Abstract
Aims
The aim of this study was to determine whether growth differentiation factor-15 (GDF-15) predicts mortality and morbidity after cardiac resynchronization therapy (CRT). Growth differentiation factor-15, a transforming growth factor-β-related cytokine which is up-regulated in cardiomyocytes via multiple stress pathways, predicts mortality in patients with heart failure treated pharmacologically.
Methods and results
Growth differentiation factor-15 was measured before and 360 days (median) after implantation in 158 patients with heart failure [age 68 ± 11 years (mean ± SD), left ventricular ejection fraction (LVEF) 23.1 ± 9.8%, New York Class Association (NYHA) class III (n = 117) or IV (n = 41), and QRS 153.9 ± 28.2 ms] undergoing CRT and followed up for a maximum of 5.4 years for events. In a stepwise Cox proportional hazards model with bootstrapping, adopting log GDF-15, log NT pro-BNP, LVEF, and NYHA class as independent variables, only log GDF-15 [hazard ratio (HR), 3.76; P = 0.0049] and log NT pro-BNP (HR, 2.12; P = 0.0171) remained in the final model. In the latter, the bias-corrected slope was 0.85, the optimism (O) was −0.06, and the c-statistic was 0.74, indicating excellent internal validity. In univariate analyses, log GDF-15 [HR, 5.31; 95% confidence interval (CI), 2.31–11.9; likelihood ratio (LR) χ2 = 14.6; P < 0.0001], NT pro-BNP (HR, 2.79; 95% CI, 1.55–5.26; LR χ2 = 10.4; P = 0.0004), and the combination of both biomarkers (HR, 7.03; 95% CI, 2.91–17.5; LR χ2 = 19.1; P < 0.0001) emerged as significant predictors. The biomarker combination was associated with the highest LR χ2 for all endpoints.
Conclusion
Pre-implant GDF-15 is a strong predictor of mortality and morbidity after CRT, independent of NT pro-BNP. The predictive value of these analytes is enhanced by combined measurement.
Keywords: Growth differentiation factor-15, Cardiac resynchronization therapy, Heart failure, Mortality
Introduction
The benefits of cardiac resynchronization therapy (CRT) for patients with heart failure are well established.1,2 Predicting mortality and morbidity on the basis pre-implant assessments, however, remains a challenge. Numerous echocardiographic measures of dyssynchrony have been explored,3 but none has emerged as a predictor of outcome in multicentre studies.4
Increasing interest is being focused on the role of biomarkers in the risk stratification of patients with cardiac disease.5 Growth differentiation factor-15 (GDF-15), which is a member of the transforming growth factor-β (TGF-β) cytokine superfamily,6,7 has been implicated in the regulation of cell survival, proliferation, and differentiation as well as in protection from ischaemia/reperfusion injury.8 Alterations in TGF-βs have been observed in association with atherosclerosis9 and in patients who are at risk of coronary events.8,10
In patients with heart failure, GDF-15 is an independent predictor of mortality, adding prognostic information to that obtained from New York Class Association (NYHA) class, left ventricular ejection fraction (LVEF), and N-terminal pro-B-type natriuretic peptide (NT pro-BNP) levels.11 We sought to determine whether GDF-15 correlates with mortality and morbidity in patients with moderate-to-severe heart failure undergoing CRT. A secondary objective was to determine whether GDF-15, either alone or in combination with NT pro-BNP, relates to symptomatic response. Of particular interest was the possible value of pre-implant GDF-15 levels in risk-stratifying patients undergoing CRT, over and above NT pro-BNP, and the more traditional prognostic markers.
Methods
Subjects
The study group consisted of patients with heart failure undergoing CRT in a single centre, from June 2002 to February 2007. The last follow-up visit was in February 2008. Over this period, 299 patients underwent implantation. For logistical reasons, however, only patients (n = 165) who underwent a planned implantation on one given day of the week (Thursday) were eligible for the study. Four patients declined consent. There were three failed implants. A total of 158 patients were included in the study. Three patients died before a clinical review was undertaken. Complete baseline and follow-up biochemical data were available in 117 patients.
The diagnosis of heart failure was made if symptoms were associated with objective evidence of LV dysfunction on echocardiography. The diagnosis of ischaemic cardiomyopathy was made if systolic dysfunction was associated with a history of myocardial infarction12 or if there was angiographically documented coronary heart disease (>50% stenosis in ≥1 coronary arteries). Late gadolinium enhancement cardiovascular magnetic resonance was also used to distinguish between ischaemic and non-ischaemic cardiomyopathy, according to Assomull et al.13 Patients with LV dysfunction in combination with the finding of transmural or subendocardial late gadolinium uptake were classified as having ischaemic cardiomyopathy, whereas patients with LV dysfunction and no gadolinium uptake, patchy uptake, or mid-wall hyperenhancement were classified as having non-ischaemic cardiomyopathy. The study, which conforms to the Declaration of Helsinki, was approved by the local Ethics Committee.
Study design
Patients underwent pacemaker implantation during an elective admission or after stabilization during the course of an unplanned admission for acute decompensated heart failure. Patients underwent a clinical assessment on the day prior to implantation and at 1, 3, and every 6 months following pacemaker implantation. Clinical follow-up data relates to the last available follow-up before death or the end of the study period.
