Abstract
Low urinary citrate excretion is a known risk factor for the development of kidney stones. Citrate inhibits stone formation by complexing with calcium in the urine, inhibiting spontaneous nucleation, and preventing growth and agglomeration of crystals. Hypocitraturia is a common metabolic abnormality found in 20% to 60% of stone formers. It is most commonly idiopathic in origin but may be caused by distal renal tubular acidosis, hypokalemia, bowel dysfunction, and a high-protein, low-alkali diet. Genetic factors, medications, and other comorbid disorders also play a role. Hypocitraturia should be managed through a combination of dietary modifications, oral alkali, and possibly lemonade or other citrus juice-based therapy. This review concerns the pathophysiology of hypocitraturia and the management of stone formers afflicted with this abnormality.
Key words: Hypocitraturia, Citrate metabolism, Citrate pathophysiology, Hypocitraturia etiologies, Medical management of stone disease, Alkali citrate, Potassium citrate, Citrus juice, Lemonade
Low urinary citrate excretion is a known risk factor for the development of kidney stones.1 Hypocitraturia, generally defined as urinary citrate excretion less than 320 mg (1.67 mmol) per day for adults,2 is a common metabolic abnormality in stone formers, occurring in 20% to 60%.1,3–6 Citrate is a known inhibitor of stone formation, working through a variety of mechanisms. In the renal tubule citrate complexes with calcium, increasing its solubility and reducing the concentration of free calcium in the urine. This calcium-citrate complex limits calcium supersaturation and prevents nucleation of both calcium oxalate and calcium phosphate, at least partly through interactions with Tamm-Horsfall protein.7,8 Additionally, citrate prevents crystal agglomeration and growth through its ability to bind to the crystal’s surface and may also prevent adhesion of calcium oxalate to renal epithelial cells.9–11 Hypocitraturia may be corrected with dietary modifications and the administration of citrate preparations or other forms of alkali therapy. Citrate excretion is linked to urinary pH and thus may influence the generation of a number of types of stones. Herein we review the pathophysiology of hypocitraturia and the management of stone formers with this abnormality.
Citrate Physiology
Overview
Citrate, a tricarboxylic acid, is synthesized in mitochondria from oxaloacetate and acetyl-CoA (coenzyme A) by the enzyme citrate synthase; it is a central component of the tricarboxylic acid (TCA) cycle. After first describing TCA cycle reactions, Krebs and colleagues12,13 demonstrated that citrate is excreted in urine. Through its metabolism in the TCA cycle, citrate generates a significant amount of energy through reduced nicotinamide-adenine dinucleotide, guanosine triphosphate, and reduced flavin-adenine dinucleotide, creating CO2 and H2O in this process. If all the citrate in the kidney were metabolized, it would provide 10% of the renal energy requirement.14
Renal Transport
In serum, the concentration of citrate is relatively constant, ranging from 0.05 mM to 0.3 mM.15 The majority of citrate exists complexed to divalent ions, such as calcium and magnesium, and is filtered freely at the glomerulus; reabsorption takes place predominantly in the proximal tubule.16 Because there is currently no evidence that significant citrate secretion occurs in the nephron, the extent of reabsorption largely regulates citrate excretion.16 In humans, 65% to 90% of the filtered load of citrate is reabsorbed.17,18
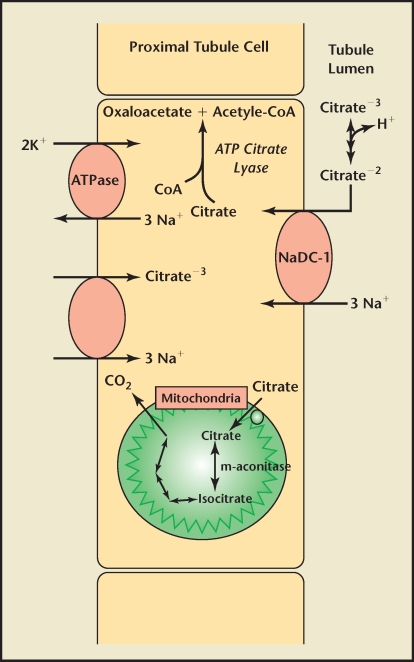
Transport of citrate across the apical membrane in the proximal tubule has been extensively studied. First cloned by Pajor,19 the dominant membrane protein is a sodium-dependent dicarboxylate transporter (NaDC-1) found not only in renal tubules, but also in the small intestine, colon, liver, and brain.18 The NaDC-1 transporter has broad specificity; it is also used by other TCA intermediates, including succinate and α-ketoglutarate (Figure 1).20,21
Figure 1.
Representation of proximal tubule citrate absorption and metabolism. CoA, coenzyme A; ATP, adenosine triphosphate.
Apical membrane reabsorption of citrate is an electrogenic process requiring secondary active transport that is highly pH dependent.18 The binding of 3 sodium ions induces a conformational change in the NaDC protein, which allows their cotransport with 1 uncomplexed, divalent citrate molecule (citrate−2).22 This movement of ions creates the net transfer of a positive charge into the cell, relying on a basolateral Na/K adenosine triphosphatase to maintain electrical neutrality. Whereas the Km for this reaction is approximately 15 µM/L, the Km for the transport of trivalent citrate (citrate−3) is far greater. Thus, more of the former is transported, whereas the latter acts as a competitive inhibitor.23 The pKa of citrate is 5.6; thus the majority of citrate exists as citrate−3 in the renal tubule.17 These properties reflect the important role that pH has in the regulation of citrate absorption in the nephron.
Once absorbed through the apical membrane of the proximal tubule, citrate does not pass through a basolateral transporter into the interstitium. In fact, there is some evidence that citrate moves in the opposite direction, though transport across this membrane is not well understood.24 Unlike the movement of citrate across the apical membrane, transport on the basolateral side into proximal tubular cells is electroneutral and not affected by changes in pH.25 This transport is also sodium dependent. Citrate is thought to be absorbed across the basolateral membrane and into proximal tubular cells in its trivalent form, reflecting its pH independence.25
Renal Citrate Metabolism
A portion of the citrate absorbed in the kidney will pass into the mitochondria for utilization in the TCA cycle.18 Adenosine triphosphate citrate lyase, however, reacts with some of the citrate while it remains in the cytoplasm.26 This enzyme catalyzes the conversion of citrate and CoA to oxaloacetate and acetyl-CoA. Acetyl-CoA produced through this pathway may be used in the synthesis of fatty acids and cholesterol and is also involved in other types of metabolism. Oxaloacetate is a substrate for gluconeogenesis, which is catalyzed by the enzyme phosphoenolpyruvate carboxykinase.26
Gastrointestinal Citrate Absorption
Information about the intestinal absorption of citrate is limited. It is thought that citrate absorption in the small intestine uses the same NaDC transporter as the proximal tubule.19,27,28 Such transporters have also been found in membranes within the intestines of several animal species.29–34 Clinically, there is strong evidence for intestinal absorption of citrate. Fegan and associates,35 using an intestinal washout technique, reported 96% to 98% absorption of an oral citrate load within 3 hours in both stone-forming and normal subjects. Others have demonstrated a significant increase in serum citrate after an oral citrate load.36 Patients with intestinal malabsorption syndromes tend to have low urinary citrate excretion,37 but this association is thought to be due to gastrointestinal bicarbonate wasting.38,39 In contrast to renal citrate transport, there is evidence for citrate efflux from intestinal enterocytes.32
pH Regulation of Citrate Excretion
Modulation of citrate excretion in the kidney is influenced by multiple factors; however, pH (systemic, tubular, and intracellular) has the strongest impact. It has long been known that acidosis decreases renal citrate excretion, whereas alkalosis increases it.40,41 There are several mechanisms through which pH exerts these effects. As noted previously, citrate is reabsorbed through the sodium citrate cotransporter as citrate−2 but exists predominately as citrate−3 within renal tubules. Lowering tubular pH increases the concentration of citrate−2 available for transport and reduces the concentration of citrate−3, thereby limiting its competitive inhibition. Even small decreases in tubular pH (7.4 to 7.2) significantly increase tubular reabsorption.42 Acute acidosis is associated with increased activity of the NaDC transporter,43 and chronic acidosis leads to increased transporter messenger ribonucleic acid and the transporter itself.44
In addition to increased capability to transport citrate in acidotic states, acidosis drives citrate metabolism. This is demonstrated by an increase in citrate reabsorption and decreased citrate tissue levels reported with acidosis.18 Intracellular acidosis has been shown to increase mitochondrial citrate transport and oxidation.18 Alkalosis, which produces the opposite effect, is thought to be modulated at least partly through pH regulation of the mitochondrial enzyme aconitase (m-aconitase).45 m-Aconitase is the enzyme responsible for the first step in citrate metabolism within the mitochondria. Alkali feeding decreases the activity and amount of this enzyme, thus inhibiting citrate metabolism; acidosis increases the activity of the enzyme.45 In addition to mitochondrial oxidation, intracellular acidosis increases citrate metabolism in the cytoplasm through increased activity of adenosine triphosphate citrate lyase.26 Products of both cytoplasmic and mitochondrial metabolism are further metabolized by phosphoenolpyruvate carboxykinase, whose activity is also increased with acidosis.46 Although these metabolic changes are also seen with reductions in systemic pH, hypokalemia produces similar effects, suggesting a strong intracellular component to pH regulation of citrate metabolism.18,26,45
Etiologies
Although the majority of patients have idiopathic hypocitraturia, there are a number of causes for this abnormality, which are reviewed here (Table 1).
