Abstract
Aims
To investigate the efficacy and safety of a cardiac resynchronization therapy with cardioverter–defibrillator (CRT-D) device with simplified ventricular tachycardia management in patients with non-ischaemic heart failure (HF) and primary prevention implantable cardioverter defibrillator (ICD) indication.
Methods and results
Prospective, controlled, parallel, multicentre, non-randomized study enrolling 324 primary prevention non-ischaemic HF patients implanted with CRT-D devices from 2004 to 2007: Protect group, 164 patients implanted with a Medtronic Insync III Protect device and Control group, 160 patients utilizing other Medtronic CRT-D devices.
Efficacy was assessed by computing appropriate and inappropriate detections and therapies during follow-up; safety compared hospitalizations and syncopal events between groups. Ninety per cent of both ventricular and supraventricular tachyarrhythmias terminated within the 13–29 beat detection interval with the Protect algorithm. The Protect group showed a significantly better event-free survival to first delivered therapy for total (P = 0.0001), appropriately treated (P = 0.002), and inappropriately treated episodes (P = 0.017). The total number of delivered shocks was significantly lower in the Protect group (22 vs. 59, P < 0.0001). In the Protect group, a significantly reduced HF hospitalization (hazard ratio 0.38, 95% CI 0.15–0.98, P = 0.044) was observed without any increase of syncope or death.
Conclusion
A simplified CRT-D device with fixed long detection reduced overall ICD therapy burden and HF hospitalizations without entailing any additional adverse events in primary prevention non-ischaemic HF patients.
Keywords: Cardiac resynchronization therapy, Implantable, Defibrillators, Non-ischaemic, Tachyarrhythmias
Introduction
Cardiac resynchronization therapy with cardioverter–defibrillator (CRT-D) back-up is indicated to reduce morbidity and mortality in optimally treated heart failure (HF) patients, with reduced ejection fraction and wide QRS.1,2
Increased interest is aimed at optimizing implantable cardioverter defibrillator (ICD) and CRT-D, by reducing shock burden without jeopardizing the efficacy of interventions, while, on the other hand, rendering these sophisticated devices more readily available by reducing device costs. Another issue hampering CRT-D diffusion may relate to the correct programming process which usually requires the collaborative expertise of physicians as well as engineers.
From studies evaluating ‘shockless’ therapy delivered through anti-tachycardia pacing (ATP),3–5 three aspects have emerged characterizing the arrhythmic profile of patients with non-ischaemic HF and primary prevention indication for an ICD. Compared with other HF patient sub-groups, these patients generally present a lower incidence of ventricular tachyarrhythmias (VA),5 a great proportion of VA in this population have a short cycle length (usually ranging from 240 to 320 ms) and these tend to be self-terminating.4,5
These notions found the rationale behind developing a CRT device presenting a full-featured biventricular pacing capabilities with ICD full-shock programmability and ‘easy-to-use fixed ATP programming’ like the Medtronic InSync III Protect CRT-D device. Such a device may potentially offer all the advantages of biventricular stimulation and protection from sudden cardiac death, while allowing a cost-effective and wide diffusion of ICD technology.
The objective of the present study was to compare two different approaches in non-ischaemic, primary prevention patients treated with CRT-D, in terms of efficacy and safety:
standard CRT-D ‘full-featured device’ with a standard short 12/16 number of intervals to detect (NID) for VA detection and treatment;
full-featured CRT pacing device combined with simplified, ‘easy-to-use’ ICD features (Medtronic InSync III Protect) with fixed, long detection intervals (30/40 NID) with one fixed 88% ATP burst in fast ventricular tachycardia (FVT) window, ‘monitor only’ VT window (32 beats detection), and full shock capability.
Methods
Study design
RELEVANT is a prospective, controlled, parallel, multicentre, non-randomized study comparing a long VT/VF detection and simplified ICD programmability vs. standard tailored ICD programming in patients with non-ischaemic cardiomyopathy and primary prevention ICD indication.
Patient selection, device programming, and follow-up
Patients were prospectively enrolled from 24 Italian Cardiological Centers from March 2004 to April 2007. Each patient provided written informed consent approved by each Hospital Ethical Committee. Eligible patients had non-ischaemic cardiomyopathy (defined as no previous history of myocardial infarction or ischaemia and no significant lesion at coronary angiography), presented a class I–IIa indication for CRT, and no previous history of VA.1,2 Enrolled patients were implanted either with the Medtronic InSync III Protect device (Protect group) or with other ‘conventional’ Medtronic CRT-D full-featured models (InSync III Marquis, InSync Maximo, InSync Sentry, Medtronic Inc., Minneapolis, MN, USA) (Control group); the former device, being a full-featured CRT pace-maker with basic ICD back-up, was sold in Europe at a price roughly 30–40% lower than other conventional ‘Medtronic CRT-D devices’.
Device programming for each study group is detailed in Table 1. The device programming for the different detection windows differed mainly for two points: (i) NID was much longer in the Protect arm (30/40) compared with the Control group (12/16); (ii) a monitor only window for VT was fixed in the Protect group and highly recommended in the Control group. In the Protect group, data on VA of 12/16 cycle length could also be retrieved, thus allowing to assess the overall ‘hypothetical detection’ of VA in the Protect arm if a short NID window would have been utilized.
Table 1.