Clinical assessment
This included assessment of NYHA functional class, quality of life (Minnesota Living with Heart Failure questionnaire),14 and a 6 min hall-walk test.15 Response to CRT was defined as survival for 1 year without heart failure hospitalizations, plus improvement by ≥1 NYHA classes or by ≥25% in 6 min walking distance.
Echocardiography
Two-dimensional echocardiography was performed at baseline using GE Systems 5 and 7 scanners with EchoPAC (General Electric Worldwide, Slough, UK). Planimetry of apical four-chamber views and Simpson's equation were used to derive LV volumes. The frame at the beginning of the QRS complex was used to estimate end-diastolic volume, whereas the frame with the smallest ventricular area just prior to mitral valve opening was used to estimate end-systolic volume.
Device therapy
Patients underwent transvenous biventricular pacemaker implantation using standard techniques under local anaesthesia. In line with national guidelines at the time of the study, no patient underwent implantation of a CRT device with implantable cardioverter defibrillator back-up. Patients in sinus rhythm underwent transmitral Doppler-directed optimization of atrioventricular delay16 prior to discharge and at every scheduled visit thereafter. In view that V-V optimization had not yet gained credence in clinical practice, we followed an empirical protocol for patients who did not respond symptomatically to CRT. Four symptomatic non-responders were programmed to a V-V delay of 30 ms (LV first). If no symptomatic response was witnessed after 6 weeks, programming was changed to LV pacing only. The pacing mode was set to DDD, with an interventricular delay of 0–4 ms, depending on the manufacturer. Back-up atrial pacing was set at 60 b.p.m. For patients in atrial fibrillation, the ventricular-triggered mode was programmed. All patients in the study were programmed to a minimum rate of 60 b.p.m. Patients were entered into the study only after a successful implantation and were followed up in a dedicated CRT clinic.
Endpoints
The clinical endpoints considered were the composite of cardiovascular mortality or an unplanned hospitalization for worsening heart failure. The first event was included in the analysis. The second endpoint considered was cardiovascular mortality, and the third, total mortality. Cardiac transplantation was considered as a cardiovascular death. Mortality data were collected through medical records, and where appropriate, from interviews with patient's caregivers. Information regarding clinical outcome was collected by an investigator who was blinded to all other study data.
Biochemical assays
Venous blood samples were collected at baseline and 360 days (median) after implantation, separated by cool centrifugation and stored at −70°C for analysis at the end of the follow-up period. Analytes were measured in two batches, one for GDF-15 and one for NT pro-BNP. The investigators were unaware of the results during the study.
Growth differentiation factor-15
The GDF-15 assay was constructed using antibodies (R&D Systems, Abingdon, Oxfordshire, UK) which have been utilized by other groups.11,17 Mouse monoclonal antibodies (200 ng/100 µL) specific for GDF-15 were coated on ELISA plates overnight at room temperature. The plates were then blocked overnight using 10% foetal calf serum. The following day, 10 µL plasma samples or standards were pipetted into the wells with 100 µL of immunoassay buffer. Plates were incubated overnight, and following washes the next day, 5 ng of biotinylated goat antibody specific for GDF-15 in 100 µL of assay buffer was pipetted into each well. Plates were incubated at room temperature on a plate shaker for 2 h and then bound biotinylated tracer antibody was detected using methyl-acridinium ester-labelled streptavidin on a Dynex MLX plate reader, with sequential injections of hydrogen peroxide in nitric acid, and sodium hydroxide with cetyl-trimethyl ammonium bromide, 4 s apart, as described previously.18 The light signal was integrated over 1 s. The lower limit of detection was 2.55 pg/mL.
N-terminal pro-B-type natriuretic peptide
Analysis for NT pro-BNP was undertaken using a non-competitive electro-chemiluminescence using the Elecsys 1010 analyzer (Roche Diagnostics, Lewes, UK).
Statistical analysis
Continuous variables are expressed as mean ± SD. Normality was tested using the Shapiro–Wilk test. Variables which were not normally distributed (GDF-15 and NT pro-BNP) were log-transformed for statistical analyses. Comparisons between normally distributed continuous variables were made using ANOVA with Scheffe's F procedure for multiple comparisons. Categorical variables were analysed using χ2 tests and Scheffe's post hoc test. Changes in variables from baseline to follow-up were analysed using paired t tests. Statistical analyses were undertaken using the SPSS 13.0 (Chicago, IL, USA), JMP 5.1 (Cary, NC, USA), and MedCalc (Mariakerke, Belgium) programs. The Harrel ‘Design’ package version 2.1-1 in R19 was used for bootstrapping and validation.
Sample size
At our centre, we had observed an annual cardiovascular mortality rate of 7% in 240 patients undergoing CRT. An arbitrary value of 2.0 was adopted as a clinically meaningful hazard ratio (HR) for GDF-15 as a predictor of cardiovascular mortality after CRT. A Cox regression of the log HR on a covariate with an SD of 1.40 times the mean, based on a sample of 130, achieved 90% power at a 0.05 significance level to detect a regression coefficient of 0.6931 (HR, 2.0), at an anticipated annual event rate of 7%. An additional 30 patients were added to this sample to allow for implantation failures and patients lost at follow-up. Power calculations were undertaken using the PASS 2008 package (NCSS, Kaysville, UT, USA).
