies focused on the right amygdala and showed central sensitization and synaptic plasticity in neurons of the latero-capsular division of the central nucleus (CeLC) in an animal model of arthritis pain (Bird et al. 2005; Fu and Neugebauer 2008; Han et al. 2005b; Ji and Neugebauer 2007; Neugebauer and Li 2003; Neugebauer et al. 2003). The localized arthritis was induced in the contralateral (left) knee only. It remains to be determined if neuronal changes depend on the side of injury (ipsi- or contralateral knee) and if they occur in the left amygdala as well.
This question is important because recent studies showed that pain is associated with biochemical changes predominantly in the right amygdala. Pain-related ERK activation was observed in the right but not left CeLC, irrespective of the side of a formalin injection in the hind paw (Carrasquillo and Gereau 2007, 2008). Accordingly, blockade of ERK activation in the right but not left CeLC significantly decreased formalin-induced mechanical hypersensitivity in both the injected and the uninjured contralateral hind paw (Carrasquillo and Gereau 2007, 2008).
Evidence for pain-related lateralization is sparse and controversial. Psychophysical studies have suggested a functional asymmetry toward the right hemisphere for pain perception based on higher pain ratings for stimuli applied to the left side, independently of handedness (Lugo et al. 2002; Merskey and Watson 1979; Schiff and Gagliese 1994). Other studies found no such difference in pain sensation (Coghill et al. 2001; Hall et al. 1981; Seltzer et al. 1992). More direct evidence for right hemispheric lateralization in pain comes from a neuroimaging (PET) study that observed right lateralized activation of several brain areas, regardless of the side of peripheral stimulation (Coghill et al. 2001). Patients with chronic complex regional pain syndrome (CRPS) showed signs of gray matter atrophy in the right hemisphere but decreased white matter connectivity in the left (Geha et al. 2008). Right amygdala activation was seen in an fMRI study in response to painful visceral (gastric) stimulation (Lu et al. 2004).
The present study tested the hypothesis that functional properties (responsiveness) of neurons in the right but not left CeLC are altered in a pain state, suggesting right-hemispheric lateralization of pain processing in a subcortical brain area. We also sought to determine if the lack of pain-related functional changes in left CeLC neurons correlates with failure to activate PKA in these neurons and if CeLC neurons in both hemispheres are capable of PKA-mediated sensitization. Our previous studies identified PKA activation as a critical mechanism of pain-related sensitization and plasticity in the amygdala (Bird et al. 2005; Fu et al. 2008; Ji and Neugebauer 2008). The present electrophysiological study is the first to show hemispheric differences in the responsiveness of individual amygdala neurons to noxious stimuli in a pain model. The results further suggest that PKA activation is necessary and sufficient for increased responsiveness of CeLC neurons but does not occur in the left CeLC in the arthritis pain model.
METHODS
Adult male Sprague–Dawley rats (250–350 g) were housed in a temperature-controlled room and maintained on a 12-h day/night cycle. Water and food were available without restriction. All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch and conform to the guidelines of the International Association for the Study of Pain and of the National Institutes of Health.
Animal preparation and anesthesia
On the day of the electrophysiological experiment, the animal was anesthetized with pentobarbital sodium (50 mg/kg ip). A cannula was inserted into the trachea for artificial respiration and to measure end-tidal CO2 levels. A catheter was placed in the jugular vein for continuous administration of anesthetic and for fluid support (3–4 ml·kg–1·h–1 lactated Ringer solution, administered intravenously). Depth of anesthesia was assessed by testing the corneal blink, hindpaw withdrawal, and tail-pinch reflexes and by continuously monitoring the end-tidal CO2 levels (kept at 4.0 ± 0.2%), heart rate, electrocardiogram (ECG) and breathing patterns. Core body temperature was maintained at 37°C by means of a homeothermic blanket system. Animals were mounted in a stereotaxic frame, paralyzed with pancuronium (induction: 0.3–0.5 mg iv; maintenance: 0.3 mg/h iv) and artificially ventilated (3–3.5 ml; 55–65 stroke/min). Constant levels of anesthesia were maintained by continuous intravenous infusion of pentobarbital (15 mg·kg–1·h–1). A craniotomy was performed at the sutura frontoparietalis level for the recording of neurons in the latero-capsular division of the central nucleus of the amygdala (CeLC) and for the administration of drugs into the central nucleus. The dura mater was opened and reflected; the pia mater was removed over the recording and drug-administration sites to allow smooth insertion of the recording electrode and microdialysis probe, respectively.
Electrophysiological recording
As described previously (Han et al. 2005b; Ji and Neugebauer 2007; Li and Neugebauer 2004a,b, 2006; Neugebauer and Li 2003), long-term extracellular recordings were made from single neurons in the CeLC with glass-insulated carbon filament electrodes (4–6 MΩ) using the following stereotaxic coordinates (Paxinos and Watson 1998): 2.1–2.8 mm caudal to bregma; 3.8–4.5 mm lateral to midline; depth 7–9 mm. The recorded signals were amplified and displayed on analog and digital storage oscilloscopes. Signals were also fed into a window discriminator the output of which was processed by an interface (CED 1401 Plus) connected to a Pentium 4 PC. Spike2 software (CED, version 4) was used to create peristimulus rate histograms on-line and to store and analyze digital records of single-unit activity off-line.
Identification of amygdala neurons
An individual CeLC neuron was identified by its background activity and by its responses to brief mechanical stimuli applied to the ipsi- and contralateral knee with a calibrated forceps (see Mechanical stimuli). Spike size and configuration were continuously monitored on the storage oscilloscopes and with the use of Spike2 software. Spikes were detected and recorded by the waveform signal that crossed a trigger level and matched a preset shape or template that was created for the individual neuron at the beginning of the recording period. Included in this study were only those neurons the spike configuration of which remained constant (matching the template) and could be clearly discriminated from activity in the background throughout the experiment, indicating that the activity of one and the same one neuron was measured.
Receptive fields
Neurons were selected that had a receptive field in the knee. Size and thresholds of the receptive fields in deep tissue and skin were mapped using graded mechanical stimuli of innocuous and noxious intensities (see Mechanical stimuli). Cutaneous input was distinguished from deep tissue input by selective stimulation of skin folds gently raised from the underlying deep tissue. Mechanical stimuli were considered to activate deep tissue (joints and muscles) if the stimulation of overlying skin evoked no or a clearly distinct response. The focus of this study was on the processing of nociceptive information from the deep tissue. Standard diagrams of the rat body were used to record the location and size of the receptive field.
Mechanical stimuli
Mechanical stimuli were applied to the deep tissue by means of a forceps equipped with a force transducer the calibrated output of which was amplified, digitized, and recorded on a Pentium PC for on- and off-line analysis. Stimulus-response functions were generated using brief (15 s) graded mechanical test stimuli of increasing intensities (100, 500, 1,000, 1,500, and 2,000 g/30 mm2 at 15-s interstimulus intervals). Stimulus intensities of 100 and 500 g/30 mm2 applied to the knee and other deep tissue are considered innocuous because they do not evoke hind limb withdrawal reflexes in awake rats and are not felt to be painful when tested on the experimenters. An intensity of 1,000 g/30 mm2 represents a firm but nonpainful stimulus that does not evoke a hind limb withdrawal reflex. Pressure stimuli >1,500 g/30 mm2 are noxious because they evoke hind limb withdrawal reflexes and vocalizations in awake rats and are distinctly painful when applied to the experimenters (Han et al. 2005a,b; Han and Neugebauer 2005; Ji et al. 2007). Background activity before stimulation was subtracted from the total response during stimulation to calculate the net response evoked by a particular stimulus.