Comparison of programmed device settings in the two study groups
| Device programming | Protect group (n = 164) | Control group (n = 160) |
|---|---|---|
| VF detection window [ms] | 120–330 | 120–330 |
| VF NID | 30/40 | 12/16 |
| VF RNID | 12/16 | 9/12 |
| Maximum energy of VF therapies, average [Joules] (SD) | 30 (0) | 34.2 (1.8) |
| Charge time [s] | 5.9 | 7.1 |
| FVT detection window [ms] | 240–330 | 240–330 |
| FVT counter | Via VF | Via VF |
| First FVT therapy, type | Burst 8 pulses at 88% CL | Burst 8 pulses at 88% CL |
| Second to last FVT therapy, type, mean energy (SD) | CV, 30 (0) | CV, 34.2 (1.8) |
| VT detection window | 330–360 | 330–360 |
| VT NID | 32 | 16 |
| VT RNID | 12 | 12 |
| VT therapies monitor, n (%) | 164 (100) | 107 (67) |
VF, ventricular fibrillation; FVT, fast ventricular tachycardia; VT, ventricular tachycardia; CL, cycle length; NID, number of intervals detected; RNID, redetection number of intervals.
Before device implantation, all patients underwent clinical evaluation, 12-lead electrocardiogram recording, and estimation of NYHA functional class. Furthermore, echocardiographic examination was performed at baseline and every 6 months and included: left ventricular end-diastolic diameter and volume, end-systolic diameter and volume, left ventricular ejection fraction (LVEF), and grading of mitral valve regurgitation (from 0 to 4). Pharmacological treatment was maintained stable throughout the follow-up as much as possible, especially with regard to anti-arrhythmic drugs.
Rhythm classification
All the devices provide extensive retrievable diagnostic data, including storage of date, time, tachycardia cycle length, ICD therapies, atrial and ventricular electrograms at episode onset, detection, before and after therapy delivery, and termination. Spontaneous arrhythmic episodes detected by the device were validated by two-blinded expert electrophysiologists. A third electrophysiologist was involved in episode review when no consensus in episode classification had been reached. Analysed episodes were classified as VA, atrial fibrillation/tachyarrhythmias (AF/AT), or other events (T-wave oversensing or noise), according to established criteria.3 Appropriately detected/treated episodes included VA detected/treated by the device that was confirmed to be ventricular in origin by the two experts. Inappropriately detected/treated episodes included AF/AT, T-wave oversensing, or electromagnetic interference.
Episodes overwritten on device memory in case of arrhythmic storm (that then could not be surely evaluated) were excluded from the analysis.
Any episodes that terminated before delivery of device therapy (either shock or ATP), or terminating spontaneously in a monitor zone, were classified as spontaneously terminating. A therapy was defined as successful when normal rhythm was restored within five beats after therapy delivery.
Assessment of adverse events
Cardiac events were considered syncope, cardiovascular hospitalizations, and deaths during follow-up. These events were computed and reviewed by an independent data and safety monitoring board composed of non-participating physicians. Syncope was defined as a transitory, complete loss of consciousness with loss of postural tone. Acceleration was defined as ≥10% cycle length reduction after therapy.3 Hospitalizations and deaths were classified as cardiac (HF or sudden), non-cardiac, or unknown according to Epstein et al.6 Patients undergoing left ventricle assist device or urgent heart transplant were classified as HF deaths.
Statistics
Descriptive statistics were reported as mean and standard deviation for normally distributed continuous variables, or median with 25th–75th inter-quartile range in the case of skewed distribution. Absolute and relative frequencies were reported for categorical variables. Comparisons of continuous variables were performed by two-tailed Student's t-test for normally distributed variables. Comparisons of categorical variables were performed by means of the Fisher exact test for extreme proportions or Chi-square otherwise.
The incidence rate ratio (IRR) of events between groups was calculated applying a Poisson regression analysis using generalized estimating equation (GEE) methodology, considering the within-patient correlation as independent. The number of events per patient was the observed outcome and the follow-up period was included in the model as offset.
Freedom from the first delivered ICD intervention (ATP and/or shock), also divided according to first appropriate or inappropriate interventions, was analysed by means of the Kaplan–Meier method and differences were evaluated by performing log-rank test. A Cox regression was performed to estimate the hazard ratio (HR) of ICD intervention, comparing observed groups.
For statistical analysis, SPSS 12.0 software (SPSS Inc., Chicago, IL, USA) and StataSE 9 (StataCorp, TX, USA) were used. A two-sided P-value < 0.05 was considered statistically significant.
Results
Three hundred and twenty-four patients with non-ischaemic HF and primary prevention ICD indication were implanted with CRT-D devices between March 2004 and September 2007: 164 patients were implanted with Medtronic Insync III Protect devices (Protect group, NID 30/40); the other 160 patients were implanted with ‘conventional’ Medtronic CRT-D models (InSync III Marquis, InSync Maximo, InSync Sentry, Medtronic Inc., Minneapolis, MN, USA) (Control group, NID 12/16).
No significant differences in baseline demographic and clinical variables (Table 2) or in mean follow-up (mean 14 months) were detected between the two groups. Also, baseline echocardiographic parameters as well as medical therapy were similar between the groups. Medications were stable throughout the follow-up without significant changes within the same group and between the two study groups.
Table 2.