Table 1.
Etiologies of Hypocitraturia
| Acid-base balance |
| Renal tubular acidosis |
| Other systemic acidosis |
| Diarrhea/malabsorption |
| Exercise |
| Hypokalemia |
| Diet |
| Dietary animal protein |
| High sodium intake |
| Ketosis promoting diets |
| Low fruit/vegetable intake |
| Starvation |
| Medications |
| ACE inhibitors |
| Acetazolamide |
| Amiloride |
| Calcitonin |
| Calcium |
| Ethacrynic acid |
| Lithium |
| Topiramate |
| Vitamin D |
| Genetic influence |
| VDR polymorphisms |
| NaDC-1 gene polymorphisms |
| Other associated disorders |
| Renal insufficiency |
| Hyperaldosteronism |
| Type I glycogen storage disease |
| Hypocalciuria, hypomagnesuria |
| Precursor compounds |
| Metabolic inhibitors |
ACE, angiotensin-converting enzyme; VDR, vitamin D receptor.
Renal Tubular Acidosis
Distal renal tubular acidosis (dRTA) significantly reduces citrate excretion, producing urinary citrate less than 100 mg/d in the complete form of the disease.47,48 Incomplete dRTA poses less risk for stone development49; however, it is still associated with hypocitraturia in the absence of systemic acidosis.50 Compared with complete dRTA, incomplete dRTA is not as clinically apparent and may be the pathology underlying many “idiopathic” hypocitraturic patients.51 This diagnosis has been made with acid load testing. However, this is not a clinically necessary test because the treatment is similar to that of idiopathic hypocitraturia. The systemic and intracellular acidosis produced in both these disorders leads to decreased citrate excretion. The systemic acidosis occurring in complete dRTA may also cause increased calcium excretion due to the release of calcium from bone and reduced reabsorption in the nephron. These patients have increased urine pH. This and the other aforementioned abnormalities place them at risk for calcium phosphate stone formation. Other forms of renal tubular acidosis are not associated with the same stone risks. Type II RTA is not associated with either increased stone formation or hypocitraturia,48 and type IV RTA may be protective against stone formation through decreased concentration of calcium and uric acid in the urine.52
Potassium
Low urinary potassium is associated with hypocitraturia.53 Hypokalemia produces both intracellular acidosis54,55 and a decrease in tubular pH.56 As described above, these changes are associated with increased citrate uptake and metabolism. Additionally, similar to acidosis, hypokalemia has been shown to stimulate the NaDC cotransporter.57
Gastrointestinal Disorders
Chronic diarrheal states and gastrointestinal malabsorption due to a variety of anatomic and functional bowel disorders are associated with hypocitraturia.37,49 A primary defect in citrate absorption has been suggested.58 However, the primary mechanism for this is thought to be gastrointestinal bicarbonate wasting resulting in acidosis and reduced citrate excretion.38,39,51,53
Diet
Diets high in animal protein provide an acid load. This promotes mild metabolic acidosis, leading to reduced citrate excretion, hypercalciuria, and a reduction in urine pH.38,59,60 Severely carbohydrate-restricted and animal protein-rich diets, such as the Atkins diet, further exacerbate this metabolic acidosis through the creation of ketones. Compared with a normal diet, both the induction and maintenance phases of an Atkinstype diet promote lower urine pH and citrate excretion.60 Other forms of dietary protein may also influence citrate excretion. For example, rats fed a high-casein diet have lower citrate and increased calcium excretion. The reduced citrate excretion in these animals is thought to be due to increased activity of the NaDC transporter induced by casein.61 Fruits, vegetables, and dietary fiber, often under-consumed in stone patients, provide a source of alkali with the potential to reverse the effects of protein consumption.38,51 When these foods are removed from the diets of normal patients, they have decreased urinary citrate excretion and higher saturations of calcium oxalate and calcium phosphate; opposite changes are seen with increased consumption of fruits and vegetables.62 High-sodium diets, possibly through a mild expansion acidosis, decrease urinary citrate.63 Finally, starvation has been shown to increase citrate absorption through an increase in NaDC transporters and systemic acidosis.64
Genetic Associations
Vitamin D receptor (VDR) gene polymorphisms are epidemiologically associated with recurrent and familial stone disease.65–67 In the nephron, 1,25(OH)2D3, the active form of vitamin D, utilizes the VDR to modulate citrate metabolism and transport.68 It also plays a role in the protein kinase pathway, altering the function of NaDC transporters.69 Recently, certain VDR gene polymorphisms, specifically the bb and TT subtypes, have been demonstrated with higher frequency in hypocitraturic stone formers when compared with both normocitraturic stone formers and normal controls.68 Certain VDR haplotypes are also associated with higher familial incidence of stone disease and lower mean age of onset.70
Shah and colleagues71 suggest further genetic influences on citrate handling. They propose a codominant inheritance of alleles at a single locus based on their trimodality frequency distribution for citrate excretion. This theory would account for the apparent “low,” “medium,” and “high” phenotypic expression found in their study, which is similar to what they found when investigating the genetics of calcium oxalate stone disease.72,73 There has also been recent identification of a single nucleotide polymorphism in the gene encoding NaDC-1, which may be associated with reduced urinary citrate excretion in recurrent stone formers.74 Ethnic background does not seem to play a role in citrate excretion.75
Other Factors
Many drugs are associated with altered citrate excretion. Acetazolamide, a carbonic anhydrase inhibitor, is known to inhibit citrate excretion, increase urine pH, and place patients at risk for developing calcium phosphate stones.76 These changes have been prevented in rat proximal tubules by controlling luminal pH and in other animals by reversing systemic acidosis.17,42 Topiramate, a commonly prescribed antiepileptic drug, is also a carbonic anhydrase inhibitor and associated with increased risk of developing calcium phosphate stones.77,78 It has recently been shown to have a dose-dependent effect on urinary citrate.79 Patients experienced an average 40% reduction in citrate at their starting dose and a 65% reduction at higher doses. Angiotensin-converting enzyme inhibitors are associated with decreased urinary citrate, thought to be promoted by increased adenosine triphosphate citrate lyase activity.80 Thiazide-induced hypokalemia leads to decreased citrate excretion, whereas lithium, through its inhibition of citrate transport, has been shown to increase urinary citrate.81,82 Other medications noted to alter renal citrate excretion include amiloride, calcitonin, and ethacrynic acid.83