Classification and response thresholds
All neurons selected for this study were multireceptive (MR) neurons according to our classification of CeLC neurons with deep tissue input (Han et al. 2005b; Ji and Neugebauer 2007; Li and Neugebauer 2004a,b, 2006; Neugebauer and Li 2002, 2003) MR neurons represent the predominant type of neurons in the CeLC. This classification is primarily based on the responses to mechanical stimulation of the knee joint and other deep tissue. MR neurons respond consistently to innocuous stimuli (<500 g/30 mm2) but are more strongly activated by noxious stimuli (>1500 g/30 mm2). Mechanical threshold was defined as the minimum stimulus intensity that evoked an excitatory response (spike frequency higher than the upper 95% confidence interval of background activity).
Experimental protocol
In each experiment, one CeLC neuron was recorded in the left or right CeLC before and for several hours after arthritis induction in the ipsi- or contralateral knee joint (see Arthritis). Background activity, evoked responses, and receptive-field size were measured repeatedly before and after induction of arthritis and before and during drug administration into the CeLC (see Drugs). Background activity was recorded for >10 min to calculate means ± SE and 95% confidence intervals (CI; GraphPad Prism 3.0). Before arthritis induction and during the development of arthritis, mechanical test stimuli were applied to the knee and other deep tissue in the receptive field at regular intervals of ∼30 min. Before and during drug applications, intervals between the test stimuli were 5–10 min. Number of stimulations was kept at a minimum to avoid any “sensitization” that might be produced by repeated stimulation. Sufficiently long control periods were used to establish consistent baseline responses before arthritis induction and drug application. A paired paradigm was used to determine arthritis pain-related changes. Rather than comparing neuronal activity in arthritis with saline-injected control groups, each neuron served as its own control and was recorded continuously before and after arthritis induction in the same animal. A previous study found no difference between saline-injected and untreated normal rats on synaptic transmission and excitability in CeLC neurons (Neugebauer et al. 2003).
Arthritis
Arthritis was induced as described in detail previously (Neugebauer et al. 2007; Schaible and Schmidt 1990). A kaolin suspension (4%, 100 μl) was slowly injected into the joint cavity through the patellar ligament with the use of a syringe and needle (1 ml, 25 gauge, 5/8 in). After repetitive flexions and extensions of the knee for 15 min, a carrageenan solution (2%, 100 μl) was injected into the knee joint cavity, and the leg was flexed and extended for another 5 min. This treatment paradigm reliably leads to inflammation and swelling of the knee within 1–3 h and the inflammation persists for weeks (Neugebauer et al. 2007).
Drugs and drug administration by microdialysis
KT5720, a potent and selective membrane-permeable PKA inhibitor (Bird et al. 2005; Cabell and Audesirk 1993), and forskolin, a membrane-permeable activator of adenylyl cyclase (Awad et al. 1983; Laurenza et al. 1989), were purchased from Tocris Bioscience, Ellisville, MO. Drugs were administered into the CeLC by microdialysis at a rate of 5 μl/min for 15 min. Several hours before the start of the electrophysiological recordings a microdialysis probe (CMA11; CMA/Microdialysis; membrane diameter: 250 μm; membrane length: 1 mm) was positioned stereotaxically in the left or right CeLC, using the following coordinates: 1.6 mm caudal to bregma; 4.0 mm lateral to midline; depth of tip 9.0 mm (Han et al. 2005b; Ji and Neugebauer 2007; Li and Neugebauer 2004a,b, 2006). Using PE-50 tubing, the microdialysis probe was connected to an infusion pump (Harvard) and perfused with artificial cerebrospinal fluid (ACSF) containing (in mM) 125.0 NaCl, 2.6 KCl, 2.5 NaH2PO4, 1.3 CaCl2, 0.9 MgCl2, 21.0 NaHCO3, and 3.5 glucose; oxygenated and equilibrated to pH = 7.4. Before the recordings, ACSF was pumped through the fiber for >1 h to establish equilibrium in the tissue. ACSF was present throughout the experiment and also served as a vehicle control.
KT5720 and forskolin were dissolved in ACSF on the day of the experiment at a concentration of 100 times that predicted to be needed based on data from our previous studies (Bird et al. 2005; Fu et al. 2008; Han et al. 2005b; Ji and Neugebauer 2008). Drug concentration in the tissue is ≥100 times lower than in the microdialysis probe as a result of the concentration gradient across the dialysis membrane and diffusion in the tissue (Fu et al. 2008; Ji and Neugebauer 2008). The numbers given in this article refer to the drug concentrations in the microdialysis fiber.
Histology
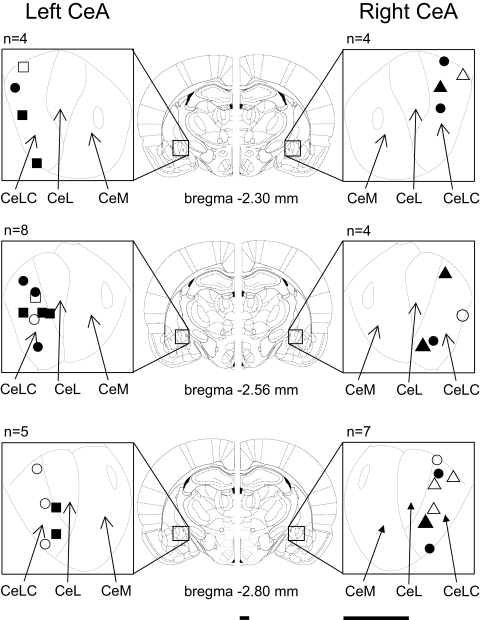
At the end of each experiment, the recording site in the CeLC was marked by injecting DC (250 μA for 3 min) through the carbon filament recording electrode. The brain was removed and submerged in 10% formalin and potassium ferrocyanide. Tissues were stored in 20% sucrose before they were frozen-sectioned at 50 μm. Sections were stained with Neutral Red, mounted on gel-coated slides, and cover-slipped. The boundaries of the different amygdala nuclei were easily identified under the microscope. Lesion/recording sites were verified histologically and plotted on standard diagrams adapted from Paxinos and Watson (1998) (see Fig. 1). The positions of the microdialysis probes in the CeLC were also verified histologically (not shown; they were virtually identical and the same stereotaxic coordinates were used in every experiment).
Fig. 1.
Histologically verified recording sites of 32 neurons in the laterocapsular division of the central nucleus of the amygdala (CeLC) in the left and right brain hemisphere. ■, ●, ▴, the locations of neurons that were recorded before and after arthritis induction; □, ○, ▵, neurons that were recorded only under normal conditions. Symbols also differentiate between the different types of receptive fields (see Fig. 2): ■, □, contralateral hindlimb only; ●, ○, bilateral hindlimbs; ▴, ▵, whole body. Diagrams (adapted from Paxinos and Watson 1998) show coronal brain sections at different levels posterior to bregma (−2.30 to −2.80). Next to each section is shown in detail the central nucleus and its medial (CeM), lateral (CeL), and latero-capsular (CeLC) subdivisions. Calibration bars are 1 mm.
Data analysis
Extracellularly recorded single-unit action potentials were analyzed off-line from peristimulus rate histograms using Spike2 software (CED, version 4). Responses to mechanical stimuli were measured and expressed as spikes per second (Hz). Background activity was subtracted from the total activity during the stimulus to obtain the “net” stimulus-evoked activity. A two-way ANOVA with Tukey posttests (SigmaStat 3.1) was used to evaluate statistically the effect of lateralization (left vs. right amygdala) and treatment (arthritis vs. normal) on neuronal activity (data in Fig. 3). A paired t-test was used to compare in four different experimental paradigms (left CeLC/right knee; left CeLC/left knee; right CeLC/left knee; left CeLC/right knee; data in Fig. 4) each neuron's activity in arthritis with prearthritis normal control values (paired experimental paradigm; Prism 3.0, GraphPad Software). A paired t-test was also used to determine significant differences of neuronal activity before and during drug administration (Prism 3.0, GraphPad Software). Statistical analysis was performed on raw data (firing rate measured as spikes per second). Statistical significance was accepted at the level P < 0.05.
Fig. 3.