Baseline characteristics
| Protect group (n = 164) | Control group (n = 160) | P-value | |
|---|---|---|---|
| Follow-up period (SD) | 14.0 (10.4) | 14.4 (8.5) | 1.000 |
| Male gender, n (%) | 126 (76.8) | 120 (75) | 0.700 |
| Age (SD) | 64.2 (11.7) | 64.2 (10.8) | 0.989 |
| Diabetes history, n (%) | 32 (19.5) | 25 (15.6) | 0.372 |
| NYHA class (SD) | 2.67 (0.59) | 2.66 (0.57) | 0.947 |
| Permanent AF, n (%) | 29 (17.7) | 23 (14.4) | 0.703 |
| QRS width (ms), (25th–75th) | 163 (135–183) | 160 (140–170) | 0.081 |
| LVEF (SD) | 24.6 (5.9) | 25.4 (5.3) | 0.269 |
| LVEDD (SD) | 70 (9) | 71 (13) | 0.727 |
| LVESD (SD) | 58 (10) | 58 (11) | 0.950 |
| LVEDV (SD) | 232 (70) | 246 (94) | 0.275 |
| LVESV (SD) | 168 (58) | 184 (76) | 0.184 |
| Mitral regurgitation, n (%) | 105 (64) | 108 (67.5) | 0.696 |
| ACE-inhibitors and/or ARB, n (%) | 121 (74) | 125 (78) | 0.220 |
| Beta-blockers, n (%) | 131 (80) | 117 (73) | 0.120 |
| Amiodarone, n (%) | 40 (26) | 49 (31) | 0.216 |
| Diuretics, n (%) | 139 (85) | 132 (83) | 0.514 |
| Digitalis, n (%) | 57 (35) | 51 (32) | 0.510 |
NYHA, New York Heart Association; AF, atrial fibrillation; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume.
Table 1 details the differences in programmed tachycardia detection and therapy between the two groups.
Analysis of recorded episodes
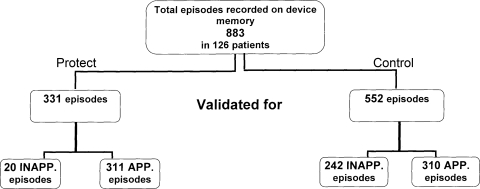
A total of 883 episodes (331 episodes in Protect and 552 in Control group) were validated and considered for the analysis. Distribution of all (appropriate and inappropriate) recorded episodes is presented in Figure 1.
Figure 1.
Distribution and numbers of all detected episodes (appropriate and inappropriate) in the two study groups.
Appropriately detected ventricular tachyarrhythmias
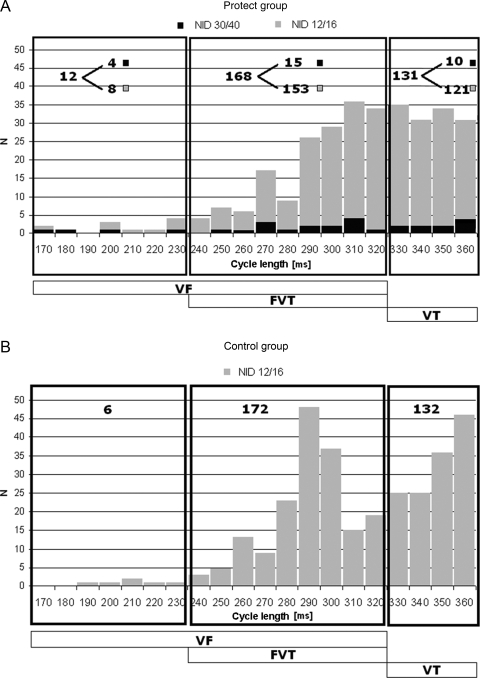
In order to estimate if the arrhythmic burden in the two groups was similar, detected VA were evaluated in the Protect group considering both actual programming (NID 30/40) and 12/16 NID hypothetical programming, as utilized in the Control group. The analysis obtained with an equivalent ‘short’ NID (12/16) pointed out that the two groups presented very similar ‘true ventricular arrhythmic burden’: utilizing an equal short NID, in fact, the number of appropriately detected episodes would have been 311 in the Protect and was 310 in the Control groups (IRR = 0.96, 95% CI 0.82–1.12, P = 0.592) (grey bars in Figure 2A and B).
Figure 2.
Distribution of all appropriately detected episodes in the Protect (A) and in the Control (B) groups are presented. The distribution is distinguished according short to 12/16 NID setting (grey bars) and long 30/40 NID (black bars).
Distribution of VA was comparable between groups: VF episodes were 12 in the Protect group and 6 in the Control group, FVT 168 and 172, VT 131 and 132, respectively. However, prolonged NID used in the Protect group allowed to correctly identify 282/311 (91%) as VA episodes which self-terminated in the interval between 13 and 29 beats.
More specifically in the Protect group, 8 out of 12 (66%) VF, and 153/168 (91%) FVT episodes terminated spontaneously within 29 beats, while 121/131 (92%) VT episodes self-terminated within 31 beats (black bars in Figure 2A). While within the Control group (with FVT/VF detection at NID 12/16), most of these episodes were considered sustained and treated, in the Protect arm with NID 30/40, the great majority (282/311 episodes, 91%) of these episodes were not treated as these were self-terminating ventricular arrhythmias.