Several other disorders are known to affect citrate handling. Chronic renal insufficiency produces a stepwise reduction in filtered citrate, with decreases in glomerular filtration rate; however, increased fractional excretion keeps urinary citrate stable until the development of severe renal dysfunction.84 Primary hyperaldosteronism, through a sodium-dependent volume expansion and chronic hypokalemia, is associated with both hypercalciuria and hypocitraturia.85 An association between type I glycogen storage disease and hypocitraturia has been reported.86 Paradoxically, there is a positive correlation between body mass index and urinary excretion of citrate, despite obesity being a risk factor for stone formation.87
Exercise has been shown to cause a mild reduction in urinary citrate, but dehydration increases free water re-absorption in the kidney, maintaining citrate concentration.88
Certain electrolytes and other organic acids alter citrate excretion. Increased urinary calcium and magnesium are both associated with increased excretion of citrate,17 and replacing magnesium in hypocitraturic patients has been shown to raise urinary citrate levels.89 Both of these ions complex with citrate in the urine and prevent its interaction with the NaDC transporter. Additionally, a deficiency in magnesium places patients at risk for hypokalemia and makes potassium replacement less effective. Finally, metabolic inhibitors (malonate and maleate), as well as precursor compounds (malate, succinate, and fumarate), increase excretion of citrate.18
Medical Management
Dietary modifications benefit the majority of patients with nephrolithiasis. These include high fluid and citrus fruit intake, normal calcium consumption, and restriction of sodium, oxalate, animal protein, and fructose intake.87,90 Increased consumption of fruits and vegetables has been demonstrated to significantly increase citrate excretion in hypocitraturic stone formers.62
The administration of citrate preparations or other alkali has been demonstrated to benefit hypocitraturic stone formers.91,92 Although many forms of citrate have been used for these patients (potassium citrate, sodium citrate, potassium-magnesium-citrate), potassium citrate has emerged as the most tolerable and beneficial.93,94 These citrate preparations raise urinary citrate by providing an alkali load. An advantage of the potassium preparations is that they may prevent or correct hypokalemia. They also increase urine pH, which benefits uric acid and cystine stone formers.48 Potassium citrate has also been demonstrated to prevent stone formation in patients with infection-related stones.95 It can be administered in conjunction with thiazide agents being used to reduce calcium excretion. Pak and associates81 demonstrated a reduction in stone formation from 4.69 to 0.57 stones per patient per year when potassium citrate was added to the medical regimen of thiazide-unresponsive patients. They also showed a significant increase in urinary citrate excretion and pH in these patients. Others have shown that the addition of either potassium citrate or potassium-magnesium-citrate was more beneficial than potassium chloride for stone prevention in thiazide-resistant patients.96,97 Potassium-magnesium-citrate has not yet been approved for use in the United States.
There have been 4 prospective randomized controlled trials aimed at preventing stone recurrence through the administration of citrate preparations. Treatment was for 3 years in 3 of these trials and for only 1 year for the other (Table 2).93,98–100 Two of these studies used potassium citrate, 1 used sodium-potassium-citrate, and 1 used magnesium-potassium-citrate. Citrate doses ranged from 60 to 90 mEq/d. Urinary citrate excretion on average increased 31% to 115% in the treatment groups, compared with no change in the control groups. In 3 of the 4 studies, significantly higher rates of stone remission were seen in the treatment groups (72%–100% treatment, 20%–71.4% control). However, Hofbauer and associates,99 using sodium-potassium-citrate, did not see a statistical difference in stone recurrence between the treatment and control groups. Barcelo and colleagues98 analyzed pre- and posttreatment procedure rates and found a significant 92% reduction in the treatment group, compared with a 20% reduction in the control group. In an intention-to-treat analysis of these 4 trials, Mattle and Hess101 reported that 53.5% of alkali citrate-treated patients remained stone free through at least 1 year of follow-up, compared with only 35% of control group patients.
Table 2.
Prospective, Randomized, Controlled Trials for the Treatment of Stone Recurrence With Alkali Citrate Therapy Lasting at Least 1 Year
| Study | Duration (mo) | Rx | n | Dropout Rate (%) | Remission Rate (%) | Average Increase in Citrate Excretion (%) |
| Barcelo et al.98 | 36 | K-Citrate | 18 | 36 | 72.2 | 77 |
| Control | 20 | 31 | 20 | 0 | ||
| Hofbauer et al.99 | 36 | Na-K-Citrate | 22 | 36 | 31* | 75 |
| Control | 16 | 12 | 27 | 0 | ||
| Ettinger et al.93 | ≦37 | K-Mg-Citrate | 31 | 48 | 87.1 | 31 |
| Control | 33 | 24 | 36.5 | 0 | ||
| Soygur et al.100 | 12 | K-Citrate | 28 | NS | 100 | 115 |
| Control | 28 | NS | 71.4 | NS |
No statistically significant difference.
NS, not stated.
Two prospective randomized trials lasting at least 1 year looked at rates of stone clearance after shock wave lithotripsy (Table 3). Soygur and colleagues100 used potassium citrate therapy in patients with lower-pole renal stones. Cicerello and associates95 administered sodium-potassium-citrate to subjects with stones in various locations in the kidney. Both groups found that significantly more patients in the treatment groups were stone free at 1 year of follow-up (44%–86% treatment, 12.5%–40% control). Additionally, they both found that a significantly higher percentage of control group patients had stone growth at 1 year. Less than 5% of the alkali citrate-treated patients, compared with up to 62.5% of the control patients, had an increase in the size of their residual stones on follow-up radiographic and ultrasound examinations. Interestingly, Cicerello and colleagues95 reported that neither the treatment nor control patients with infection-related stones showed any progression in stone burden. On intention-to-treat analysis, 66% of alkali citrate-treated patients became stone free at 1 year, compared with only 27.5% of control patients, a statistically significant difference.95
Table 3.
Prospective, Randomized, Controlled Trials for Elimination of Residual Calculi After Shock Wave Lithotripsy With Alkali Citrate Therapy Lasting at Least 1 Year
| Study | Rx | Duration (Months) | n | Drop Out Rate (%) | Stone Free (%) | Stone Size Increased (%) |
| Cicerello et al.95 | Na-K-Citrate | 12 | 35 | 3 | 75 (Ca-Ox)/86 (Inf) | 5 (Ca-Ox)/0 (Inf) |
| Control | 35 | 3 | 32 (Ca-Ox)/40 (Inf) | 47 (Ca-Ox)/0 (Inf) | ||
| Soygur et al.100 | K-Citrate | 12 | 18 | NS | 44.4 | 0 |
| Control | 16 | NS | 12.5 | 62.5 |
Ca-Ox, calcium oxalate stone formers; Inf, infection-related stone formers; NS, not stated.