Right-hemispheric lateralization of arthritis pain-related changes. A: unchanged background and evoked activity of 1 left CeLC neuron in arthritis. B: increased background and evoked activity of 1 right CeLC neuron after arthritis induction. A and B: line graphs show the time course of extracellularly recorded responses (number of spikes per second) to brief (15 s) innocuous (500 g/30 mm2) and noxious (2,000 g/30 mm2) stimulation of the knee and background activity. Symbols show the mean activity during a 15-s period before stimulation (= background activity) or the difference between mean activity during and before 15-s stimuli (= net activity evoked by noxious or innocuous stimuli; see methods). Peristimulus time histograms (insets) show individual responses (spikes per second) before and 5 h after induction of arthritis. Top traces show recordings of the force (g/30 mm2) applied to the knee joint with a calibrated forceps (see methods). C: comparison of left vs. right CeLC neurons. Under normal conditions before arthritis, there was no significant difference of background and evoked activity between left (n = 11) and right (n = 9) CeLC neurons (P > 0.05, Tukey test). Five hours after arthritis induction, activity of right CeLC neurons was significantly higher than that of left CeLC neurons (P < 0.01–0.05, Tukey test). Bar histograms show background activity and responses to innocuous (500 g/30 mm2) and noxious (2,000 g/30 mm2) stimulation of the knee averaged for the sample of neurons (means ± SE). Single asterisk, P < 0.05; double asterisks, P < 0.01 (Tukey test, comparing values in right vs. left CeLC neurons).
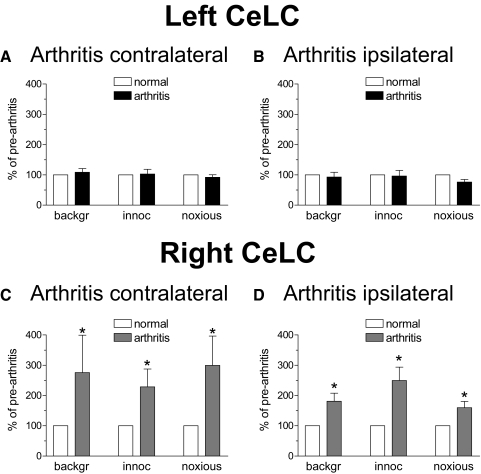
Fig. 4.
Pain-related lateralization is independent of the side of arthritis. A and B: there was no significant change of background and evoked activity of left CeLC neurons after induction of arthritis in the right (contralateral, A; n = 5) or left (ipsilateral, B; n = 6) knee. C and D: background and evoked activity of CeLC neurons in the right hemisphere increased 5 h after arthritis was induced in the left (contralateral, C; n = 5) or right (ipsilateral, D; n = 4) knee. Bar histograms show background activity and responses to brief (15 s) innocuous (500 g/30 mm2) and noxious (2,000 g/30 mm2) stimulation of the knee expressed as percent of prearthritis values (set to 100%). Data were averaged for the sample of neurons (means ± SE). *, P < 0.05 (paired t-test comparing values in arthritis with prearthritis values under normal conditions; statistical analysis was performed on raw data).
For quantification of the receptive field size, the body map was divided into 21 different areas. A score of 1 was assigned to each area that was part of the total receptive field. Addition of the scores yielded a value for the total receptive field of a neuron. Averaged values for left versus right amygdala neurons were compared using a Mann-Whitney U test (unpaired experimental paradigm; Prism 3.0, GraphPad Software). Averaged values for normal versus arthritis state were compared using a Wilcoxon signed-rank test (paired experimental paradigm; Prism 3.0, GraphPad Software). For multiple comparisons in nonparametric tests, the alpha level was adjusted and statistical significance accepted at the level P < 0.025.
RESULTS
Extracellular single-unit recordings were made from 17 neurons in the left and 15 neurons in the right CeLC of anesthetized adult male rats (Fig. 1). Only one neuron was recorded in each rat. Neurons were selected that had a receptive field in the knee joint as in our previous studies. Neurons were multireceptive (MR, see Classification) and responded more strongly to brief noxious than innocuous stimuli applied to the knee and other parts of the receptive field. In this study, we included only MR neurons because our previous studies showed that they consistently and reliably become sensitized in the arthritis pain model (Han et al. 2005b; Ji and Neugebauer 2007; Li and Neugebauer 2004a,b, 2006; Neugebauer and Li 2003) and are believed to integrate nociceptive and affective information (Neugebauer 2006; Neugebauer et al. 2004). Continuous recordings before and after arthritis induction were made from 11 neurons in the left and 9 neurons in the right CeLC. The remaining neurons (6 in the left and 6 in the right CeLC) were only recorded under normal conditions to determine the effects of forskolin alone and in the presence of a PKA inhibitor (KT5720).
Properties of left and right CeLC neurons under normal conditions
RECEPTIVE FIELD SIZE.
All neurons were activated by mechanical stimulation (compression) of the knee joint, which served as the search stimulus. The receptive field of neurons in the left CeLC (n = 17) was smaller than that of neurons in the right CeLC (n = 15; see Fig. 2). Receptive fields of left CeLC neurons were either confined to the contralateral hindlimb (n = 9) or included an additional high-threshold receptive field in the ipsilateral hindlimb (n = 8; Fig. 2B, normal). In contrast, receptive fields of right CeLC neurons were always (n = 15) bilateral and symmetrical in the deep tissue of the hindlimbs and tail; some of these neurons (n = 8) had additional receptive fields in the forepaws and trunk (Fig. 2A, normal).
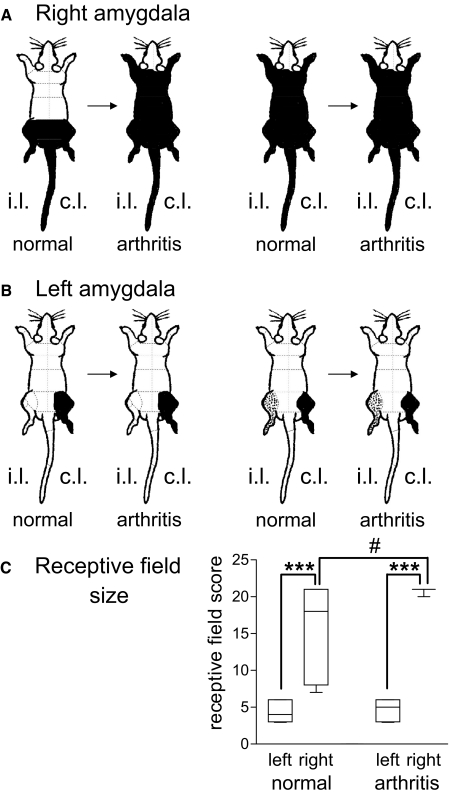
Fig. 2.
Receptive fields of neurons in the right (A) and left (B) CeLC. Receptive fields in the deep tissue are shown before (normal) and 5 h postinduction of arthritis. Changes of receptive field size were observed in right, not left, CeLC neurons. A and B: all neurons showed increasing responses to graded innocuous and noxious stimulation of areas colored black (= multireceptive neurons, see methods).  , high-threshold receptive fields, stimulation of which activated the neuron weakly. i.l., ipsilateral; c.l., contralateral to recording site. A: under normal conditions, receptive fields of right CeLC neurons (n = 15) were symmetrical in the deep tissue of both hindlimbs and the tail (n = 7, left) or covered the whole body (n = 8, right). B: receptive fields of left CeLC neurons (n = 17) were either confined to the contralateral hindlimb (n = 9) or included an additional high-threshold receptive field in the ipsilateral hindlimb (n = 8). C: semi-quantitative analysis of the receptive field size in neurons that were recorded continuously before and after arthritis induction. The body map was divided into 21 areas (A and B, - - -). The total number of areas that contained part of the receptive field was calculated for each neuron and averaged for left (n = 11) and right (n = 9) CeLC neurons (see Data analysis). Only neurons that were recorded before and after arthritis induction are included in the analysis. In the graph, each box extends from the 25th to the 75th percentile, with a line at the median (50th percentile). The whiskers extend above and below the box to show the highest and lowest values. ***, P < 0.0005 (receptive field size of right compared with left CeLC neurons; Mann-Whitney U test), #, P < 0.025 (receptive field size after arthritis compared with normal; Wilcoxon signed-rank test), alpha level adjusted for multiple comparisons.