Inappropriately detected episodes
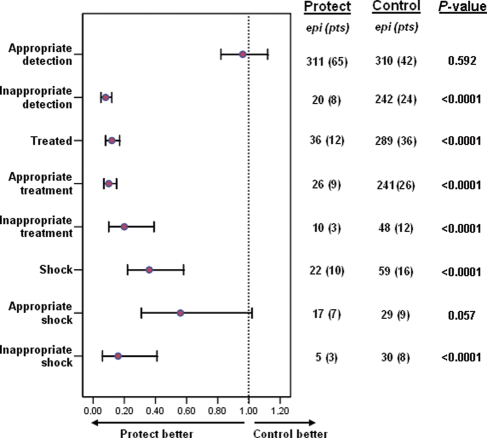
The number of inappropriately detected episodes (AF/AT, T-wave over-sensing or noise) differed significantly between the two groups, amounting to only 20 in Protect and 242 episodes in Control groups (IRR = 0.08, 95% CI 0.05–0.12, P < 0.0001) (Figures 1 and 3). In both groups, less than 10% of inappropriate episodes were due to noise or oversensing, while most inappropriately recorded episodes were determined by supraventricular tachyarrhythmias (AT/AF); specifically 18/20 (90% of inappropriate detections) episodes for Protect group and 225/242 episodes (93%) for Control group.
Figure 3.
Poisson regression estimates (GEE adjusted) of incidence rate ratio (IRR) values of ICD interventions between Protect and Control groups.
Analysis of treated episodes
A total of 325 treated episodes were recorded in 48 patients: 12 patients in Protect group and 36 patients in Control group. Overall, event-free survival to first delivered therapy (ATP or shock, appropriate and inappropriate) was dramatically lower in the Protect arm (log-rank = 15.02, P < 0.0001 for all therapies). A significantly lower number of interventions were observed in Protect compared with Control, respectively, 36 episodes vs. 289 episodes (IRR = 0.12, 95% CI 0.08–0.17, P < 0.0001) (Figure 3); also, few shocks occurred in the Protect group (Protect 22 shocks vs. 59 shocks for Control, IRR = 0.36, 95% CI 0.22–0.58, P < 0.0001) (Figure 3).
Appropriately treated VA episodes
Appropriately treated VA amounted to 267. Appropriately treated episodes were significantly lower for the Protect arm (only 26 episodes in nine patients) compared with Control (241 episodes in 26 patients, IRR = 0.10, 95% CI 0.07–0.15, P < 0.0001) (Figure 3). Considering appropriate shock therapy, a trend towards a significantly lower number of shocks was observed in the Protect arm (Protect 17 episodes vs. Control 29, IRR = 0.56, 95% CI 0.31–1.02, P = 0.057) (Figure 3).
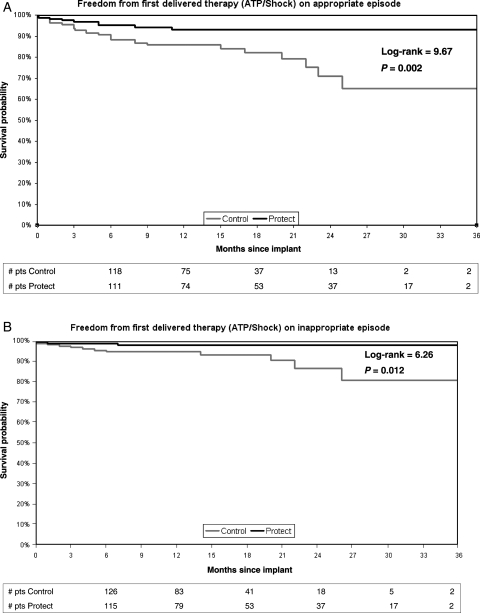
Kaplan–Meier analysis (Figure 4A) further supported these findings by showing a significantly superior event-free survival to first delivered therapy in the Protect group (log-rank = 9.67, P = 0.002).
Figure 4.
Kaplan–Meier analysis of event-free survival to first delivered therapy for appropriate (A) or inappropriate episodes (B).
Inappropriately treated episodes
Due to the longer NID utilized in the Protect arm, the number of inappropriately treated episodes were remarkably lower for the Protect group, numbering only 10 events compared with 48 events for the Control group (IRR = 0.20, 95% CI 0.10–0.39, P < 0.0001) (Figure 3). Likewise, Protect group presented a significantly lower number of shocks compared with Control group (5 and 30 shocks, respectively, IRR = 0.16, 95% CI 0.06–0.41, P < 0.0001) (Figure 3). Kaplan–Meier analysis (Figure 4B) confirmed significantly superior event-free survival to first inappropriate therapy (log-rank = 6.26, P = 0.012) in Protect arm.