Development and validation of the prediction model
According to Harrell et al.'s20,21 recommendations on multivariate prognostic modelling, no more than m/10 parameters were considered, where m is the number of uncensored events, in this case cardiovascular deaths (n = 40). In accordance with Harrell et al.'s20,21 recommendations on multivariable prognostic modelling, variables were selected on the basis of their proven clinical, pathophysiological, and epidemiological relevance to the endpoint in question. As the purpose of this study was to explore whether GDF-15, alone or in combination with NT pro-BNP, predicts mortality in the study cohort, these variables were included in the analyses. Two more variables, namely NYHA class and LVEF, were also chosen on the basis of evidence showing inverse correlations between both NYHA class and LVEF, and mortality in patients with heart failure.22 Linearity assumptions were checked using Martingale residuals. The proportional hazards assumption was checked using Schoenfeld23 residuals. Validation of the Cox proportional hazards models was implemented using bootstrapping.20,21,24 For each group of the bootstrap samples, the model was refitted and tested against the observed sample in order to derive an estimate of the predictive accuracy and bias. The following parameters were derived: (i) the β coefficients from Cox proportional hazards models; (ii) Somer's D rank correlation index, and (iii) an estimate of the slope shrinkage.20 The apparent Somer's D (Dapp) was derived using stepwise Cox proportional analyses. The bootstrap-corrected performance of the predictor equation, or ‘optimism’,21 was quantified by assessing the difference between Somer's D in the original sample (Dorig) and D in the bootstrap sample (Dboot). The average optimism, termed O, was derived by repeating the above steps 500 times over. The bootstrap-corrected performance of the original stepwise model, Dapp− O, is, effectively, an honest estimate of internal validity.21
Kaplan–Meier survival curves were constructed for GDF-15 and NT pro-BNP and statistical significance was tested using the log-rank test. Cut-off values for these variables were those which, on receiver-operator characteristic curves, were found to have the highest product of the sensitivity and specificity in relation to the endpoint of cardiovascular mortality at the end of the study. The estimate of the confidence interval (CI) for the cut-off was calculated using the sensitivity and specificity estimates. We applied error propagation assuming statistical independency of specificity and sensitivity. Local slopes d(cut-off)/d(sensitivity) and d(cut-off)/d(specificity) around the optimal cut-off were obtained by linear regression. Slopes obtained were multiplied by the CI for sensitivity and specificity, respectively. To determine the predictive accuracy of GDF-15 and NT pro-BNP over the follow-up period, we adopted the approach suggested by Heagerty and Zheng.25 This involved calculation of the AUCs and the associated 90% CI as a measure of predictive accuracy at 1, 2, and 3 years for each endpoint and for each biomarker. Linear predictions were based on a Cox proportional hazards model.
Calculation of the combined biomarker index
The combined biomarker index was expressed as the sum of the products of the variables and their β coefficients, or weights, identified in the final stepwise multivariable Cox proportional hazards model:20,21 |β1x1 + β2x2 + ··· + βnxn|, where β1, β2,… ,βn are the β coefficients for each variable and x1, x2,… ,xn are the absolute values for each of the variables. Thus, the combined biomarker index using the β coefficients from the final bootstrapped Cox proportional hazards model was calculated as follows: [0.7533 × NT pro-BNP (pg/mL] + [(1.3257 × GDF-15 (ng/L)].
Results
The characteristics of the study group are shown in Table 1. As shown in Table 2, significant improvements in NYHA class, 6 min walking distance, and quality-of-life scores were observed (P < 0.0001). Response to CRT, defined as survival for 1 year without hospitalizations for heart failure plus improvement by ≥1 NYHA classes or a 25% in 6 min walking distance, was 72%.
Table 1.
Characteristics of the study group
| All | |
|---|---|
| N | 158 |
| Age (years) | 68.3 ± 10.7 |
| Male gender, n (%) | 131 (83) |
| NYHA class | 3.3 ± 0.4 |
| Ischaemic cardiomyopathy, n (%) | 114 (72) |
| Atrial rhythm, n (%) | |
| Sinus rhythm | 122 (77) |
| Atrial fibrillation | 36 (23) |
| Co-morbidity, n (%) | |
| Diabetes mellitus | 28 (18) |
| Hypertension | 43 (27) |
| CABG | 34 (22) |
| NT pro-BNP (pg/mL) | 3476.8 ± 4098.6 |
| GDF-15 (ng/L) | 3838.7 ± 4081.9 |
| Medication, n (%) | |
| Loop diuretics | 144 (91) |
| ACE-inhibitors or ARBs | 147 (93) |
| Beta-blockers | 93 (59) |
| Spironolactone | 73 (46) |
| ECG and echocardiography | |
| QRS duration (ms) | 153.9 ± 28.2 |
| LVEF (%) | 23.1 ± 9.8 |
Continuous variables are expressed as mean ± SD. NYHA, New York Heart Association class; CABG, coronary artery bypass grafting; ACE, angiotensin-converting enzyme; ARBs, angiotensin II receptor blockers.
Table 2.