Side effects of alkali citrate therapy are usually minimal and often gastrointestinally related. Among randomized controlled trial patients, approximately 33% of treated patients, compared with 17% of placebo-treated patients, reported side effects from the medication.101 Complaints included abdominal bloating, diarrhea, nausea, and abdominal pain. Potassium citrate is available in 3 formulations: tablets, crystals for dilution, and oral solution. Liquid preparations have been shown to produce more gastrointestinal side effects than tablets (33% vs 9.3%).91 Tablet potassium citrate, however, is not absorbed as completely as liquid preparations,35 and patients with chronic diarrheal syndromes typically have short gastrointestinal transit, which limits the effectiveness of potassium citrate tablets.102 Sodium-based alkali (sodium citrate, sodium bicarbonate) may need to be used in hypocitraturic patients who have diminished renal function or those who use potassium-sparing medications. This is mandated in those who are hyperkalemic.
The dose of alkali is based on the degree of hypocitraturia and acidosis, as well as on patient size (pediatric patients). Typical doses of potassium citrate for adults with idiopathic hypocitraturia and normal renal function range from 40 to 60 mEq per day. The dose for those with dRTA may need to be higher because these individuals have profound hypocitraturia and acidosis and are frequently hypokalemic.
Patients being administered alkali therapy warrant follow-up with blood and urine testing. Serum electrolytes should be checked, urine pH measured, and 24-hour urine metabolic testing obtained. Urine pH may increase to a level that places the patient at risk for developing calcium phosphate stones. The latter stone type is becoming more prevalent, and some have questioned whether this could be due to the widespread use of potassium citrate.103,104 Therefore, if the pH is 7 or greater, the dose of alkali may need to be decreased. An exception is those with dRTA who have high baseline urine pH. Adjustments of alkali dose in these patients are mainly based on serum electrolytes and 24-hour urine testing.
Patients who either cannot tolerate or cannot afford potassium citrate may benefit from consuming citrus juices, which contain significant amounts of citrate. Wabner and Pak105 compared orange juice consumption (1.2 L/d) with potassium citrate (60 mEq/d). Increases in urinary pH and citrate excretion were similar for both. Orange juice, however, did not decrease urinary saturation of calcium oxalate, whereas potassium citrate did. Grapefruit juice, although shown to have significantly higher levels of citrate than orange juice,106,107 does not seem to reduce urinary risk factors for stone formation.108 Additionally, grapefruit inhibits cytochrome p-450, thereby altering the metabolism of many commonly used medications.
Lemonade has been reported to increase citrate consumption. This was initially reported by Seltzer and associates,109 who had hypocitraturic patients consume 2 L of a lemonade preparation per day (120 mL of concentrated lemon diluted up to 2 L with water). Citrate excretion increased 144%. Kang and associates110 retrospectively compared the outcomes of patients receiving lemonade therapy (120 mL concentrated lemon juice diluted to 2 L with water) with those of patients receiving potassium citrate (40 mEq/d). They reported significant increases in renal excretion of citrate in both the lemonade and the potassium citrate groups; however, compared with lemonade consumption, patients taking potassium citrate had significantly greater increases in urinary citrate excretion, as well as increases in urine pH. Urine pH was not altered by lemonade.110 However, not all studies on lemonade therapy have demonstrated positive results. Penniston and associates111 compared lemonade therapy (either 120 mL of concentrated lemon juice diluted in water or 1 L of sugar-free lemonade) with lemonade therapy (same preparation) combined with potassium citrate (20–90 mEq per day). Although both regimens initially promoted a significant increase in citrate excretion, only the potassium citrate cohort had a durable response. Koff and colleagues112 performed a cross-over comparison of potassium citrate (60 mEq/d) and lemonade (90 mL of concentrated lemon juice diluted in 532 mL of water). Potassium citrate consumption significantly increased pH and citrate excretion, whereas this did not occur with lemonade. Odvina and colleagues97 performed a study in which other nutrients were controlled. Lemonade (1200 mL/d), distilled water (1200 mL/d), and orange juice (1200 mL/d) were administered in a cross-over design. Only orange juice resulted in an increase in urine pH and citrate excretion. This investigator thought that this difference was due to orange juice having high potassium citrate content as compared with lemonade, which has a high level of citric acid. The former would theoretically result in an increased alkali load, whereas with the latter the benefits of citrate would be negated by the accompanying hydrogen proton that neutralizes the effects of citrate.
The citric acid contents of various juice preparations have been measured. Penniston and colleagues107 quantified citrate content in natural and commercially available fruit juices, using ion chromatography. They found that natural lemon and lime juice contain the greatest quantity of citric acid, followed closely by lemon and lime juice concentrates. Grapefruit juice and orange juice contained significant amounts of citrate; however, both contained less than lemon and lime juices. In a similar study, Haleblian and associates106 used nuclear magnetic resonance spectroscopy to quantify citrate content. Using this technique, they found grapefruit juice to have the highest citrate content, followed by lemon juice and orange juice. They also found that among low-calorie beverages, Crystal Light® lemonade (Kraft Foods, Glenview, IL) had the highest citrate levels.
Conclusions
Citrate is a urinary inhibitor of the crystallization of several stone-forming salts. Citrate excretion is modulated by systemic factors, including acidosis. It may also be impacted by nephron dysfunction, such as dRTA, and may be influenced by medications and diet.
Hypocitraturia is a risk factor for stone formation. There is solid evidence that correction of this abnormality with medical therapy reduces stone risk. Dietary modifications should also be used in conjunction with medical therapy.
Main Points.
Hypocitraturia, generally defined as urinary citrate excretion less than 320 mg (1.67 mmol) per day for adults, is a common metabolic abnormality in stone formers, occurring in 20% to 60%.
Modulation of citrate excretion in the kidney is influenced by multiple factors; however, pH (systemic, tubular, and intracellular) has the strongest impact.
Although the majority of patients have idiopathic hypocitraturia, there are a number of causes for this abnormality, including distal renal tubular acidosis, hypokalemia, bowel dysfunction, and a high-protein, low-alkali diet.
Other factors associated with altered citrate excretion include genetic factors, certain drugs (eg, acetazolamide, topiramate, angiotensin-converting enzyme inhibitors, and thiazide), renal insufficiency, hyperaldosteronism, type I glycogen storage disease, and exercise.
Dietary modifications benefit the majority of patients with nephrolithiasis. These include high fluid and citrus fruit intake, normal calcium consumption, and restriction of sodium, oxalate, animal protein, and fructose intake.
The administration of citrate preparations or other alkali has been demonstrated to benefit hypocitraturic stone formers. Although many forms of citrate have been used for these patients (potassium citrate, sodium citrate, potassium-magnesium-citrate), potassium citrate has emerged as the most beneficial.
Patients who either cannot tolerate or cannot afford potassium citrate may benefit from consuming citrus juices, which contain significant amounts of citrate.