, high-threshold receptive fields, stimulation of which activated the neuron weakly. i.l., ipsilateral; c.l., contralateral to recording site. A: under normal conditions, receptive fields of right CeLC neurons (n = 15) were symmetrical in the deep tissue of both hindlimbs and the tail (n = 7, left) or covered the whole body (n = 8, right). B: receptive fields of left CeLC neurons (n = 17) were either confined to the contralateral hindlimb (n = 9) or included an additional high-threshold receptive field in the ipsilateral hindlimb (n = 8). C: semi-quantitative analysis of the receptive field size in neurons that were recorded continuously before and after arthritis induction. The body map was divided into 21 areas (A and B, - - -). The total number of areas that contained part of the receptive field was calculated for each neuron and averaged for left (n = 11) and right (n = 9) CeLC neurons (see Data analysis). Only neurons that were recorded before and after arthritis induction are included in the analysis. In the graph, each box extends from the 25th to the 75th percentile, with a line at the median (50th percentile). The whiskers extend above and below the box to show the highest and lowest values. ***, P < 0.0005 (receptive field size of right compared with left CeLC neurons; Mann-Whitney U test), #, P < 0.025 (receptive field size after arthritis compared with normal; Wilcoxon signed-rank test), alpha level adjusted for multiple comparisons.
In an attempt to quantify the differences, we divided the body map into 21 different sectors (see Fig. 2). The total number of areas that contained part of the receptive field of a neuron was calculated and averaged for neurons in the left and for those in the right CeLC. The comparison (Fig. 2C) showed that the average receptive field size of right CeLC neurons was significantly larger than that of left CeLC neurons under normal conditions (P < 0.0005, Mann-Whitney U test).
BACKGROUND ACTIVITY AND EVOKED RESPONSES.
No evidence of lateralization was found for background activity and responses to innocuous and noxious stimuli under normal conditions. Figure 3 shows the background activity and evoked responses of individual CeLC neurons in the left (A) and right (B) CeLC before arthritis induction (and changes after arthritis; see Properties of left and right CeLC neurons in the arthritis pain model). The averaged values for the samples of left (n = 11) and right (n = 9) CeLC neurons under normal conditions are shown in Fig. 3C. The analysis only includes neurons that were recorded before and after arthritis induction to allow the direct comparison. Statistical analysis revealed no significant differences between left and right CeLC neurons under normal conditions (P > 0.05, Tukey test).
Properties of left and right CeLC neurons in the arthritis pain model
RECEPTIVE FIELD SIZE.
After the induction of a knee joint arthritis (see methods) the size of the receptive field of neurons in the right CeLC expanded (Fig. 2A). This change was observed in the majority of right CeLC neurons (6 of 9 neurons); the receptive fields of the remaining right CeLC neurons covered the whole body before arthritis and no apparent increase was detected. In contrast, the receptive field size of neurons in the left CeLC did not change (Fig. 2B). The quantitative analysis of the receptive field size on the body map (Fig. 2C) also revealed significant increases for right (P < 0.025) but not left (P > 0.05) CeLC neurons in the arthritis pain model (Wilcoxon signed-rank test). The receptive field size of right CeLC neurons was significantly greater than that of left CeLC neurons (P < 0.0005, Mann-Whitney U test). The analysis only included neurons that were recorded before and after arthritis induction to allow the direct comparison of the receptive field size.
BACKGROUND ACTIVITY AND EVOKED RESPONSES.
Activity of right but not left CeLC neurons increased in the arthritis pain model. Figure 3B shows an individual example of a neuron in the right CeLC. In agreement with our previous studies (Han et al. 2005b; Ji and Neugebauer 2007; Li and Neugebauer 2004a,b, 2006; Neugebauer and Li 2003), background activity and responses to innocuous and noxious stimulation of the knee (see Mechanical stimuli) increased after arthritis induction and reached a plateau at 4–5 h. In contrast, background activity and evoked responses of a neuron in the left CeLC did not change for several hours after arthritis induction (Fig. 3A). In both cases, arthritis was induced in the knee contralateral to the recording site because our previous studies showed sensitization of right CeLC neurons when arthritis was induced in the contralateral (left) knee (Han et al. 2005b; Ji and Neugebauer 2007; Li and Neugebauer 2004a,b, 2006; Neugebauer and Li 2003). Both neurons were recorded continuously before and after arthritis induction.
Figure 3C shows significant right-hemispheric lateralization of arthritis pain-related changes. The activity of left (n = 11) versus right (n = 9) CeLC neurons are compared under normal conditions and in the arthritis pain model. Two-way ANOVA revealed significant main effects of lateralization on background activity [P < 0.05, F(1,36) = 5.06] and on responses to innocuous [P < 0.01, F(1,36) = 8.28] and noxious [P < 0.05, F(1,36) = 5.76] stimuli. Tukey posttests showed significant differences between left and right CeLC neurons in the arthritis pain model (background, P < 0.05; innocuous, P < 0.01; noxious, P < 0.01) but not under normal conditions (P > 0.05). Two-way ANOVA also revealed significant main effects of treatment on the responses to innocuous [P < 0.05, F(1,36) = 4.24] and noxious ([P < 0.05, F(1,36) = 4.15] stimuli but not on background activity [P > 0.05, F(1,36) = 2.97].
Importantly, the differential effects of arthritis pain on right and left CeLC neurons were independent of the side of the monoarthritis. Significant (P < 0.05, paired t-test performed on raw data) changes of background and evoked activity were observed in right CeLC neurons after arthritis was induced in the contralateral (left) knee (n = 5 neurons; Fig. 4C) or ipsilateral (right) knee (n = 4 neurons; D). Left CeLC neurons showed no changes after arthritis was induced either in the right knee (n = 5; A) or left knee (n = 6; B). These results suggest that pain-related lateralization is independent of the side of arthritis.
Effects of a PKa inhibitor
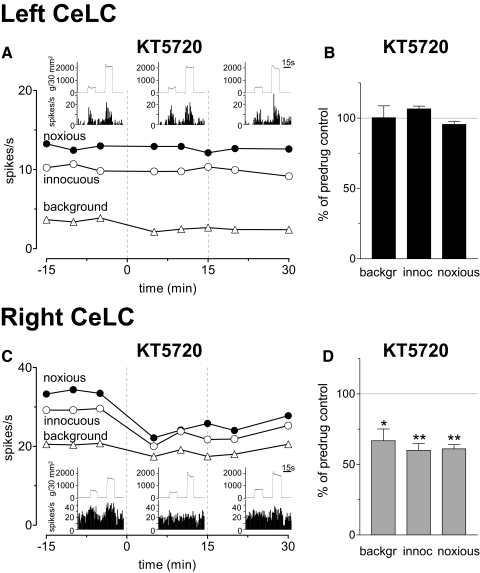
Our previous studies showed an important contribution of PKA to central sensitization and synaptic plasticity in the right CeLC (Bird et al. 2005; Fu et al. 2008). Here we addressed the hypothesis that lack of PKA activation in the left CeLC contributes to pain-related right-hemispheric lateralization. A selective PKA inhibitor (KT5720, KT; 100 μM, concentration in the microdialysis fiber; 15 min; see Drugs and drug administration by microdialysis) was administered into the CeLC 5–6 h postinduction of arthritis. The positions of the microdialysis probes in the CeLC were verified histologically. Administration of KT5720 into the left CeLC had no effect on CeLC neurons in the left hemisphere. An individual example is shown in Fig. 5 A. Figure 5B summarizes the lack of effect of KT5720 on background and evoked activity in the sample of left CeLC neurons (n = 5) after arthritis induction in the contralateral knee. Administration of KT5720 into the right CeLC significantly (P < 0.05, paired t-test) inhibited the increased activity of neurons in the right CeLC (n = 5; see individual example in Fig. 5C and summary of data in D). The data suggest that PKA is not activated endogenously in the left CeLC in the arthritis pain model.