Clinical variables at baseline and follow-up
| P-value | ||
|---|---|---|
| NYHA class | ||
| Baseline | 3.3 (0.4) | |
| Follow-up | 2.1 (0.9) | <0.0001 |
| 6 min walk test (m) | ||
| Baseline | 236.6 (115.2) | |
| Follow-up | 312.0 (120.1) | <0.0001 |
| Quality-of-life scores | ||
| Baseline | 53.5 (19.9) | |
| Follow-up | 34.2 (23.6) | <0.0001 |
| Responders, n (%) | 72% | |
P-values refer changes from baseline.
Growth differentiation factor-15 and survival
After a maximum follow-up period of 1958 days (median 950 days), 40 (25%) patients died from cardiovascular causes (including 1 heart transplantation) and 11 from non-cardiovascular causes. A total of 52 (33%) patients reached the composite endpoint of cardiovascular mortality or heart failure hospitalization.
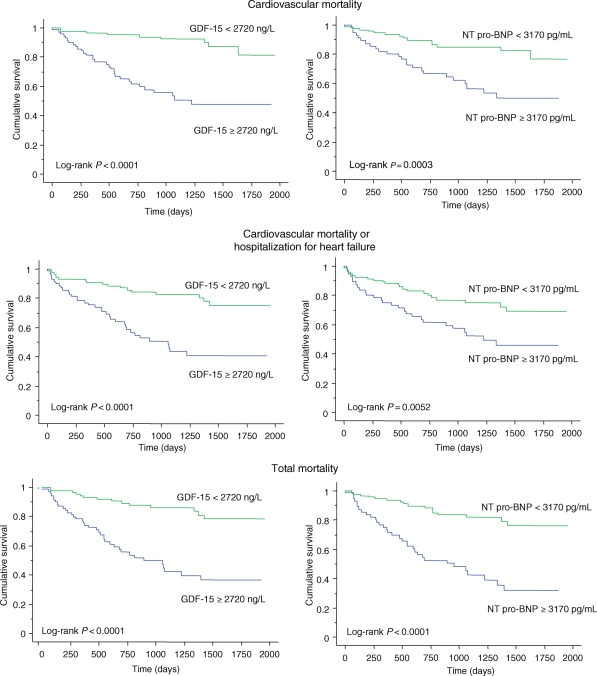
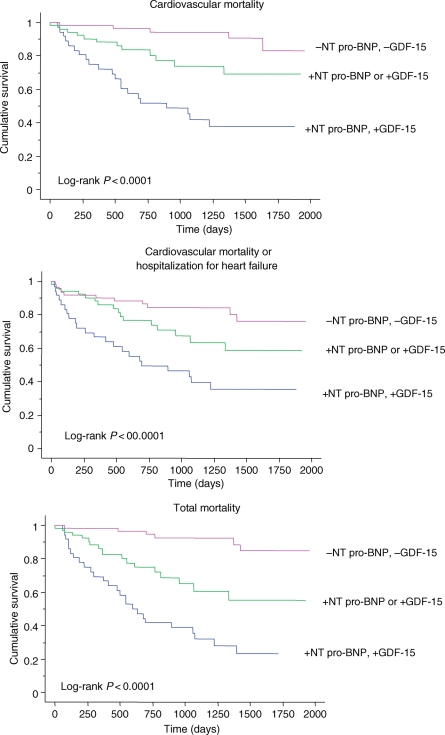
The utility of NT pro-BNP and GDF-15 in predicting the various endpoints was assessed using Kaplan–Meier analyses. As shown in Figure 1, a GDF-15 ≥2720 ng/L (95% CI: 1983 and 3545 ng/L) was associated with a higher risk of cardiovascular mortality, cardiovascular mortality or hospitalization for heart failure, and total mortality (all P < 0.0001). The ability of this cut-off of GDF-15 to discriminate between the risk groups was comparable to NT pro-BNP at a cut-off of 3170 pg/mL (95% CI: 1596 and 6763 pg/mL). Combining GDF-15 and NT pro-BNP resulted in a significant split between the risk groups with respect to the cardiovascular mortality, total mortality, and the composite endpoint of cardiovascular mortality or hospitalizations for heart failure (Figure 2). Analysis of the AUCs over 1, 2, and 3 years for each endpoint and for GDF-15, NT pro-BNP, and the combined biomarker score revealed that their predictive accuracy remained stable over time. Cox proportional models predicted outcome up to 3 years accurately and in a stable fashion (data not shown).
Figure 1.
Kaplan–Meier survival curves for elevations in growth differentiation factor-15 and N-terminal pro-B-type natriuretic peptide in relation to outcome.
Figure 2.
Kaplan–Meier survival curves for elevations in growth differentiation factor-15 and N-terminal pro-B-type natriuretic peptide in relation to outcome, using dichotomous variables.
Derivation and validation of predictive risk model
The objective in predictive modelling was to determine whether GDF-15, in isolation or in combination with NT pro-BNP, predicted cardiovascular mortality in the whole cohort. A stepwise Cox proportional hazards model with bootstrapping was used to assess the ability of biomarkers to reliably predict cardiovascular mortality. In a stepwise model adopting log GDF-15, log NT pro-BNP, LVEF, and NYHA class as independent variables, only log GDF-15 [HR, 3.76; coefficient, 1.33; standard error (SE), 0.47; z-score, 2.80; P = 0.0049] and log NT pro-BNP (HR, 2.12; coefficient, 0.75; SE, 0.32; z-score, 2.38; P = 0.0171) remained in the final model. In this model, the bias-corrected slope was 0.85, the optimism (O) was −0.06, and the c-statistic was 0.74.