References
- 1.Curhan GC, Taylor EN. 24-h uric acid excretion and the risk of kidney stones. Kidney Int. 2008;73:489–496. doi: 10.1038/sj.ki.5002708. [DOI] [PubMed] [Google Scholar]
- 2.Chow K, Dixon J, Gilpin S, et al. Citrate inhibits growth of residual fragments in an in vitro model of calcium oxalate renal stones. Kidney Int. 2004;65:1724–1730. doi: 10.1111/j.1523-1755.2004.00566.x. [DOI] [PubMed] [Google Scholar]
- 3.Nicar MJ, Skurla C, Sakhaee K, Pak CY. Low urinary citrate excretion in nephrolithiasis. Urology. 1983;21:8–14. doi: 10.1016/0090-4295(83)90113-9. [DOI] [PubMed] [Google Scholar]
- 4.Rudman D, Kutner MH, Redd SC, 2nd, et al. Hypocitraturia in calcium nephrolithiasis. J Clin Endocrinol Metab. 1982;55:1052–1057. doi: 10.1210/jcem-55-6-1052. [DOI] [PubMed] [Google Scholar]
- 5.Pak CY. Etiology and treatment of urolithiasis. Am J Kidney Dis. 1991;18:624–637. doi: 10.1016/s0272-6386(12)80602-0. [DOI] [PubMed] [Google Scholar]
- 6.Pak CY, Poindexter JR, Adams-Huet B, Pearle MS. Predictive value of kidney stone composition in the detection of metabolic abnormalities. Am J Med. 2003;115:26–32. doi: 10.1016/s0002-9343(03)00201-8. [DOI] [PubMed] [Google Scholar]
- 7.Nicar MJ, Hill K, Pak CY. Inhibition by citrate of spontaneous precipitation of calcium oxalate in vitro. J Bone Miner Res. 1987;2:215–220. doi: 10.1002/jbmr.5650020308. [DOI] [PubMed] [Google Scholar]
- 8.Hess B, Zipperle L, Jaeger P. Citrate and calcium effects on Tamm-Horsfall glycoprotein as a modifier of calcium oxalate crystal aggregation. Am J Physiol. 1993;265:F784–F791. doi: 10.1152/ajprenal.1993.265.6.F784. [DOI] [PubMed] [Google Scholar]
- 9.Ryall RL. Urinary inhibitors of calcium oxalate crystallization and their potential role in stone formation. World J Urol. 1997;15:155–164. doi: 10.1007/BF02201852. [DOI] [PubMed] [Google Scholar]
- 10.Sheng X, Jung T, Wesson JA, Ward MD. Adhesion at calcium oxalate crystal surfaces and the effect of urinary constituents. Proc Natl Acad Sci U S A. 2005;102:267–272. doi: 10.1073/pnas.0406835101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kok DJ, Papapoulos SE, Bijvoet OL. Excessive crystal agglomeration with low citrate excretion in recurrent stone-formers. Lancet. 1986;1:1056–1058. doi: 10.1016/s0140-6736(86)91329-2. [DOI] [PubMed] [Google Scholar]
- 12.Krebs HA, Johnson WA. The role of citric acid in intermediate metabolism in animal tissues. FEBS Lett. 1980;117(suppl):K1–K10. doi: 10.4159/harvard.9780674366701.c143. [DOI] [PubMed] [Google Scholar]
- 13.Krebs HA, Salvin E, Johnson WA. The formation of citric and alpha-ketoglutaric acids in the mammalian body. Biochem J. 1983;32:113–117. doi: 10.1042/bj0320113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nieth H, Schollmeyer P. Substrate-utilization of the human kidney. Nature. 1966;209:1244–1245. doi: 10.1038/2091244a0. [DOI] [PubMed] [Google Scholar]
- 15.Minisola S, Rossi W, Pacitti MT, et al. Studies on citrate metabolism in normal subjects and kidney stone patients. Miner Electrolyte Metab. 1989;15:303–308. [PubMed] [Google Scholar]
- 16.Brennan TS, Klahr S, Hamm LL. Citrate transport in rabbit nephron. Am J Physiol. 1986;251:F683–F689. doi: 10.1152/ajprenal.1986.251.4.F683. [DOI] [PubMed] [Google Scholar]
- 17.Hamm LL. Renal handling of citrate. Kidney Int. 1990;38:728–735. doi: 10.1038/ki.1990.265. [DOI] [PubMed] [Google Scholar]
- 18.Simpson DP. Citrate excretion: a window on renal metabolism. Am J Physiol. 1983;244:F223–F234. doi: 10.1152/ajprenal.1983.244.3.F223. [DOI] [PubMed] [Google Scholar]
- 19.Pajor AM. Sequence and functional characterization of a renal sodium/dicarboxylate cotransporter. J Biol Chem. 1995;270:5779–5785. doi: 10.1074/jbc.270.11.5779. [DOI] [PubMed] [Google Scholar]
- 20.Pajor AM. Sodium-coupled transporters for Krebs cycle intermediates. Annu Rev Physiol. 1999;61:663–682. doi: 10.1146/annurev.physiol.61.1.663. [DOI] [PubMed] [Google Scholar]
- 21.Wright SH, Kippen I, Klinenberg JR, Wright EM. Specificity of the transport system for tricarboxylic acid cycle intermediates in renal brush borders. J Membr Biol. 1980;57:73–82. doi: 10.1007/BF01868987. [DOI] [PubMed] [Google Scholar]
- 22.Hirayama B, Wright EM. Coupling between sodium and succinate transport across renal brush border membrane vesicles. Pflugers Arch. 1986;407(suppl 2):S174–S179. doi: 10.1007/BF00584948. [DOI] [PubMed] [Google Scholar]
- 23.Barac-Nieto M. Effects of pH, calcium, and succinate on sodium citrate cotransport in renal microvilli. Am J Physiol. 1984;247:F282–F290. doi: 10.1152/ajprenal.1984.247.2.F282. [DOI] [PubMed] [Google Scholar]
- 24.Chen X, Tsukaguchi H, Chen XZ, et al. Molecular and functional analysis of SDCT2, a novel rat sodium-dependent dicarboxylate transporter. J Clin Invest. 1999;103:1159–1168. doi: 10.1172/JCI5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jørgensen KE, Kragh-Hansen U, Røigaard-Petersen H, Sheikh MI. Citrate uptake by basolateral and luminal membrane vesicles from rabbit kidney cortex. Am J Physiol. 1983;244:F686–F695. doi: 10.1152/ajprenal.1983.244.6.F686. [DOI] [PubMed] [Google Scholar]
- 26.Melnick JZ, Srere PA, Elshourbagy NA, et al. Adenosine triphosphate citrate lyase mediates hypocitraturia in rats. J Clin Invest. 1996;98:2381–2387. doi: 10.1172/JCI119051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pajor AM. Molecular cloning and functional expression of a sodium-dicarboxylate cotransporter from human kidney. Am J Physiol. 1996;270:F642–F648. doi: 10.1152/ajprenal.1996.270.4.F642. [DOI] [PubMed] [Google Scholar]
- 28.Pajor AM, Sun N. Characterization of the rabbit renal Na(+)-dicarboxylate cotransporter using antifusion protein antibodies. Am J Physiol. 1996;271:C1808–C1816. doi: 10.1152/ajpcell.1996.271.6.C1808. [DOI] [PubMed] [Google Scholar]
- 29.Wolffram S, Badertscher M, Scharrer E. Carrier-mediated transport is involved in mucosal succinate uptake by rat large intestine. Exp Physiol. 1994;79:215–226. doi: 10.1113/expphysiol.1994.sp003754. [DOI] [PubMed] [Google Scholar]
- 30.Wolffram S, Bisang B, Grenacher B, Scharrer E. Transport of tri- and dicarboxylic acids across the intestinal brush border membrane of calves. J Nutr. 1990;120:767–774. doi: 10.1093/jn/120.7.767. [DOI] [PubMed] [Google Scholar]