Outcome data
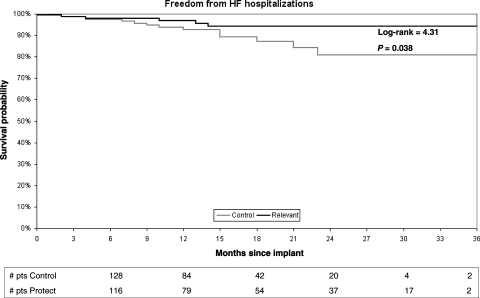
Data concerning NYHA class and EF at 6 months were not available in 18 and 33 patients, respectively, while 28 patients were lost to follow-up. All other outcome variables were available in both groups. No significant differences in clinical outcomes were observed between the two groups at 6 months: mean NYHA class was 1.96 ± 0.55 in Protect group vs. 2.03 ± 0.59 in Control group, P = 0.276; mean LVEF (%) was 29.1 ± 10.9 in Protect group vs. 27.5 ± 8.1 in Control group (P = 0.316). During follow-up, two syncope were observed in Protect group (one due to prolonged detection on a FVT and one due to FVT acceleration after ATP), while three episodes occurred in Control group (all due to VT acceleration after ATP) (P = 0.465). Freedom from the first HF hospitalization was significantly better in Protect than in Control patients (log-rank = 4.31, P = 0.038) (Figure 5) and there was a 60% risk reduction of total HF hospitalizations for the Protect group (HR = 0.38, 95% CI 0.15–0.98, P = 0.044).
Figure 5.
Kaplan–Meier analysis of event-free survival to first heart failure hospitalizations.
At the end of the follow-up period, 6 deaths were observed: in both Protect and Control groups three patients died (P = 1.000), with a similar incidence of 1.8 per 100 patient years and 1.9 per 100 patient years, respectively.
Discussion
This study investigated, for the first time, the efficacy and safety of a simplified ICD strategy in a multicentre, prospective, comparative, parallel scheme enrolling only patients with non-ischaemic HF and primary prevention indication treated with CRT-D. The potential advantages of an ‘empiric’ programming with longer NID in the FVT/VF windows have already been demonstrated in patients with LV dysfunction and ICD indication.7,8 However, the present study differs from previous ones in several aspects. Compared with the present study, the EMPIRIC study7 tested detection set at NID 18/24 and not 30/40. Also, in EMPIRIC, only half of the patients enrolled were implanted in primary prevention and most patients presented ischaemic aetiology. The PREPARE study8 tested long detection algorithm in patients implanted with conventional and biventricular ICD in primary prevention against a retrospective control cohort gathered from previous EMPIRIC and MIRACLE-ICD studies.9 Moreover, as in EMPIRIC, most patients included presented ischaemic aetiology. Very different from these previous studies,7,8 the present one considered a specific HF patient population treated with CRT-D and evaluated a strategy, mainly characterized by a long 30/40 NID FVT detection window, compared with a Control group with conventional ‘tailored’ ICD programming. The study demonstrated that this strategy with long detection reduced the number of overall interventions (appropriate and inappropriate), without undermining effective ICD therapy. Moreover, patients treated with this algorithm did not exhibit increased adverse effects (such as increased syncopal episodes) but, on the contrary, HF hospitalization was found to be significantly lower compared with Control.
Self-terminating ventricular tachyarrhythmias in primary prevention non-ischaemic heart failure patients
The comparison of the two groups with different detection settings allowed to provide some fundamental information on the natural history of VA in this patient sub-group.
These data have been obtained not only by an inter-group comparison, but also through an intra-group analysis inside the Protect group by discriminating between the shorter FVT episodes, which would have been hypothetically detected within NID 12/16, and the more prolonged episodes that reached 30/40 NID. This distinction allowed to confirm the very similar VA profile between the two groups, thus emphasizing that a dramatic reduction of the incidence of detected and treated VA can be obtained by setting a long 30/40 NID.
In fact, the great majority of true VT and FVT (more than 90%), which in the Control arm entered the treatment window, were found to self-terminate within 30 beats in the Protect arm, and, more importantly, in the Protect group, only one-third of VF actually reached 30 beats, thus necessitating shock therapy.
The concept that long detection offers the possibility for VA to terminate spontaneously is not a novel one and has been previously raised, mainly in patients treated with single or dual chamber ICDs.4,7,8,10 The simple additional detection delay of mean 3.3 s present during charge in the shock therapy group in PainFREE Rx II4 allowed to observe that a high proportion of FVT (34%) would terminate spontaneously in the 3 s time interval following 18/24 NID. In the present study, the different NID windows determined a mean detection time of 3.7 s for Control group, and 9.3 s for Protect group before any therapy was delivered; this almost 6 s delay may explain the dramatic reduction of arrhythmias really necessitating treatment. More recent studies performed on large numbers of heterogeneous patients with LV dysfunction and ICD indication observed that standardized ICD programming, that included long detection, effectively reduced shock-related morbidity.7,8
The present study found that appropriate ICD interventions were significantly lower for the Protect group (P < 0.0001), and happened to be virtually one-tenth of the number of interventions in the Control group. This suggests vice versa that almost 90% of the appropriate interventions observed in the Control group were probably applied on VA which may have otherwise self-terminated with a longer NID window.
The importance of self-terminating supraventricular tachyarrhythmias in primary prevention non-ischaemic heart failure patients
One of the most remarkable features derived from the comparison of the two ICD strategies is the dramatically lower number of inappropriate detections in the Protect arm (Protect only 20 episodes vs. 242 episodes in the Control arm, P <0.0001). The long NID strategy may have substantially contributed to effectively abolish inappropriate detections in this patient population. Similar to what has been already reported, most inappropriate interventions are determined by high-rate AT/AF,4,11,12 especially in HF patients with history of AF, representing 20–40% of all inappropriate therapies. In our series, the comparison between the different NID allowed to assume that around 90% of AT/AF were found to resume in a 12–30 beat interval. Even this information on atrial arrhythmias, to our knowledge, has never been reported before and may play an important role in optimizing ICD detection and therapy in these patients. In fact, the number of inappropriate interventions was significantly lower for the Protect group both in terms of total interventions (10 episodes in Protect vs. 48 episodes in Control group, P = 0.0001) and shocks (5 episodes in the Protect group and 30 episodes for Control, P < 0.0001).