Univariate analyses
Univariate Cox proportional hazards analyses were undertaken in order to compare NT pro-BNP and GDF-15 to other variables22,26 which are known to be of value in predicting mortality in patients with heart failure (Table 3). In univariate Cox proportional hazards analyses adopting cardiovascular mortality as the dependent variable, log GDF-15 was associated with a HR of 5.31 (95% CI, 2.31–11.9; LR χ2 = 14.6; P < 0.0001), compared with a HR of 2.79 (95% CI, 1.55–5.26; LR χ2 = 10.4; P = 0.0004) for log NT pro-BNP and a HR of 7.03 (95% CI, 2.91–17.5; LR χ2 = 19.1; P < 0.0001) for the combined biomarker index. Importantly, the latter was associated with the highest LR χ2.
Table 3.
Univariate Cox proportional hazards analyses of candidate predictors of cardiovascular mortality in patients with heart failure undergoing cardiac resynchronization therapy
| Univariate |
|||
|---|---|---|---|
| HR (95% CI) | LR χ2 | P-value | |
| Age | 1.03 (1.00–1.06) | 2.48 | 0.0914 |
| Female gender | 0.24 (0.06–0.99) | 3.9 | 0.0483 |
| Ischaemic aetiology | 1.56 (0.72–3.40) | 1.28 | 0.2580 |
| NYHA class | 2.79 (1.49–5.23) | 10.4 | 0.0013 |
| Creatinine | 1.01 (1.00–1.02) | 4.15 | 0.0417 |
| Log NT pro-BNP | 2.79 (1.55–5.26) | 12.4 | 0.0004 |
| Log GDF-15 | 5.31 (2.31–11.9) | 14.6 | 0.0001 |
| Log combined biomarker index | 7.03 (2.91–17.5) | 19.1 | <0.00001 |
| QRS duration | 1.00 (0.99–1.01) | 0.25 | 0.6181 |
| Atrial fibrillation | 0.99 (0.47–2.08) | 0.00 | 0.9752 |
| LVEF | 0.96 (0.93–0.99) | 5.51 | 0.0189 |
| Medication | |||
| Diuretic | 0.73 (0.22–2.38) | 0.28 | 0.6146 |
| ACE-I or ARA | 1.34 (0.47–3.76) | 0.30 | 0.5848 |
| Beta-blockers | 1.77 (0.94–3.35) | 3.17 | 0.0751 |
| Spironolactone | 0.98 (0.52–1.83) | 0.01 | 0.9368 |
LR χ2, likelihood ratio chi-squared.
In univariate Cox proportional hazards analyses adopting cardiovascular mortality or heart failure hospitalizations as the dependent variable, log GDF-15 was associated with a HR of 3.77 (95% CI, 1.75–7.90; LR χ2 = 11.0; P = 0.0009), compared with a HR of 2.40 (95% CI, 1.46–4.08; LR χ2 = 12.5; P = 0.0004) for NT pro-BNP and a HR of 4.93 (95% CI, 2.27–10.9; LR χ2 = 16.3; P = 0.0001) for the combined biomarker index. Again, the combined biomarker score was associated with the highest LR χ2.
In univariate Cox proportional hazards analyses adopting total mortality as the dependent variable, log GDF-15 was associated with a HR of 5.59 (95% CI, 2.69–11.4; LR χ2 = 19.9; P < 0.0001), compared with a HR of 4.30 (95% CI, 2.43–7.97; LR χ2 = 28.2; P < 0.0001) for NT pro-BNP and a HR of 10.5 (95% CI, 4.74–24.1; LR χ2 = 34.3; P < 0.0001) for the combined biomarker index. The combined biomarker score was therefore associated with the highest LR χ2 for total mortality.
Two patients derived a symptomatic benefit from a V-V delay of 30 ms (LV first) and one patient derived a symptomatic benefit from LV pacing only. Two patients derived a symptomatic benefit from a V-V delay of 30 ms (LV first) and one patient derived a symptomatic benefit from LV pacing only. Exclusion of these patients in the analyses made no difference in the relationship between GDF-15 or NT pro-BNP and the various mortality/morbidity endpoints.