- 31.Wolffram S, Hagemann C, Grenacher B, Scharrer E. Characterization of the transport of tri- and dicarboxylates by pig intestinal brush-border membrane vesicles. Comp Biochem Physiol Comp Physiol. 1992;101:759–767. doi: 10.1016/0300-9629(92)90355-t. [DOI] [PubMed] [Google Scholar]
- 32.Wolffram S, Unternährer R, Grenacher B, Scharrer E. Transport of citrate across the brush border and basolateral membrane of rat small intestine. Comp Biochem Physiol Physiol. 1994;109:39–52. doi: 10.1016/0300-9629(94)90310-7. [DOI] [PubMed] [Google Scholar]
- 33.Wolffram S, Zimmermann W, Scharrer E. Transport of tricarballylate by intestinal brush-border membrane vesicles from steers. Exp Physiol. 1993;78:473–484. doi: 10.1113/expphysiol.1993.sp003699. [DOI] [PubMed] [Google Scholar]
- 34.Browne JL, Sanford PA, Smyth DH. Transfer and metabolism of citrate, succinate, alpha-ketoglutarate and pyruvate by hamster small intestine. Proc R Soc Lond B Biol Sci. 1978;200:117–135. doi: 10.1098/rspb.1978.0010. [DOI] [PubMed] [Google Scholar]
- 35.Fegan J, Khan R, Poindexter J, Pak CY. Gastrointestinal citrate absorption in nephrolithiasis. J Urol. 1992;147:1212–1214. doi: 10.1016/s0022-5347(17)37520-1. [DOI] [PubMed] [Google Scholar]
- 36.Sakhaee K, Alpern R, Poindexter J, Pak CY. Citraturic response to oral citric acid load. J Urol. 1992;147:975–976. doi: 10.1016/s0022-5347(17)37437-2. [DOI] [PubMed] [Google Scholar]
- 37.Rudman D, Dedonis JL, Fountain MT, et al. Hypocitraturia in patients with gastrointestinal malabsorption. N Engl J Med. 1980;303:657–661. doi: 10.1056/NEJM198009183031201. [DOI] [PubMed] [Google Scholar]
- 38.Sakhaee K, Williams RH, Oh MS, et al. Alkali absorption and citrate excretion in calcium nephrolithiasis. J Bone Miner Res. 1993;8:789–794. doi: 10.1002/jbmr.5650080703. [DOI] [PubMed] [Google Scholar]
- 39.Usui Y, Matsuzaki S, Matsushita K, Shima M. Urinary citrate in kidney stone disease. Tokai J Exp Clin Med. 2003;28:65–70. [PubMed] [Google Scholar]
- 40.Crawford MA, Milne MD, Scribner BH. The effects of changes in acid-base balance on urinary citrate in the rat. J Physiol. 1959;149:413–423. doi: 10.1113/jphysiol.1959.sp006348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dedmon RE, Wrong O. The excretion of organic anion in renal tubular acidosis with particular reference to citrate. Clin Sci. 1962;22:19–32. [PubMed] [Google Scholar]
- 42.Brennan S, Hering-Smith K, Hamm LL. Effect of pH on citrate reabsorption in the proximal convoluted tubule. Am J Physiol. 1988;255:F301–F306. doi: 10.1152/ajprenal.1988.255.2.F301. [DOI] [PubMed] [Google Scholar]
- 43.Jenkins AD, Dousa TP, Smith LH. Transport of citrate across renal brush border membrane: effects of dietary acid and alkali loading. Am J Physiol. 1985;249:F590–F595. doi: 10.1152/ajprenal.1985.249.4.F590. [DOI] [PubMed] [Google Scholar]
- 44.Aruga S, Wehrli S, Kaissling B, et al. Chronic metabolic acidosis increases NaDC-1 mRNA and protein abundance in rat kidney. Kidney Int. 2000;58:206–215. doi: 10.1046/j.1523-1755.2000.00155.x. [DOI] [PubMed] [Google Scholar]
- 45.Melnick JZ, Preisig PA, Moe OW, et al. Renal cortical mitochondrial aconitase is regulated in hypo- and hypercitraturia. Kidney Int. 1998;54:160–165. doi: 10.1046/j.1523-1755.1998.00974.x. [DOI] [PubMed] [Google Scholar]
- 46.Hwang JJ, Curthoys NP. Effect of acute alterations in acid-base balance on rat renal glutaminase and phosphoenolpyruvate carboxykinase gene expression. J Biol Chem. 1991;266:9392–9396. [PubMed] [Google Scholar]
- 47.Coe FL, Parks JH. Stone disease in hereditary distal renal tubular acidosis. Ann Intern Med. 1980;93:60–61. doi: 10.7326/0003-4819-93-1-60. [DOI] [PubMed] [Google Scholar]
- 48.Hamm LL, Hering-Smith KS. Pathophysiology of hypocitraturic nephrolithiasis. Endocrinol Metab Clin North Am. 2002;31:885–893. doi: 10.1016/s0889-8529(02)00031-2. [DOI] [PubMed] [Google Scholar]
- 49.Pak CY. Citrate and renal calculi: an update. Miner Electrolyte Metab. 1994;20:371–377. [PubMed] [Google Scholar]
- 50.Preminger GM, Sakhaee K, Pak CY. Hypercalciuria and altered intestinal calcium absorption occurring independently of vitamin D in incomplete distal renal tubular acidosis. Metabolism. 1987;36:176–179. doi: 10.1016/0026-0495(87)90014-x. [DOI] [PubMed] [Google Scholar]
- 51.Hess B, Michel R, Takkinen R, et al. Risk factors for low urinary citrate in calcium nephrolithiasis: low vegetable fibre intake and low urine volume to be added to the list. Nephrol Dial Transplant. 1994;9:642–649. doi: 10.1093/ndt/9.6.642. [DOI] [PubMed] [Google Scholar]
- 52.Uribarri J, Oh MS, Pak CY. Renal stone risk factors in patients with type IV renal tubular acidosis. Am J Kidney Dis. 1994;23:784–787. doi: 10.1016/s0272-6386(12)80129-6. [DOI] [PubMed] [Google Scholar]
- 53.Domrongkitchaiporn S, Stitchantrakul W, Kochakarn W. Causes of hypocitraturia in recurrent calcium stone formers: focusing on urinary potassium excretion. Am J Kidney Dis. 2006;48:546–554. doi: 10.1053/j.ajkd.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 54.Adam WR, Koretsky AP, Weiner MW. 31P-NMR in vivo measurement of renal intracellular pH: effects of acidosis and K+ depletion in rats. Am J Physiol. 1986;251:F904–F910. doi: 10.1152/ajprenal.1986.251.5.F904. [DOI] [PubMed] [Google Scholar]
- 55.Adler S, Fraley DS. Potassium and intracellular pH. Kidney Int. 1977;11:433–442. doi: 10.1038/ki.1977.61. [DOI] [PubMed] [Google Scholar]
- 56.Soleimani M, Bergman JA, Hosford MA, McKinney TD. Potassium depletion increases luminal Na+/H+ exchange and basolateral Na+:CO3=: HCO3− cotransport in rat renal cortex. J Clin Invest. 1990;86:1076–1083. doi: 10.1172/JCI114810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Levi M, McDonald LA, Preisig PA, Alpern RJ. Chronic K depletion stimulates rat renal brush-border membrane Na-citrate cotransporter. Am J Physiol. 1991;261:F767–F773. doi: 10.1152/ajprenal.1991.261.5.F767. [DOI] [PubMed] [Google Scholar]
- 58.Cowley DM, McWhinney BC, Brown JM, Chalmers AH. Chemical factors important to calcium nephrolithiasis: evidence for impaired hydroxycarboxylic acid absorption causing hyperoxaluria. Clin Chem. 1987;33:243–247. [PubMed] [Google Scholar]