Fig. 5.
Lateralized effects of a protein kinase A (PKA) inhibitor in the arthritis pain model. A: administration of KT5720 (100 μM, concentration in the microdialysis probe; 15 min) into the left CeLC had no effect on background and evoked activity of a neuron in the left CeLC. B: normalized data summarize the lack of effects of KT5720 on background activity and evoked responses of left CeLC neurons (n = 5) 5–6 h postinduction of arthritis in the contralateral (right) knee. C: KT5720 (100 μM) administered into the right CeLC inhibited the increased background activity and evoked responses of a neuron in the right CeLC. D: normalized data summarize the significant inhibition of background activity and evoked responses right CeLC neurons (n = 5) 5–6 h postinduction of arthritis in the contralateral (left) knee. A and C: symbols show background activity (mean activity during a 15-s period before stimulation) and responses to innocuous (500 g/30 mm2) and noxious (2,000 g/30 mm2) stimuli before [in arificial cerebrospinal fluid (ACSF)], during and after KT5720 administration (see - - -). Evoked responses are calculated as the difference between mean activity during and before 15-s stimuli (see methods). Insets: individual responses (spike/s, peristimulus-time histograms, bin width: 1 s) and recordings of the force (g/30 mm2, top traces) applied to the knee joint with a calibrated forceps (see methods) before, during, and after administration of KT5720 into the CeLC. B and D: bar histograms show averaged values (means ± SE) during drug administration normalized to predrug control values (in ACSF, set to 100% as indicated, - - -). *, P < 0.05; **, P < 0.01 (paired t-test).
Effects of forskolin
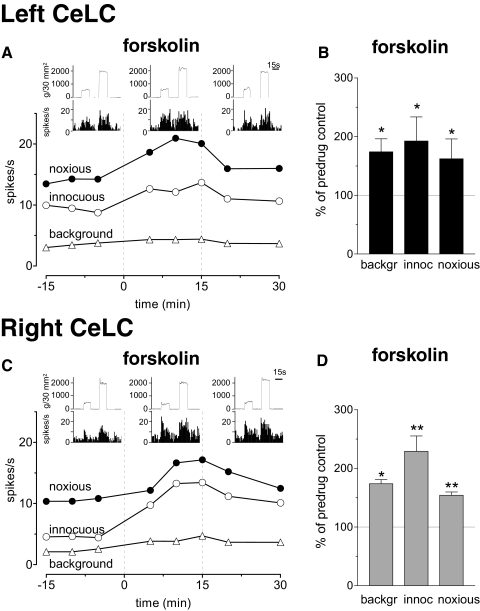
Next we sought to determine if the exogenous activation of signal transduction pathways could sensitize neurons in the left CeLC. A widely used cell-permeable activator of adenylyl cyclase (forskolin, 1 mM, concentration in microdialysis fiber; 15 min) was administered into the left or right CeLC under normal conditions (no arthritis). The positions of the microdialysis probes in the CeLC were verified histologically. Forskolin increased background activity and evoked responses of left and right CeLC neurons. The receptive field size did not change. Figure 6 shows individual neurons in the left (A) and right (C) CeLC and summarizes the significant effects of forskolin on the sample of neurons in the left (n = 4, B) and right (n = 4, D) CeLC. In the presence of a PKA inhibitor (KT5720, 100 μM, concentration in the microdialysis fiber; 15 min), forskolin had no significant effect (P > 0.05, compared with predrug control values; paired t-test; n = 4 neurons; Supplementary Fig. S11). The data demonstrate that in principle the activity of left CeLC neurons can be modulated (increased) like that of right CeLC neurons, suggesting that PKA activation is sufficient for increased responsiveness of CeLC neurons but does not occur in the left CeLC in the arthritis pain model.
Fig. 6.
Forskolin effects are not lateralized. A: administration of forskolin (1 mM, concentration in the microdialysis probe; 15 min) into the left CeLC increased background activity and evoked responses of a neuron in the left CeLC. B: normalized data summarize the significant effects of forskolin in the sample of left CeLC neurons (n = 4). C: forskolin (1 mM) administered into the right CeLC had similar facilitatory effects on a right CeLC neuron. D: normalized data summarize the significant facilitatory effects of forskolin in the right CeLC (n = 4). A and C: symbols show background and evoked net activity before, during, and after forskolin administration (same display as in Fig. 5, A and C). B and D: bar histograms show averaged values (means ± SE) during drug administration normalized to predrug control values (same display as in Fig. 5, B and D). Recordings were made under normal conditions (no arthritis). *, P < 0.05; **, P < 0.01 (paired t-test).
DISCUSSION
The key findings of this study are as follows. Unlike CeLC neurons in the right amygdala, neurons in the left CeLC do not develop increased responsiveness in a rodent model of arthritis pain. This hemispheric lateralization is independent of the side of the peripheral injury (ipsi- or contralateral to the recording site). No significant difference was found in the magnitude of the responses of left and right CeLC neurons to brief physiological noxious stimuli under normal conditions, indicating that individual inputs have comparable effects on neurons in the left and right CeLC. The smaller receptive field size of left compared with right CeLC neurons may suggest the tonic control of effective inputs. The contribution of PKA in the right but not left amygdala to pain-related changes is in agreement with the findings of another group (Carrasquillo and Gereau 2007, 2008) that pain-related lateralization involves differences in the endogenous activation of signaling pathways in the CeLC. Importantly, the exogenous activation of intracellular effectors in the left or right CeLC by forskolin produced activity changes that resembled those observed in the arthritis pain model. The results suggest that PKA activation is necessary and sufficient for increased responsiveness of CeLC neurons but does not occur in the left CeLC in the arthritis pain model.
The results shows that nociceptive information reaches both left and right amygdala (CeLC), but it is the right CeLC that plays a major role in the processing of prolonged nociceptive inputs and develops sensitization. Neurons in the left CeLC are capable of activity changes when intracellular signing pathways are stimulated directly (forskolin experiment), but an unknown mechanism prevents their activation in the arthritis pain model. This result is novel and significant because there has been little evidence for hemispheric lateralization of brain functions related to pain (see introduction). Two recent biochemical and behavioral studies (Carrasquillo and Gereau 2007, 2008) were the first to show lateralization of amygdala function in pain. Activation of the MAP kinase ERK was observed in the right, but not left, CeLC in the formalin pain model. Conversely, blockade of ERK activation in the right, but not the left, CeLC inhibited pain behavior. Results from the present study suggest that hemispheric lateralization is not restricted to the function of a single molecule (ERK) but also involves PKA and possibly other effectors. Failure to activate these signaling pathways appears to prevent the left CeLC from developing pain-related activity increases, therefore contributing to right-hemispheric lateralization.
A consequence of pain-related changes in the right, but not left CeLC, would be “asymmetric” output from the amygdala to target structures such as the periaqueductal gray (PAG) (Heinricher and McGaraughty 1999; Neugebauer et al. 2004; Rizvi et al. 1991; Shipley et al. 1991; Tracey and Mantyh 2007). The PAG is an important brain stem center for the descending modulation of pain and other behaviors (Heinricher and McGaraughty 1999; Mason 2005; Tracey and Mantyh 2007). Importantly, increased neural transmission in this largely ipsilateral output pathway during stress-induced anxiety was observed in the right but not left hemisphere (Adamec et al. 2005a,b). Consistent with this finding, pain behavior was modified by manipulating ERK activation in the right but not left CeLC (Carrasquillo and Gereau 2007, 2008).