Growth differentiation factor-15 and N-terminal pro-B-type natriuretic peptide changes after cardiac resynchronization therapy
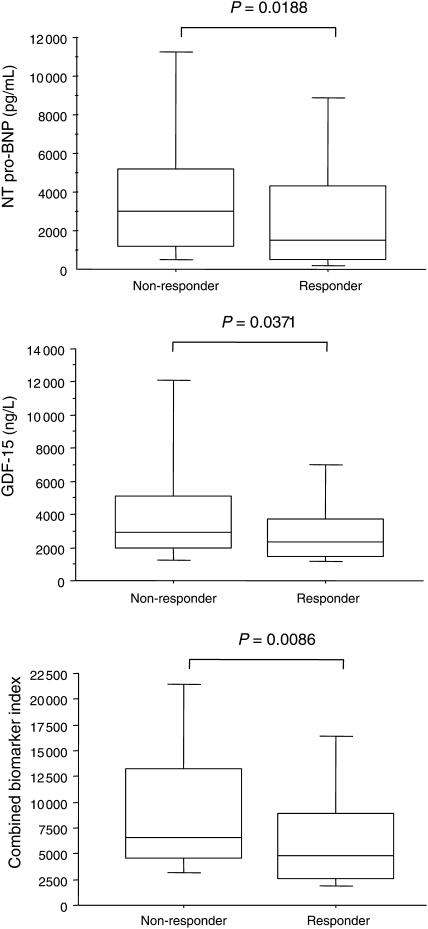
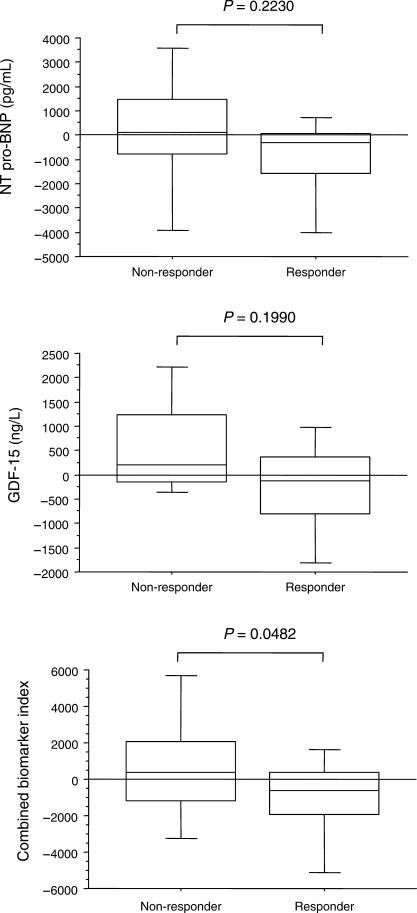
As shown in Figure 3, pre-implant NT pro-BNP and GDF-15 levels, in isolation and together as a combined biomarker index, were higher in non-responders. With respect to changes from baseline, only the combination, reflected in the combined biomarker index, showed a significant reduction (Figure 4).
Figure 3.
Box-and-whisker plot for pre-implant growth differentiation factor-15 and N-terminal pro-B-type natriuretic peptide in relation to response or non-response to cardiac resynchronization therapy. The five horizontal lines represent the 10th, 25th, 50th, 75th, and 90th percentiles, from bottom to top. For calculation of the combined biomarker index, see text.
Figure 4.
Box-and-whisker plot for changes from baseline in growth differentiation factor-15 and N-terminal pro-B-type natriuretic peptide in relation to response or non-response to cardiac resynchronization therapy. The five horizontal lines represent the 10th, 25th, 50th, 75th, and 90th percentiles, from bottom to top. For calculation of the combined biomarker index, see text.
Discussion
As a member of the TGF-β cytokine superfamily,6,7 GDF-15 has a number of effects on cardiomyocyte biology. Recent evidence suggests that it has autocrine and endocrine functions, such as antagonizing the hypertrophic response and loss of ventricular performance.27 Although GDF-15 is not normally expressed in the healthy adult myocardium, a dramatic induction of expression occurs following injury, hypoxia, and cytokine/growth factor stimulation.28 Accordingly, high circulating levels of GDF-15 are observed in conditions such as heart failure. Amidst the current interest in risk-stratifying patients with heart failure undergoing CRT, we have shown that pre-implant levels of GDF-15 are a strong predictor of long-term mortality following CRT device implantation, independent of NT pro-BNP and other factors that are known to be of prognostic value in patients with heart failure. Furthermore, we have shown that the combination of these analytes provides a better risk stratification than each analyte in isolation. Reductions in a combined biomarker index were also witnessed in responders to CRT.
In the present study of patients undergoing CRT, GDF-15 was a strong independent predictor of total mortality. These findings are consistent with those of Kempf et al.11 in patients with heart failure treated pharmacologically. In the present study, GDF-15 also emerged as a powerful predictor of cardiovascular mortality and the composite endpoint of cardiovascular mortality or hospitalizations for heart failure. Furthermore, it was comparable or superior to NT pro-BNP with respect to some of the endpoints. With regard to cardiovascular mortality, for example, the LR χ2 from univariate Cox proportional hazards models were 14.6 for GDF-15 and 10.4 for NT pro-BNP. The LR χ2 for GDF-15 was also higher than that for NT pro-BNP with respect to the composite endpoint of cardiovascular mortality or heart failure hospitalizations or the endpoint of total mortality.
It is apparent from our analyses that combined biomarker index is superior to either GDF-15 or NT pro-BNP alone in risk stratification. In this respect, the LR χ2 associated with the combined biomarker index as was as high as 19.1 for cardiovascular mortality, 16.3 for the combined endpoint of cardiovascular mortality or heart failure hospitalizations, and 34.3 for total mortality. This indicates that the combination of pre-implant GDF-15 and NT pro-BNP is particularly useful in defining a high-risk group of patients after CRT.
We have found that changes in either GDF-15 or NT pro-BNP after CRT were not predictive of clinical response. This might be expected from the very high biological variability of these analytes. In a study of 43 patients with stable heart failure assessed within 1 week, on consecutive days, and at weekly intervals over a 6-week period, serial change values of 32%, 74%, and 113% for within-day, day-to-day, and week-to-week sampling were observed.29 In healthy individuals, percentage serial change values of up to 92% for NT pro-BNP have been described.30 Such high biological variability is likely to hamper the use of changes in NT pro-BNP for guiding clinical management. Although the day-to-day variation of GDF-15 has not been explored, the case is likely to be similar to that of NT pro-BNP, thus limiting its use in isolation as a predictor of clinical benefit. Importantly, however, the combined biomarker index was higher in non-responders.