- 59.Breslau NA, Brinkley L, Hill KD, Pak CY. Relationship of animal protein-rich diet to kidney stone formation and calcium metabolism. J Clin Endocrinol Metab. 1988;66:140–146. doi: 10.1210/jcem-66-1-140. [DOI] [PubMed] [Google Scholar]
- 60.Reddy ST, Wang CY, Sakhaee K, et al. Effect of low-carbohydrate high-protein diets on acid-base balance, stone-forming propensity, and calcium metabolism. Am J Kidney Dis. 2002;40:265–274. doi: 10.1053/ajkd.2002.34504. [DOI] [PubMed] [Google Scholar]
- 61.Amanzadeh J, Gitomer WL, Zerwekh JE, et al. Effect of high protein diet on stone-forming propensity and bone loss in rats. Kidney Int. 2003;64:2142–2149. doi: 10.1046/j.1523-1755.2003.00309.x. [DOI] [PubMed] [Google Scholar]
- 62.Meschi T, Maggiore U, Fiaccadori E, et al. The effect of fruits and vegetables on urinary stone risk factors. Kidney Int. 2004;66:2402–2410. doi: 10.1111/j.1523-1755.2004.66029.x. [DOI] [PubMed] [Google Scholar]
- 63.Sakhaee K, Harvey JA, Padalino PK, et al. The potential role of salt abuse on the risk for kidney stone formation. J Urol. 1993;150:310–312. doi: 10.1016/s0022-5347(17)35468-x. [DOI] [PubMed] [Google Scholar]
- 64.Windus DW, Cohn DE, Heifets M. Effects of fasting on citrate transport by the brush-border membrane of rat kidney. Am J Physiol. 1986;251:F678–F682. doi: 10.1152/ajprenal.1986.251.4.F678. [DOI] [PubMed] [Google Scholar]
- 65.Jackman SV, Kibel AS, Ovuworie CA, et al. Familial calcium stone disease: TaqI polymorphism and the vitamin D receptor. J Endourol. 1999;13:313–316. doi: 10.1089/end.1999.13.313. [DOI] [PubMed] [Google Scholar]
- 66.Nishijima S, Sugaya K, Naito A, et al. Association of vitamin D receptor gene polymorphism with urolithiasis. J Urol. 2002;167:2188–2191. [PubMed] [Google Scholar]
- 67.Ruggiero M, Pacini S, Amato M, et al. Association between vitamin D receptor gene polymorphism and nephrolithiasis. Miner Electrolyte Metab. 1999;25:185–190. doi: 10.1159/000057443. [DOI] [PubMed] [Google Scholar]
- 68.Mossetti G, Vuotto P, Rendina D, et al. Association between vitamin D receptor gene polymorphisms and tubular citrate handling in calcium nephrolithiasis. J Intern Med. 2003;253:194–200. doi: 10.1046/j.1365-2796.2003.01086.x. [DOI] [PubMed] [Google Scholar]
- 69.Pajor AM, Sun N. Protein kinase C-mediated regulation of the renal Na(+)/dicarboxylate cotransporter, NaDC-1. Biochim Biophys Acta. 1999;1420:223–230. doi: 10.1016/s0005-2736(99)00102-9. [DOI] [PubMed] [Google Scholar]
- 70.Mossetti G, Rendina D, Viceconti R, et al. The relationship of 3′ vitamin D receptor haplotypes to urinary supersaturation of calcium oxalate salts and to age at onset and familial prevalence of nephrolithiasis. Nephrol Dial Transplant. 2004;19:2259–2265. doi: 10.1093/ndt/gfh273. [DOI] [PubMed] [Google Scholar]
- 71.Shah O, Assimos DG, Holmes RP. Genetic and dietary factors in urinary citrate excretion. J Endourol. 2005;19:177–182. doi: 10.1089/end.2005.19.177. [DOI] [PubMed] [Google Scholar]
- 72.Goodman HO, Brommage R, Assimos DG, Holmes RP. Genes in idiopathic calcium oxalate stone disease. World J Urol. 1997;15:186–194. doi: 10.1007/BF02201856. [DOI] [PubMed] [Google Scholar]
- 73.Goodman HO, Holmes RP, Assimos DG. Genetic factors in calcium oxalate stone disease. J Urol. 1995;153:301–307. doi: 10.1097/00005392-199502000-00003. [DOI] [PubMed] [Google Scholar]
- 74.Okamoto N, Aruga S, Matsuzaki S, et al. Associations between renal sodium-citrate cotransporter (hNaDC-1) gene polymorphism and urinary citrate excretion in recurrent renal calcium stone formers and normal controls. Int J Urol. 2007;14:344–349. doi: 10.1111/j.1442-2042.2007.01554.x. [DOI] [PubMed] [Google Scholar]
- 75.Maloney ME, Springhart WP, Ekeruo WO, et al. Ethnic background has minimal impact on the etiology of nephrolithiasis. J Urol. 2005;173:2001–2004. doi: 10.1097/01.ju.0000159076.70638.1e. [DOI] [PubMed] [Google Scholar]
- 76.Gordon EE, Sheps SG. Effect of acetazolamide on citrate excretion and formation of renal calculi. N Engl J Med. 1957;256:1215–1219. doi: 10.1056/NEJM195706272562602. [DOI] [PubMed] [Google Scholar]
- 77.Welch BJ, Graybeal D, Moe OW, et al. Biochemical and stone-risk profiles with topiramate treatment. Am J Kidney Dis. 2006;48:555–563. doi: 10.1053/j.ajkd.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 78.Kuo RL, Moran ME, Kim DH, et al. Topiramate-induced nephrolithiasis. J Endourol. 2002;16:229–231. doi: 10.1089/089277902753752188. [DOI] [PubMed] [Google Scholar]
- 79.Warner BW, LaGrange CA, Tucker T, et al. Induction of progressive profound hypocitraturia with increasing doses of topiramate. Urology. 2008;72:29–32. doi: 10.1016/j.urology.2008.01.042. discussion 32–33. [DOI] [PubMed] [Google Scholar]
- 80.Melnick JZ, Preisig PA, Haynes S, et al. Converting enzyme inhibition causes hypocitraturia independent of acidosis or hypokalemia. Kidney Int. 1998;54:1670–1674. doi: 10.1046/j.1523-1755.1998.00150.x. [DOI] [PubMed] [Google Scholar]
- 81.Pak CY, Peterson R, Sakhaee K, et al. Correction of hypocitraturia and prevention of stone formation by combined thiazide and potassium citrate therapy in thiazide-unresponsive hypercalciuric nephrolithiasis. Am J Med. 1985;79:284–288. doi: 10.1016/0002-9343(85)90305-5. [DOI] [PubMed] [Google Scholar]
- 82.Wright EM, Wright SH, Hirayama B, Kippen I. Interactions between lithium and renal transport of Krebs cycle intermediates. Proc Natl Acad Sci U S A. 1982;79:7514–7517. doi: 10.1073/pnas.79.23.7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hamm LL, Alpern RJ. Regulation of acid-base balance, citrate, and urine pH. In: Coe FL, Pak CYC, Parks JH, Preminger GM, editors. Kidney Stones: Medical and Surgical Management. Philadelphia: Lippincott-Raven; 1996. [Google Scholar]
- 84.Marangella M, Vitale C, Manganaro M, et al. Renal handling of citrate in chronic renal insufficiency. Nephron. 1991;57:439–443. doi: 10.1159/000186347. [DOI] [PubMed] [Google Scholar]
- 85.Shey J, Cameron MA, Sakhaee K, Moe OW. Recurrent calcium nephrolithiasis associated with primary aldosteronism. Am J Kidney Dis. 2004;44:e7–e12. doi: 10.1053/j.ajkd.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 86.Weinstein DA, Somers MJ, Wolfsdorf JI. Decreased urinary citrate excretion in type 1a glycogen storage disease. J Pediatr. 2001;138:378–382. doi: 10.1067/mpd.2001.111322. [DOI] [PubMed] [Google Scholar]