Taken together, the evidence presented herein shows that AT/AF in this particular population tend to terminate spontaneously, further reinforcing the concept that setting long NID and ‘VT monitor only zone’ is advisable.13
Reducing the burden of ICD interventions using long detection intervals
The present study found that prolonged 30/40 detection significantly reduced the burden of ICD interventions without increasing the incidence of syncope: in this regard, the observed incidence of syncope in the Protect arm was low and comparable with the Control group (1.2 vs. 1.9%). These incidences are in line with previous observations derived from ICD studies3,4 and confirm the safety of long detection programming.7,8 The overall burden of shocks (appropriate and inappropriate), evaluated through Poisson regression analysis (Figure 3), was significantly lower for the Protect group (<0.0001). More specifically, in the Protect group the proportion of inappropriate shocks was significantly lower (P < 0.0001) compared with the Control group; concerning appropriate shocks a trend towards a lower proportion of these events was also found (P = 0.057).
It is well known that direct current (DC) shock may have a profound negative impact on quality of life,14 morbidity, and even survival.11,15,16 This is particularly true in patients with refractory HF implanted with CRT-D, in whom both appropriate and inappropriate shocks may increase morbidity and mortality in different ways, including myocardial depression,17,18 pro-arrhythmic effect of DC shock,15,16 thromboembolic complications after DC shock,19 and increased stress-related sympathetic tone which is pro-arrhythmogenic and pro-thrombotic.20
In the present study, as a result of the lower number of appropriate and inappropriate therapies delivered in the Protect arm, significantly lower HF hospitalizations were observed in this group, suggesting that too much ICD therapy and interventions may be detrimental in this patient sub-group. Of note, this intriguing finding runs in line with the recent results of a sub-study of the SCD-HeFT trial that identified ICD shocks (whether appropriate or inappropriate) as independent predictors of death, particularly HF death,21 in HF patients treated with conventional ICD.
Clinical implications
The findings herein support the use of a simplified programming (FVT with long detection and single burst ATP, monitor only VT window) in a specific HF patient sub-group with non-ischaemic HF aetiology, ventricular conduction delay, and primary prevention indication for an ICD. In this patient sub-group, too sensitive anti-tachycardia diagnostic and therapeutic settings may dramatically increase ICD therapy burden and may, paradoxically, increase HF hospitalizations. It would be reasonable to evaluate whether such a simplified approach with long detection would be effective in patients with ischaemic HF patients implanted for primary prevention as these patients usually have a low incidence of ventricular arrhythmic events.22 The diffusion of such a strategy incorporated in a simplified ICD device with fixed programming to ischaemic patients as well may entail a remarkable reduction of costs while ensuring a wider access to ICD technology. Also, in light of the present study findings, the possibility of programming even longer NID may be evaluated in the future.
In the present study, the use of a simplified device for CRT-D, with a lower price, was associated with favourable results and this may have important implications in an economic perspective. A series of cost-effectiveness studies23,24 demonstrated that lowering the cost of an ICD would improve the incremental cost-effectiveness of device therapy. Focusing on CRT, a series of studies have provided varying estimates of the cost-effectiveness of CRT-P and CRT-D relative to medical treatment,25 but the incremental cost-effectiveness of CRT-P vs. CRT-D remains not well defined. A reduction in the incremental cost of CRT-D vs. CRT-P, as obtained in our experience by simpler CRT-D devices, may substantially improve the incremental cost-effectiveness of CRT-D vs. CRT-P. The important 60% reduction of HF hospitalization observed in the Protect group, combined with an upfront cost reduction of CRT-D devices by roughly 30–40%, may determine an improvement in cost effectiveness that could be the basis for promoting a more homogeneous implementation of current guidelines on CRT-D, trying to overcome current inequalities in the ‘real world’.26,27
Study limitations
This is a prospective, multicentre, controlled, parallel study. The major limitation is that treatment assignment was not randomized, but it was centrally based with availability of the first or second type of device mainly determined on the basis of regional device allocation policy. This centralized treatment assignment modality was even dictated by difficulties to obtain a full echonomical support for a randomized study. It is worthwhile to consider, however, that the participating Centers were all regional referral Centers for device implant and that the experience in CRT implantation between Centers enrolling Protect vs. Control patients was found to be equivalent, both in terms of volume, success rate of implants, as well as mean number of years of experience in CRT device implant. For the above-mentioned reasons, no major bias was observed in the study, nonetheless the non-randomized nature of the study could not permit to exclude the presence of minor selection biases.
Data on quality of life were obtained only in a minority of the study population, and no statistical evaluation was therefore possible.
Finally, the comparison with the Protect (30/40 NID) group was performed against Control group utilizing 12/16 and not 18/24 NID; though this latter NID has recently shown to be effective and safe in ICD recipients,7,8 the present study was started at a time when NID was conventionally programmed at 12/16.
Conclusions
In patients with non-ischaemic HF and conventional primary prevention indication for a CRT-D, a simplified CRT-D with full shock capability, and pre-set long detection window (30/40 NID) determined a dramatic reduction of ICD interventions without either jeopardizing ICD therapy capabilities or entailing increased morbidity. Further studies confirming that the effectiveness of this simplified and cheaper strategy in HF primary prevention ischaemic patients could allow a wider diffusion and availability of this life-saving device therapy.