Limitations of the study
This study has several limitations. First, biomarkers were assessed at baseline and at a median of 360 days after CRT. The time required for CRT to have its full therapeutic effect has not been ascertained and therefore, it is possible that a longer time period is required for biomarkers to decrease following implantation. Secondly, the temporal variability of plasma levels of GDF-15 has not been established, either in this study population or in patients with heart failure who are not treated with CRT. Thirdly, this study was undertaken in patients undergoing CRT without defibrillator back-up. Our findings may not apply to patients undergoing CRT with defibrillator back-up. Although we have established that GDF-15 is a good prognostic biomarker in patients with advanced heart failure undergoing CRT, it does not appear to be a good biomarker for identifying symptomatic responders to this therapy. Its clinical application is therefore limited. To be confident on the accuracy of the predictive model described herein, external validation is required.
Conclusions
Pre-implant GDF-15 levels are strong predictors of poor long-term outcome after CRT, independent of NT pro-BNP, QRS duration, and LVEF. In terms of relative risk, combined elevations in GDF-15 and NT pro-BNP carry a particularly high risk of mortality and morbidity after CRT. At a time when pre-implant echocardiography has failed to prove useful in risk-stratifying patients undergoing CRT,4 further studies should focus on the possible value of pre-implant GDF-15 levels, alone or in combination with NT pro-BNP, in tailoring device therapy.
Funding
Funding to pay the Open Access publication charges for this article was provided by the Cardiovascular Research Fund, Heart of England NHS Trust.
Conflict of interest: none declared.
Acknowledgements
We are grateful to Medtronic Inc. and St Jude Medical for their continued support. We are also grateful to Lisa Ball, Janet Brashaw-Smith, and Nick Irwin for their input in the follow-up of patients included in this study.
References
- 1.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass D, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM for the Comparison of Medical Therapy, Pacing and Defibrillation in Heart Failure (COMPANION) Investigators. Cardiac resynchronization therapy with or without an implantable defibrillator in advanced heart failure. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 2.Cleland JGF, Daubert J-C, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L for the Cardiac Resynchronization-Heart Failure (CARE-HF) study investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 3.Bax JJ, Abraham T, Barold SS, Breithardt OA, Fung JWH, Garrigue S, Gorcsan J, III, Hayes DL, Kass DA, Knuuti J, Leclercq C, Linde C, Mark DB, Monaghan MJ, Nihoyannopoulos P, Schalij MJ, Stellbrink C, Yu C-M. Cardiac resynchronization therapy: part 1—issues before device implantation. J Am Coll Cardiol. 2005;46:2153–2167. doi: 10.1016/j.jacc.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 4.Chung E, Leon A, Tavazzi L, Sun J, Nihoyannopoulos P, Merlino J, Abraham W, Guio S, Leclerq C, Bax J, Yu C-M, Gorcsan J, III, Sutton M, De Sutter J, Murillo J. Results of the Predictors of Response to CRT (PROSPECT) Trial. Circulation. 2008;117:2608–2616. doi: 10.1161/CIRCULATIONAHA.107.743120. [DOI] [PubMed] [Google Scholar]
- 5.Maisel A. Biomarkers in heart failure. Does prognostic utility translate to clinical futility? J Am Coll Cardiol. 2007;50:1061–1063. doi: 10.1016/j.jacc.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 6.Bootcov M, Basuskin A, Valenzuela S, Moore A, Bansal M, He X, Zhang H, Donnellan M, Mahler S, Pryor K, Walsh B, Nicholson R, Fairlie W, Por S, Robbins J, Breit S. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci USA. 1997;94:11514–11519. doi: 10.1073/pnas.94.21.11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bottner M, Laaff M, Schechinger B, Rappold G, Unsicker K, Suter-Crazzolara C. Characterization of the rat, mouse, and human genes of growth/differentiation factor-15/macrophage inhibiting cytokine-1 (GDF-15/MIC-1) Gene. 1999;237:105–111. doi: 10.1016/s0378-1119(99)00309-1. [DOI] [PubMed] [Google Scholar]
- 8.Kempf T, Eden M, Strelau J, Naguib M, Willenbockel C, Tongers J, Heineke J, Kotlarz D, Xu J, Molkentin JD, Niessen HW, Drexler H, Wollert KC. The transforming growth factor-beta superfamily member growth-differentiation factor-15 protects the heart from ischemia/reperfusion injury. Circ Res. 2006;98:351–360. doi: 10.1161/01.RES.0000202805.73038.48. [DOI] [PubMed] [Google Scholar]
- 9.Blobe G, Schiemann W, Lodish H. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 10.Wollert KC, Kempf T, Lagerqvist B, Lindahl B, Olofsson S, Allhoff T, Peter T, Siegbahn A, Venge P, Drexler H, Wallentin L. Growth differentiation factor 15 for risk stratification and selection of an invasive treatment strategy in non ST-elevation acute coronary syndrome. Circulation. 2007;116:1540–1548. doi: 10.1161/CIRCULATIONAHA.107.697714. [DOI] [PubMed] [Google Scholar]