- 87.Ekeruo WO, Tan YH, Young MD, et al. Metabolic risk factors and the impact of medical therapy on the management of nephrolithiasis in obese patients. J Urol. 2004;172:159–163. doi: 10.1097/01.ju.0000128574.50588.97. [DOI] [PubMed] [Google Scholar]
- 88.Sakhaee K, Nigam S, Snell P, et al. Assessment of the pathogenetic role of physical exercise in renal stone formation. J Clin Endocrinol Metab. 1987;65:974–979. doi: 10.1210/jcem-65-5-974. [DOI] [PubMed] [Google Scholar]
- 89.Reungjui S, Prasongwatana V, Premgamone A, et al. Magnesium status of patients with renal stones and its effect on urinary citrate excretion. BJU Int. 2002;90:635–639. doi: 10.1046/j.1464-410x.2002.03015.x. [DOI] [PubMed] [Google Scholar]
- 90.Pak CY. Kidney stones. Lancet. 1998;351:1797–1801. doi: 10.1016/S0140-6736(98)01295-1. [DOI] [PubMed] [Google Scholar]
- 91.Pak CY, Fuller C, Sakhaee K, et al. Long-term treatment of calcium nephrolithiasis with potassium citrate. J Urol. 1985;134:11–19. doi: 10.1016/s0022-5347(17)46962-x. [DOI] [PubMed] [Google Scholar]
- 92.Pak CY, Fuller C. Idiopathic hypocitraturic calcium-oxalate nephrolithiasis successfully treated with potassium citrate. Ann Intern Med. 1986;104:33–37. doi: 10.7326/0003-4819-104-1-33. [DOI] [PubMed] [Google Scholar]
- 93.Ettinger B, Pak CY, Citron JT, et al. Potassium-magnesium citrate is an effective prophylaxis against recurrent calcium oxalate nephrolithiasis. J Urol. 1997;158:2069–2073. doi: 10.1016/s0022-5347(01)68155-2. [DOI] [PubMed] [Google Scholar]
- 94.Preminger GM, Sakhaee K, Pak CY. Alkali action on the urinary crystallization of calcium salts: contrasting responses to sodium citrate and potassium citrate. J Urol. 1988;139:240–242. doi: 10.1016/s0022-5347(17)42374-3. [DOI] [PubMed] [Google Scholar]
- 95.Cicerello E, Merlo F, Gambaro G, et al. Effect of alkaline citrate therapy on clearance of residual renal stone fragments after extracorporeal shock wave lithotripsy in sterile calcium and infection nephrolithiasis patients. J Urol. 1994;151:5–9. doi: 10.1016/s0022-5347(17)34858-9. [DOI] [PubMed] [Google Scholar]
- 96.Nicar MJ, Peterson R, Pak CY. Use of potassium citrate as potassium supplement during thiazide therapy of calcium nephrolithiasis. J Urol. 1984;131:430–433. doi: 10.1016/s0022-5347(17)50438-3. [DOI] [PubMed] [Google Scholar]
- 97.Odvina CV, Mason RP, Pak CY. Prevention of thiazide-induced hypokalemia without magnesium depletion by potassium-magnesium-citrate. Am J Ther. 2006;13:101–108. doi: 10.1097/01.mjt.0000149922.16098.c0. [DOI] [PubMed] [Google Scholar]
- 98.Barcelo P, Wuhl O, Servitge E, et al. Randomized double-blind study of potassium citrate in idiopathic hypocitraturic calcium nephrolithiasis. J Urol. 1993;150:1761–1764. doi: 10.1016/s0022-5347(17)35888-3. [DOI] [PubMed] [Google Scholar]
- 99.Hofbauer J, Höbarth K, Szabo N, Marberger M. Alkali citrate prophylaxis in idiopathic recurrent calcium oxalate urolithiasis-a prospective randomized study. Br J Urol. 1994;73:362–365. doi: 10.1111/j.1464-410x.1994.tb07597.x. [DOI] [PubMed] [Google Scholar]
- 100.Soygur T, Akbay A, Kupeli S. Effect of potassium citrate therapy on stone recurrence and residual fragments after shockwave lithotripsy in lower caliceal calcium oxalate urolithiasis: a randomized controlled trial. J Endourol. 2002;16:149–152. doi: 10.1089/089277902753716098. [DOI] [PubMed] [Google Scholar]
- 101.Mattle D, Hess B. Preventive treatment of nephrolithiasis with alkali citrate-a critical review. Urol Res. 2005;33:73–79. doi: 10.1007/s00240-005-0464-8. [DOI] [PubMed] [Google Scholar]
- 102.Shenoy C. Hypocitraturia despite potassium citrate tablet supplementation. MedGenMed. 2006;8:8. [PMC free article] [PubMed] [Google Scholar]
- 103.Mandel N, Mandel I, Fryjoff K, et al. Conversion of calcium oxalate to calcium phosphate with recurrent stone episodes. J Urol. 2003;169:2026–2029. doi: 10.1097/01.ju.0000065592.55499.4e. [DOI] [PubMed] [Google Scholar]
- 104.Parks JH, Worcester EM, Coe FL, et al. Clinical implications of abundant calcium phosphate in routinely analyzed kidney stones. Kidney Int. 2004;66:777–785. doi: 10.1111/j.1523-1755.2004.00803.x. [DOI] [PubMed] [Google Scholar]
- 105.Wabner CL, Pak CY. Effect of orange juice consumption on urinary stone risk factors. J Urol. 1993;149:1405–1408. doi: 10.1016/s0022-5347(17)36401-7. [DOI] [PubMed] [Google Scholar]
- 106.Haleblian GE, Leitao VA, Pierre SA, et al. Assessment of citrate concentrations in citrus fruit-based juices and beverages: implications for management of hypocitraturic nephrolithiasis. J Endourol. 2008;22:1359–1366. doi: 10.1089/end.2008.0069. [DOI] [PubMed] [Google Scholar]
- 107.Penniston KL, Nakada SY, Holmes RP, Assimos DG. Quantitative assessment of citric acid in lemon juice, lime juice, and commercially-available fruit juice products. J Endourol. 2008;22:567–570. doi: 10.1089/end.2007.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Goldfarb DS, Asplin JR. Effect of grapefruit juice on urinary lithogenicity. J Urol. 2001;166:263–267. [PubMed] [Google Scholar]
- 109.Seltzer MA, Low RK, McDonald M, et al. Dietary manipulation with lemonade to treat hypocitraturic calcium nephrolithiasis. J Urol. 1996;156:907–909. [PubMed] [Google Scholar]
- 110.Kang DE, Sur RL, Haleblian GE, et al. Long-term lemonade based dietary manipulation in patients with hypocitraturic nephrolithiasis. J Urol. 2007;177:1358–1362. doi: 10.1016/j.juro.2006.11.058. discussion 1362; quiz 1591. [DOI] [PubMed] [Google Scholar]
- 111.Penniston KL, Steele TH, Nakada SY. Lemonade therapy increases urinary citrate and urine volumes in patients with recurrent calcium oxalate stone formation. Urology. 2007;70:856–860. doi: 10.1016/j.urology.2007.06.1115. [DOI] [PubMed] [Google Scholar]
- 112.Koff SG, Paquette EL, Cullen J, et al. Comparison between lemonade and potassium citrate and impact on urine pH and 24-hour urine parameters in patients with kidney stone formation. Urology. 2007;69:1013–1016. doi: 10.1016/j.urology.2007.02.008. [DOI] [PubMed] [Google Scholar]