Funding
Funding to pay the Open Access publication charges for this article was provided by Medtronic Italia.
Conflict of interest: M.G., C.M., A.P., M.L., S.I., A.C., E.L., F.R., M.B. and G.B. had nothing to be declared, V.B. and A.D. are Medtronic employees.
References
- 1.Vardas PE, Auricchio A, Blanc JJ, Daubert JC, Drexler H, Ector H, Gasparini M, Linde C, Morgado FB, Oto A, Sutton R, Trusz-Gluza M European Society of Cardiology. Guidelines for cardiac pacing and cardiac resynchronization therapy: The Task Force for Cardiac Pacing and Cardiac Resynchronization Therapy of the European Society of Cardiology. Developed in Collaboration with the European Heart Rhythm Association. Eur Heart J. 2007;28:2256–2295. doi: 10.1093/eurheartj/ehm305. [DOI] [PubMed] [Google Scholar]
- 2.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NAM, III, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): Developed in Collaboration With the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation. 2008;117:e350–e408. doi: 10.1161/CIRCUALTIONAHA.108.189742. [DOI] [PubMed] [Google Scholar]
- 3.Wathen MS, Sweeney MO, DeGroot PJ, Stark AJ, Koehler JL, Chisner MB, Machado C, Adkisson WO. Shock reduction using antitachycardia pacing for spontaneous rapid ventricular tachycardia in patients with coronary artery disease. Circulation. 2001;104:796–801. doi: 10.1161/hc3101.093906. [DOI] [PubMed] [Google Scholar]
- 4.Wathen MS, DeGroot PJ, Sweeney MO, Stark AJ, Otterness MF, Adkisson WO, Canby RC, Khalighi K, Machado C, Rubenstein DS, Volosin KJ for the Pain Free Rx II Investigators. Prospective randomized multicenter trial of empirical antitachycardia pacing versus shocks for spontaneous rapid ventricular tachycardia in patients with implantable cardioverter-defibrillators: Pacing Fast Ventricular Tachycardia Reduces Shock Therapies (PainFREE Rx II) Trial Results. Circulation. 2004;110:2591–2596. doi: 10.1161/01.CIR.0000145610.64014.E4. [DOI] [PubMed] [Google Scholar]
- 5.Boriani G, Gasparini M, Lunati M, Santini M, Landolina M, Vincenti A, Curnis A, Bocchiardo M, Padeletti L, Biffi M, Allaria L, Denaro A. Characteristics of ventricular tachyarrhythmias occurring in ischemic versus nonischemic patients implanted with a biventricular cardioverter-defibrillator for primary or secondary prevention of sudden death. Am Heart J. 2006;152:527.e521–527.e511. doi: 10.1016/j.ahj.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Epstein AE, Carlson MD, Fogoros RN, Higgins SL, Venditti FJ. Classification of death in antiarrhythmia trials. J Am Coll Cardiol. 1996;27:433–442. doi: 10.1016/0735-1097(95)00482-3. [DOI] [PubMed] [Google Scholar]
- 7.Wilkoff BL, Ousdigian KT, Sterns LD, Wang ZJ, Wilson RD, Morgan JM. A comparison of empiric to physician-tailored programming of implantable cardioverter-defibrillators: results from the Prospective Randomized Multicenter EMPIRIC Trial. J Am Coll Cardiol. 2006;48:330–339. doi: 10.1016/j.jacc.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 8.Wilkoff B, Stern R, Williamson B, Wathen M, Holloman K, Fieberg A, Brown M. Strategic programming of detection and therapy parameters in implantable cardioverter defibrillators reduces shocks in primary prevention patients: results from the PREPARE (Primary Prevention Parameters Evaluation) study. J Am Coll Cardiol. 2008;52:541–550. doi: 10.1016/j.jacc.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Young JB, Abraham WT, Smith AL, Leon AR, Lieberman R, Wilkoff B, Canby RC, Schroeder JS, Liem LB, Hall S, Wheelan K Multicenter InSync ICD Randomized Clinical Evaluation (MIRACLE ICD) Trial Investigators. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. JAMA. 2003;289:2685–2694. doi: 10.1001/jama.289.20.2685. [DOI] [PubMed] [Google Scholar]
- 10.Maloney J, Masterson M, Khoury D, Trohman R, Wilkoff B, Simmons T, Morant V, Castle L. Clinical performance of the implantable cardioverter defibrillator: electrocardiographic documentation of 101 spontaneous discharges. Pacing Clin Electrophysiol. 1991;14:280–285. doi: 10.1111/j.1540-8159.1991.tb05107.x. [DOI] [PubMed] [Google Scholar]
- 11.Daubert JP, Zareba W, Cannom DS, McNitt S, Rosero SZ, Wang P, Schuger C, Steinberg JS, Higgins SL, Wilber DJ, Klein H, Andrews ML, Hall WJ, Moss AJ. Inappropriate implantable cardioverter-defibrillator shocks in MADIT II: frequency, mechanisms, predictors, and survival impact. J Am Coll Cardiol. 2008;51:1357–1365. doi: 10.1016/j.jacc.2007.09.073. [DOI] [PubMed] [Google Scholar]