- 11.Kempf T, von Haehling S, Peter T, Allhoff T, Cicoira M, Doehner W, Ponikowski P, Filippatos G, Rozentryt P, Drexler H, Anker S, Wollert K. Prognostic utility of growth-differentiation factor-15 in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:1054–1060. doi: 10.1016/j.jacc.2007.04.091. [DOI] [PubMed] [Google Scholar]
- 12.Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined—a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36:959–969. doi: 10.1016/s0735-1097(00)00804-4. [DOI] [PubMed] [Google Scholar]
- 13.Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, Khan M, Sheppard MN, Poole-Wilson PA, Pennell DJ. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006;48:1977–1985. doi: 10.1016/j.jacc.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 14.Rector TS, Kubo SH, Cohn JN. Patient's self-assessment of their congestive heart failure. Content, reliability and validity of a new measure—The Minnesota living with heart failure questionnaire. Heart Failure. 1987;3:198–207. [Google Scholar]
- 15.Guyatt GH, Sullivan MJ, Thompson PJ. The 6 min walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132:919–923. [PMC free article] [PubMed] [Google Scholar]
- 16.Ritter P, Padeletti L, Gillio-Meina L, Gaggini G. Determination of the optimal atrioventricular delay in DDD pacing: comparison between echo and peak endocardial acceleration measurements. Europace. 1999;1:126–130. doi: 10.1053/eupc.1998.0032. [DOI] [PubMed] [Google Scholar]
- 17.Wollert KC, Kempf T, Peter T, Olofsson S, James S, Johnston N, Lindahl B, Horn-Wichmann R, Brabant G, Simoons ML, Armstrong PW, Califf RM, Drexler H, Wallentin L. Prognostic value of growth-differentiation factor-15 in patients with non-ST-elevation acute coronary syndrome. Circulation. 2007;115:962–971. doi: 10.1161/CIRCULATIONAHA.106.650846. [DOI] [PubMed] [Google Scholar]
- 18.Ng L, O'Brien R, Demme B, Jennings S. Non-competitive immunochemiluminometric assay for cardiotrophin-1 detects elevated plasma levels in human heart failure. 2002;102:411–416. [PubMed] [Google Scholar]
- 19.Harrell FJ. The Design Package. 2007. [Google Scholar]
- 20.Harrell FJ, Lee K, Califf R, Pryor D, Rosati R. Regression modelling strategies for improved prognostic prediction. Stat Med. 1984;3:143–152. doi: 10.1002/sim.4780030207. [DOI] [PubMed] [Google Scholar]
- 21.Harrell FJ, Lee K, Mark D. Multivariate prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 22.Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton P, Sullivan MD, Pitt B, Poole-Wilson PA, Mann DL, Packer M. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006;113:1424–1433. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 23.Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69:239–241. [Google Scholar]
- 24.Efron B. Bootstrap method: another look at the jacknife. Ann Stat. 1979;7:1–26. [Google Scholar]
- 25.Heagerty P, Zheng Y. Survival model predictive accuracy and ROC curves. UW Biostatistics Working Paper Series. 2003 doi: 10.1111/j.0006-341X.2005.030814.x. Working Paper 219. [DOI] [PubMed] [Google Scholar]
- 26.Aaronson KD, Schwartz JS, Chen TM, Wong KL, Goin JE, Mancini DM. Development and prospective validation of a clinical index to predict survival in ambulatory patients referred for cardiac transplant evaluation. Circulation. 1997;95:2660–2667. doi: 10.1161/01.cir.95.12.2660. [DOI] [PubMed] [Google Scholar]
- 27.Xu J, Kimball TR, Lorenz JN, Brown DA, Bauskin AR, Klevitsky R, Hewett TE, Breit SN, Molkentin JD. GDF15/MIC-1 functions as a protective and antihypertrophic factor released from the myocardium in association with SMAD protein activation. Circ Res. 2006;98:342–350. doi: 10.1161/01.RES.0000202804.84885.d0. [DOI] [PubMed] [Google Scholar]
- 28.Azhar M, Schultz Jel J, Grupp I, Dorn GW, 2nd, Meneton P, Molin DG, Gittenberger-de Groot AC, Doetschman T. Transforming growth factor beta in cardiovascular development and function. Cytokine Growth Factor Rev. 2003;14:391–407. doi: 10.1016/s1359-6101(03)00044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bruins S, Fokkema MR, Romer JW, Dejongste MJ, van der Dijs FP, van den Ouweland JM, Muskiet FA. High intraindividual variation of B-type natriuretic peptide (BNP) and amino-terminal proBNP in patients with stable chronic heart failure. Clinical chemistry. 2004;50:2052–2058. doi: 10.1373/clinchem.2004.038752. [DOI] [PubMed] [Google Scholar]
- 30.Wu AH, Smith A, Wieczorek S, Mather JF, Duncan B, White CM, McGill C, Katten D, Heller G. Biological variation for N-terminal pro- and B-type natriuretic peptides and implications for therapeutic monitoring of patients with congestive heart failure. Am J Cardiol. 2003;92:628–631. doi: 10.1016/s0002-9149(03)00741-0. [DOI] [PubMed] [Google Scholar]