Relatively few studies have specifically addressed or described lateralization of amygdala function in rodents. Predominant activation or involvement of the right amygdala was found in aversively motivated learning and memory (Coleman-Mesches and McGaugh 1995a,b; Coleman-Mesches et al. 1996; Lalumiere and McGaugh 2005) and contextual fear conditioning (Baker and Kim 2004) in rats. In humans, right hemispheric lateralization of amygdala function was associated with negative emotions (Angrilli et al. 1996; Canli et al. 1998; Funayama et al. 2001; Lee et al. 2004; Yoshimura et al. 2008), fear extinction (LaBar et al. 1998), subconscious emotional learning (Morris et al. 1998), rapid automatic stimulus detection and response (Costafreda et al. 2008; Sergerie et al. 2008), and pain (Lu et al. 2004). Positive emotions tend to be lateralized to the left amygdala in humans (Canli et al. 1998; Lee et al. 2004; Yoshimura et al. 2008). However, greater right than left amygdala activation was reported for viewing happy faces and greater left amygdala activation for fearful faces (Hardee et al. 2008; Killgore and Yurgelun-Todd 2001). The left rather than right amygdala has been implicated in perceived or anticipated but not actually experienced fear (Funayama et al. 2001; Phelps et al. 2001). Sex-related hemispheric differences of amygdala activation include the preferential involvement of the right amygdala in emotional responses and emotional memory in men and of the left amygdala in women (see Cahill 2006 for review). Sex-related differences, however, may be valence dependent because they were observed for happy but not fearful faces (Killgore and Yurgelun-Todd 2001). These data strongly support the concept of hemispheric lateralization of amygdala function in emotions, but the underlying principle and mechanisms remain to be determined.
Pain-related right hemispherical lateralization of amygdala function is consistent with the predominant activation or involvement of the right amygdala in emotions. One study suggested that left sided pain, experimental or chronic, produced greater “emotional disturbance” and anxiety (Schiff and Gagliese 1994). Pain has a negative emotional-affective component and is closely related to anxiety and depression (Gallagher and Verma 2004; Grachev et al. 2001; Rhudy and Meagher 2003; Tracey and Mantyh 2007), but the role of hemispheric lateralization in this relationship remains to be determined.
Possible mechanisms of pain-related lateralization of amygdala function include differences in nociceptive inputs, neuronal properties, and control by other brain areas. The amygdala receives nociceptive information through anatomically and functionally distinct lines of input (Braz et al. 2005; Neugebauer 2006; Neugebauer et al. 2004). Purely nociceptive information reaches the CeLC directly from the spinal cord and brain stem (parabrachial area), thus bypassing the thalamus (Bernard and Besson 1990; Cliffer et al. 1991; Gauriau and Bernard 2004). Polymodal sensory, including nociceptive, inputs from thalamus (posterior areas) and cortex (insula and association cortices) reach primarily the lateral amygdala (Pare et al. 2004; Phelps and Ledoux 2005; Shi and Davis 1999). Associative processing in the lateral-basolateral amygdala network generates affect-related information that is transmitted to the central nucleus, a major output nucleus for amygdala functions (Maren 2005; Pare et al. 2004; Phelps and Ledoux 2005). The present study shows that CeLC neurons on the left and right respond to brief innocuous and noxious stimuli with similar magnitude under normal conditions, arguing against a major difference in nociceptive and nonnociceptive inputs to the CeLC.
Differences in the neuronal populations of the left and right CeLC are unlikely to account for lateralized amygdala functions. The two principal types of CeLC neurons are nociceptive-specific (NS) neurons, which receive exclusively nociceptive input, and multireceptive (MR) neurons, which respond to innocuous and noxious stimuli and integrate nociceptive signals with affective information from the lateral-basolateral circuitry (Neugebauer 2006; Neugebauer et al. 2004). MR neurons, but not NS neurons, undergo central sensitization in the arthritis pain model (Neugebauer and Li 2003). The present study did not systematically analyze the proportion of NS and MR neurons, but MR neurons were identified in the left CeLC and did not show the pain-related changes of MR neurons in the right CeLC.
Biochemical and pharmacological data suggest that lateralized amygdala function in pain involves differences in the activation of cellular signaling pathways (ERK, Carrasquillo and Gereau 2007, 2008; PKA, present study). The effects of forskolin in the present study show that effector systems can be activated in left CeLC neurons and produce increased neuronal activity. These data suggest that a yet unknown mechanism prevents the pain-related endogenous activation of signaling mechanisms and sensitization of left CeLC neurons. The smaller receptive field sizes of neurons in the left compared with the right CeLC also argues for the presence of a control mechanism of effective inputs and cellular effectors. Expansion of the receptive fields of CNS neurons is well documented in the arthritis pain model and is generally taken as evidence for central sensitization that renders normally ineffective inputs functional (Neugebauer and Li 2003; Neugebauer and Schaible 1990).
The difference in receptive field size between left and right CeLC neurons and the lack of pain-related changes in left CeLC neurons may suggest a tonic inhibitory mechanism, which could involve the well-known cortical control of amygdala functions (see Banks et al. 2007). The amygdala is reciprocally connected with prefrontal cortical areas (see Ghashghaei et al. 2007) that exert a top-down inhibitory influence on the amygdala (Carmichael and Price 1995; McDonald et al. 1996; Quirk and Beer 2006; Rosenkranz and Grace 2002). Inverse coupling of prefrontal cortex and amygdala was observed in imaging studies in humans in association with aversive and other emotional stimuli, possibly representing a neural system for cognitive regulation of emotions (Kim et al. 2003; Ochsner et al. 2002; Urry et al. 2006; but see Banks et al. 2007). Pertinent to the current study, greater left than right prefrontal cortical electroencephalographic (EEG) activity predicted an attenuated physiological response to aversive stimuli (Jackson et al. 2003).
In conclusion, the present study demonstrates differences in the processing of nociceptive information by neurons in the left versus right amygdala in a model of arthritic pain. Pain-related sensitization of left CeLC neurons is prevented by a mechanism that remains to be determined but may involve prefrontal cortical inhibition of the amygdala. Right hemispheric lateralization of pain processing in the amygdala is consistent with a predominant role of the right amygdala in negative emotions.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grants NS-38261 and NS-11255.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. William D. Willis Jr., for critical reading of and helpful comments on this manuscript. We also thank Dr. N. Bradley Keele for valuable assistance with the statistics and suggestions on the manuscript.
Footnotes
1The online version of this article contains supplemental data.