- 12.Klein RC, Raitt MH, Wilkoff BL, Beckman KJ, Coromilas J, Wyse DG, Friedman PL, Martins JB, Epstein AE, Hallstrom AP, Ledingham RB, Belco KM, Greene HL AVIS Investigators. Analysis of implantable cardioverter defibrillator therapy in the Antiarrhythmic versus Implantable Defibrillators (AVID) Trial. J Cardiovasc Electrophysiol. 2003;14:940–948. doi: 10.1046/j.1540-8167.2003.01554.x. [DOI] [PubMed] [Google Scholar]
- 13.Wilkoff BL, Hess M, Young J, Abraham WT. Differences in tachyarrhythmia detection and implantable cardioverter defibrillator therapy by primary or secondary prevention indication in cardiac resynchronization therapy patients. J Cardiovasc Electrophysiol. 2004;15:1002–1009. doi: 10.1046/j.1540-8167.2004.03625.x. [DOI] [PubMed] [Google Scholar]
- 14.Avid Investigators. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. 1997;337:1576–1583. doi: 10.1056/NEJM199711273372202. [DOI] [PubMed] [Google Scholar]
- 15.Veltmann C, Borggrefe M, Schimpf R, Wolpert C. Fatal inappropriate ICD shock. J Cardiovasc Electrophysiol. 2007;18:326–328. doi: 10.1111/j.1540-8167.2006.00692.x. [DOI] [PubMed] [Google Scholar]
- 16.Vollman D, Lüthje L, Vonhof S, Unterberg C. Inappropriate therapy and fatal proarrhythmia by an implantable cardioverter-defibrillator. Heart Rhythm. 2005:307–309. doi: 10.1016/j.hrthm.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 17.Tokano T, Bach D, Chang J, Davis J, Souza JJ, Zivin A, Knight BP, Goyal R, Man KC, Morady F, Strickberger SA. Effect of ventricular shock strength on cardiac hemodynamics. J Cardiovasc Electrophysiol. 1998;9:791–797. doi: 10.1111/j.1540-8167.1998.tb00118.x. [DOI] [PubMed] [Google Scholar]
- 18.Steinbeck G, Dorwarth U, Mattke S, Hoffmann E, Markewitz A, Kaulbach H, Tassani P. Hemodynamic deterioration during ICD implant: Predictors of high-risk patients. Am Heart J. 1994;127:1064–1067. doi: 10.1016/0002-8703(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 19.Benedini G, Marchini A, Curnis A, Bianchetti F, Gardini A, Pinetti P, Zanelli E. Implantable defibrillation and thromboembolic events. Pacing Clin Electrophysiol. 1995;18:199–202. doi: 10.1111/j.1540-8159.1995.tb02504.x. [DOI] [PubMed] [Google Scholar]
- 20.Birk AV, Leno E, Robertson HD, Bolotina VM, Szeto HH. Interaction between ATP and catecholamines in stimulation of platelet aggregation. Am J Physiol Heart Circ Physiol. 2003;284:H619–H625. doi: 10.1152/ajpheart.00110.2002. [DOI] [PubMed] [Google Scholar]
- 21.Poole JE, Johnson GW, Hellkamp AS, Anderson J, Callans DJ, Raitt MH, Reddy RK, Marchlinski FE, Yee R, Guarnieri T, Talajic M, Wilber DJ, Fishbein DP, Packer DL, Mark DB, Lee KL, Bardy GH. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359:1009–1017. doi: 10.1056/NEJMoa071098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, Brown MW, Heo M. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med. 1996;335:1933–1940. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 23.Sanders GD, Hlatky MA, Owens DK. Cost-effectiveness of implantable cardioverter-defibrillators. N Engl J Med. 2005;353:1471–1480. doi: 10.1056/NEJMsa051989. [DOI] [PubMed] [Google Scholar]
- 24.Owens DK, Sanders GD, Harris RA, McDonald KM, Heidenreich PA, Dembitzer AD, Hlatky MA. Cost-effectiveness of implantable cardioverter defibrillators relative to amiodarone for prevention of sudden cardiac death. Ann Intern Med. 1997;126:1–12. doi: 10.7326/0003-4819-126-1-199701010-00001. [DOI] [PubMed] [Google Scholar]
- 25.Boriani G, Biffi M, Martignani C, Valzania C, Diemberger I, Bertini M, Domenichini G, Branzi A. Is cardiac resynchronization therapy cost-effective? Europace. 2009 doi: 10.1093/europace/eup274. (in press) [DOI] [PubMed] [Google Scholar]
- 26.Fonarow GC, Yancy CW, Albert NM, Curtis AB, Stough WG, Gheorghiade M, et al. Heart failure care in the outpatient cardiology practice setting: findings from IMPROVE HF. Circulation: Heart Failure. 2008;1:98–106. doi: 10.1161/CIRCHEARTFAILURE.108.772228. [DOI] [PubMed] [Google Scholar]
- 27.Piccini JP, Hernandez AF, Dai D, Thomas KL, Lewis WR, Yancy CW, Peterson ED, Fonarow GC Get With the Guidelines Steering Committee, Hospitals. Use of cardiac resynchronization therapy in patients hospitalized with heart failure. Circulation. 2008;118:926–933. doi: 10.1161/CIRCULATIONAHA.108.773838. [DOI] [PubMed] [Google Scholar]