REFERENCES
- Adamec R, Blundell J, Burton P. Role of NMDA receptors in the lateralized potentiation of amygdala afferent and efferent neural transmission produced by predator stress. Physiol Behav 86: 75–91, 2005a [DOI] [PubMed] [Google Scholar]
- Adamec RE, Blundell J, Burton P. Neural circuit changes mediating lasting brain and behavioral response to predator stress. Neurosci Biobehav Rev 29: 1225–1241, 2005b [DOI] [PubMed] [Google Scholar]
- Adolphs R. Neural systems for recognizing emotion. Curr Opin Neurobiol 12: 169–177, 2002 [DOI] [PubMed] [Google Scholar]
- Angrilli A, Mauri A, Palomba D, Flor H, Birbaumer N, Sartori G, di PF. Startle reflex and emotion modulation impairment after a right amygdala lesion. Brain 119: 1991–2000, 1996 [DOI] [PubMed] [Google Scholar]
- Atchley RA, Ilardi SS, Enloe A. Hemispheric asymmetry in the processing of emotional content in word meanings: the effect of current and past depression. Brain Lang 84: 105–119, 2003 [DOI] [PubMed] [Google Scholar]
- Awad JA, Johnson RA, Jakobs KH, Schultz G. Interactions of forskolin and adenylate cyclase. Effects on substrate kinetics and protection against inactivation by heat and N-ethylmaleimide. J Biol Chem 258: 2960–2965, 1983 [PubMed] [Google Scholar]
- Baker KB, Kim JJ. Amygdalar lateralization in fear conditioning: evidence for greater involvement of the right amygdala. Behav Neurosci 118: 15–23, 2004 [DOI] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci 2: 303–312, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard J-F, Besson JM. The spino(trigemino)pontoamygdaloid pathway: electrophysiological evidence for an involvement in pain processes. J Neurophysiol 63: 473–490, 1990 [DOI] [PubMed] [Google Scholar]
- Bird GC, Lash LL, Han JS, Zou X, Willis WD, Neugebauer V. Protein kinase A-dependent enhanced NMDA receptor function in pain-related synaptic plasticity in rat amygdala neurons. J Physiol 564: 907–921, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braz JM, Nassar MA, Wood JN, Basbaum AI. Parallel “pain” pathways arise from subpopulations of primary afferent nociceptor. Neuron 47: 787–793, 2005 [DOI] [PubMed] [Google Scholar]
- Cabell L, Audesirk G. Effects of selective inhibition of protein kinase C, cyclic AMP- dependent protein kinase, and Ca(2+)-calmodulin-dependent protein kinase on neurite development in cultured rat hippocampal neurons. Int J Dev Neurosci 11: 357–368, 1993 [DOI] [PubMed] [Google Scholar]
- Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci 7: 477–484, 2006 [DOI] [PubMed] [Google Scholar]
- Canli T, Desmond JE, Zhao Z, Glover G, Gabrieli JD. Hemispheric asymmetry for emotional stimuli detected with fMRI. Neuroreport 9: 3233–3239, 1998 [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol 363: 615–641, 1995 [DOI] [PubMed] [Google Scholar]
- Carrasquillo Y, Gereau RW. Activation of the extracellular signal-regulated kinase in the amygdala modulates pain perception. J Neurosci 27: 1543–1551, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasquillo Y, Gereau RW. Hemispheric lateralization of a molecular signal for pain modulation in the amygdala. Mol Pain 4: 24, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliffer KD, Burstein R, Giesler GJ., Jr Distributions of spinothalamic, spinohypothalamic, and spinotelencephalic fibers revealed by anterograde transport of PHA-L in rats. J Neurosci 11: 852–868, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghill RC, Gilron I, Iadarola MJ. Hemispheric lateralization of somatosensory processing. J Neurophysiol 85: 2602–2612, 2001 [DOI] [PubMed] [Google Scholar]
- Coleman-Mesches K, McGaugh JL. Differential effects of pretraining inactivation of the right or left amygdala on retention of inhibitory avoidance training. Behav Neurosci 109: 642–647, 1995a [DOI] [PubMed] [Google Scholar]
- Coleman-Mesches K, McGaugh JL. Differential involvement of the right and left amygdalae in expression of memory for aversively motivated training. Brain Res 670: 75–81, 1995b [DOI] [PubMed] [Google Scholar]
- Coleman-Mesches K, Salinas JA, McGaugh JL. Unilateral amygdala inactivation after training attenuates memory for reduced reward. Behav Brain Res 77: 175–180, 1996 [DOI] [PubMed] [Google Scholar]
- Costafreda SG, Brammer MJ, David AS, Fu CH. Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Res Rev 58: 57–70, 2008 [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiatry 51: 68–80, 2002 [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Shackman AJ, Maxwell JS. Asymmetries in face and brain related to emotion. Trends Cogn Sci 8: 389–391, 2004 [DOI] [PubMed] [Google Scholar]
- Demaree HA, Everhart DE, Youngstrom EA, Harrison DW. Brain lateralization of emotional processing: historical roots and a future incorporating “dominance.” Behav Cogn Neurosci Rev 4: 3–20, 2005 [DOI] [PubMed] [Google Scholar]
- Fields H. State-dependent opioid control of pain. Nat Rev Neurosci 5: 565–575, 2004 [DOI] [PubMed] [Google Scholar]
- Fu Y, Han J, Ishola T, Scerbo M, Adwanikar H, Ramsey C, Neugebauer V. PKA and ERK, but not PKC, in the amygdala contribute to pain-related synaptic plasticity and behavior. Mol Pain 4: 26, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Neugebauer V. Differential mechanisms of CRF1 and CRF2 receptor functions in the amygdala in pain-related synaptic facilitation and behavior. J Neurosci 28: 3861–3876, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funayama ES, Grillon C, Davis M, Phelps EA. A double dissociation in the affective modulation of startle in humans: effects of unilateral temporal lobectomy. J Cogn Neurosci 13: 721–729, 2001 [DOI] [PubMed] [Google Scholar]
- Gallagher RM, Verma S. Mood and anxiety disorders in chronic pain. Prog Pain Res Management 27: 139–178, 2004 [Google Scholar]
- Gauriau C, Bernard J-F. A comparative reappraisal of projections from the superficial laminae of the dorsal horn in the rat: the forebrain. J Comp Neurol 468: 24–56, 2004 [DOI] [PubMed] [Google Scholar]
- Geha PY, Baliki MN, Harden RN, Bauer WR, Parrish TB, Apkarian AV. The brain in chronic CRPS pain: abnormal gray-white matter interactions in emotional and autonomic regions. Neuron 60: 570–581, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage 34: 905–923, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grachev ID, Fredickson BE, Apkarian AV. Dissociating anxiety from pain: mapping the neuronal marker N-acetyl aspartate to perception distinguishes closely interrelated characteristics of chronic pain. Mol Psychiatry 6: 256–258, 2001 [DOI] [PubMed] [Google Scholar]
- Hall W, Hayward L, Chapman CR. On “the lateralization of pain.” Pain 10: 337–356, 1981 [DOI] [PubMed] [Google Scholar]
- Han JS, Bird GC, Li W, Neugebauer V. Computerized analysis of audible and ultrasonic vocalizations of rats as a standardized measure of pain-related behavior. J Neurosci Methods 141: 261–269, 2005a [DOI] [PubMed] [Google Scholar]
- Han JS, Li W, Neugebauer V. Critical role of calcitonin gene-related peptide 1 receptors in the amygdala in synaptic plasticity and pain behavior. J Neurosci 25: 10717–10728, 2005b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JS, Neugebauer V. mGluR1 and mGluR5 antagonists in the amygdala inhibit different components of audible and ultrasonic vocalizations in a model of arthritic pain. Pain 113: 211–222, 2005 [DOI] [PubMed] [Google Scholar]
- Hardee JE, Thompson JC, Puce A. The left amygdala knows fear: laterality in the amygdala response to fearful eyes. Soc Cogn Affect Neurosci 3: 47–54, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinricher MM, McGaraughty S. Pain-modulating neurons and behavioral state. In: Handbook of Behavioral State Control, edited by Lydic R, Baghdoyan HA. New York: CRC, 1999, p. 487–503 [Google Scholar]
- Ikeda R, Takahashi Y, Inoue K, Kato F. NMDA receptor-independent synaptic plasticity in the central amygdala in the rat model of neuropathic pain. Pain 127: 161–172, 2007 [DOI] [PubMed] [Google Scholar]
- Jackson DC, Mueller CJ, Dolski I, Dalton KM, Nitschke JB, Urry HL, Rosenkranz MA, Ryff CD, Singer BH, Davidson RJ. Now you feel it, now you don't: frontal brain electrical asymmetry and individual differences in emotion regulation. Psychol Sci 14: 612–617, 2003 [DOI] [PubMed] [Google Scholar]
- Ji G, Fu Y, Ruppert KA, Neugebauer V. Pain-related anxiety-like behavior requires CRF1 receptors in the amygdala. Mol Pain 3: 13–17, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G, Neugebauer V. Differential effects of CRF1 and CRF2 receptor antagonists on pain-related sensitization of neurons in the central nucleus of the amygdala. J Neurophysiol 97: 3893–3904, 2007 [DOI] [PubMed] [Google Scholar]
- Ji G, Neugebauer V. Pro- and anti-nociceptive effects of corticotropin-releasing factor (CRF) in central amygdala neurons are mediated through different receptors. J Neurophysiol 99: 1201–1212, 2008 [DOI] [PubMed] [Google Scholar]
- Killgore WD, Yurgelun-Todd DA. Sex differences in amygdala activation during the perception of facial affect. Neuroreport 12: 2543–2547, 2001 [DOI] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Alexander AL, Whalen PJ. Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport 14: 2317–2322, 2003 [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron 20: 937–945, 1998 [DOI] [PubMed] [Google Scholar]
- Lalumiere RT, McGaugh JL. Memory enhancement induced by post-training intrabasolateral amygdala infusions of beta-adrenergic or muscarinic agonists requires activation of dopamine receptors: involvement of right, but not left, basolateral amygdala. Learn Mem 12: 527–532, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurenza A, Sutkowski EM, Seamon KB. Forskolin: a specific stimulator of adenylyl cyclase or a diterpene with multiple sites of action? Trends Pharmacol Sci 10: 442–447, 1989 [DOI] [PubMed] [Google Scholar]
- Lee GP, Meador KJ, Loring DW, Allison JD, Brown WS, Paul LK, Pillai JJ, Lavin TB. Neural substrates of emotion as revealed by functional magnetic resonance imaging. Cogn Behav Neurol 17: 9–17, 2004 [DOI] [PubMed] [Google Scholar]
- Li W, Neugebauer V. Block of NMDA and non-NMDA receptor activation results in reduced background and evoked activity of central amygdala neurons in a model of arthritic pain. Pain 110: 112–122, 2004a [DOI] [PubMed] [Google Scholar]
- Li W, Neugebauer V. Differential roles of mGluR1 and mGluR5 in brief and prolonged nociceptive processing in central amygdala neurons. J Neurophysiol 91: 13–24, 2004b [DOI] [PubMed] [Google Scholar]
- Li W, Neugebauer V. Differential changes of group II and group III mGluR function in central amygdala neurons in a model of arthritic pain. J Neurophysiol 96: 1803–1815, 2006 [DOI] [PubMed] [Google Scholar]
- Lu CL, Wu YT, Yeh TC, Chen LF, Chang FY, Lee SD, Ho LT, Hsieh JC. Neuronal correlates of gastric pain induced by fundus distension: a 3T-fMRI study. Neurogastroenterol Motil 16: 575–587, 2004 [DOI] [PubMed] [Google Scholar]
- Lugo M, Isturiz G, Lara C, Garcia N, Eblen-Zaijur A. Sensory lateralization in pain subjective perception for noxious heat stimulus. Somatosens Mot Res 19: 207–212, 2002 [DOI] [PubMed] [Google Scholar]
- Maren S. Synaptic mechanisms of associative memory in the amygdala. Neuron 47: 783–786, 2005 [DOI] [PubMed] [Google Scholar]
- Mason P. Deconstructing endogenous pain modulations. J Neurophysiol 94: 1659–1663, 2005 [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience 71: 55–75, 1996 [DOI] [PubMed] [Google Scholar]
- Merskey H, Watson GD. The lateralization of pain. Pain 7: 271–280, 1979 [DOI] [PubMed] [Google Scholar]
- Morris JS, Ohman A, Dolan RJ. Conscious and unconscious emotional learning in the human amygdala. Nature 393: 467–470, 1998 [DOI] [PubMed] [Google Scholar]
- Neugebauer V. Subcortical processing of nociceptive information: basal ganglia and amygdala. In: Pain, edited by Cervero F, Jensen TS. Amsterdam: Elsevier, 2006, p. 141–158 [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Han JS, Adwanikar H, Fu Y, Ji G. Techniques for assessing knee joint pain in arthritis. Mol Pain 3: 8–20, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V, Li W. Processing of nociceptive mechanical and thermal information in central amygdala neurons with knee-joint input. J Neurophysiol 87: 103–112, 2002 [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Li W. Differential sensitization of amygdala neurons to afferent inputs in a model of arthritic pain. J Neurophysiol 89: 716–727, 2003 [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Li W, Bird GC, Bhave G, Gereau RW. Synaptic plasticity in the amygdala in a model of arthritic pain: differential roles of metabotropic glutamate receptors 1 and 5. J Neurosci 23: 52–63, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. Neuroscientist 10: 221–234, 2004 [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Schaible HG. Evidence for a central component in the sensitization of spinal neurons with joint input during development of acute arthritis in cat's knee. J Neurophysiol 64: 299–311, 1990 [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci 14: 1215–1229, 2002 [DOI] [PubMed] [Google Scholar]
- Pare D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol 92: 1–9, 2004 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic, 1998 [DOI] [PubMed] [Google Scholar]
- Pedersen LH, Scheel-Kruger J, Blackburn-Munro G. Amygdala GABA-A receptor involvement in mediating sensory-discriminative and affective-motivational pain responses in a rat model of peripheral nerve injury. Pain 127: 17–26, 2007 [DOI] [PubMed] [Google Scholar]
- Phelps EA, O'Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M. Activation of the left amygdala to a cognitive representation of fear. Nat Neurosci 4: 437–441, 2001 [DOI] [PubMed] [Google Scholar]
- Phelps EA, Ledoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron 48: 175–187, 2005 [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr Opin Neurobiol 16: 723–727, 2006 [DOI] [PubMed] [Google Scholar]
- Rhudy JL, Meagher MW. The role of emotion in pain modulation. Curr Opin Psychiatry 14: 241–245, 2001 [Google Scholar]
- Rhudy JL, Meagher MW. Negative affect: effects on an evaluative measure of human pain. Pain 104: 617–626, 2003 [DOI] [PubMed] [Google Scholar]
- Rizvi TA, Ennis M, Behbehani MM, Shipley MT. Connections between the central nucleus of the amygdala and the midbrain periaqueductal gray: topography and reciprocity. J Comp Neurol 303: 121–131, 1991 [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Cellular mechanisms of infralimbic and prelimbic prefrontal cortical inhibition and dopaminergic modulation of basolateral amygdala neurons in vivo. J Neurosci 22: 324–337, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible HG, Schmidt RF. From articular nociception to pain: peripheral and spinal mechanisms. In: From Neuron to Action, edited by Deecke L, Eccles JC, Mountcastle VB. Berlin: Springer-Verlag, 1990, p. 305–311 [Google Scholar]
- Schiff BB, Gagliese L. The consequences of experimentally induced and chronic unilateral pain: reflections of hemispheric lateralization of emotion. Cortex 30: 255–267, 1994 [DOI] [PubMed] [Google Scholar]
- Seltzer SF, Yarczower M, Woo R, Seltzer JL. Laterality and modality-specific effects of chronic pain. Percept Psychophys 51: 500–503, 1992 [DOI] [PubMed] [Google Scholar]
- Sergerie K, Chochol C, Armony JL. The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev 32: 811–830, 2008 [DOI] [PubMed] [Google Scholar]
- Shi C, Davis M. Pain pathways involved in fear conditioning measured with fear- potentiated startle: lesion studies. J Neurosci 19: 420–430, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley MT, Ennis M, Rizvi TA, Behbehani MM. Topographical specificity of forebrain inputs to the midbrain periaqueductal gray: evidence for discrete longitudinally organized input columns. In: The Midbrain Periaqueductal Gray Matter, edited by Depaulis A, Bandler R. New York: Plenum, 1991, p. 417–448 [Google Scholar]
- Stephan KE, Fink GR, Marshall JC. Mechanisms of hemispheric specialization: insights from analyses of connectivity. Neuropsychologia 45: 209–228, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron 55: 377–391, 2007 [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci 26: 4415–4425, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura S, Ueda K, Suzuki SI, Onoda K, Okamoto Y, Yamawaki S. Self-referential processing of negative stimuli within the ventral anterior cingulate gyrus and right amygdala. Brain Cogn 69: 218–225, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.