Abstract
Urinary incontinence (UI) in community-dwelling men affects quality of life and increases the risk of institutionalization. Observational studies and randomized, controlled trials published in English from 1990 to November 2007 on the epidemiology and prevention of UI were identified in several databases to abstract rates and adjusted odds ratios (OR) of incontinence, calculate absolute risk difference (ARD) after clinical interventions, and synthesize evidence with random-effects models. Of 1083 articles identified, 126 were eligible for analysis. Pooled prevalence of UI increased with age to 21% to 32% in elderly men. Poor general health, comorbidities, severe physical limitations, cognitive impairment, stroke (pooled OR 1.54; 95% confidence interval [CI], 1.14–2.1), urinary tract infections (pooled OR 3.49; 95% CI, 2.33–5.23), prostate diseases, and diabetes (pooled OR 1.36; 95% CI, 1.14–1.61) were associated with UI. Treatment with tolterodine alone (ARD 0.17; 95% CI, 0.02–0.32) or combined with tamsulosin (ARD 0.17; 95% CI, 0.08–0.25) resulted in greater self-reported benefit compared with placebo. Radical prostatectomy or radiotherapy for prostate cancer compared with watchful waiting increased UI. Short-term prevention of UI with pelvic floor muscle rehabilitation after prostatectomy was not consistently seen across randomized, controlled trials. The prevalence of incontinence increased with age and functional dependency. Stroke, diabetes, poor general health, radiation, and surgery for prostate cancer were associated with UI in community-dwelling men. Men reported overall benefit from drug treatments. Limited evidence of preventive effects of pelvic floor rehabilitation requires future investigation.
Key words: Urinary incontinence, Risk factors, Rehabilitation, Drug therapy
Urinary incontinence (UI) affects substantial proportions of men1; the estimated prevalence of UI varied from 11% among those aged 60 to 64 years to 31% in older men, and from 16% among white men to 21% among African American men.2 Daily UI was reported by 30% to 47% and weekly UI by 15% to 37% of community-dwelling men.2 A small proportion (22%) of men with weekly UI episodes ever sought medical care for this problem, whereas 40% of treated men reported moderate to great frustration with continued urine leakage.3
Baseline mechanisms of UI include overactive bladder that may result in urge UI and poor urethral sphincter function that can result in primary urethral incompetence and stress UI.4,5 Baseline mechanisms of incontinence lead to variable definitions, risk factors, and effective interventions to prevent and treat UI.5
This review was commissioned as background material for a National Institutes of Health Office of Medical Applications of Research State of the Science Conference on Incontinence. We aimed to synthesize evidence of the effectiveness of different clinical interventions to prevent the occurrence and progression of UI in community-dwelling men.
Methods
Literature Search Strategy and Eligibility Criteria
Studies were sought from a wide variety of sources, including MEDLINE via PubMed, the Cumulative Index to Nursing and Allied Health Literature, Cochrane databases, and manual searches of reference lists from systematic reviews. Search strategies are described in the full-text report, available at http://www.ahrq.gov/downloads/pub/evidence/pdf/fuiad/fuiad.pdf.
Three investigators independently decided on the eligibility of the studies.6 Full texts of the original epidemiologic studies published in English after 1989 were examined to include studies with eligible outcomes, defined as prevalence and incidence of incontinence, absolute and adjusted relative risk (RR) of incidence, and progression of urinary incontinence in community-dwelling men. We included randomized, controlled trials (RCTs) of clinical interventions on incontinence. We excluded studies with children and adolescents, studies with no information relevant to incidence and progression of incontinence, and case series with fewer than 100 men and no control. We also excluded observational studies of men in nursing homes, case series to describe incontinence after different treatments for prostate diseases, and randomized, controlled clinical trials that did not report patient outcomes but did report changes in instrumental tests (these studies are included in the full report, available at http://www.ahrq.gov/downloads/pub/evidence/pdf/fuiad/fuiad.pdf).
Quality Assessment and Rating the Body of Evidence
Study quality was analyzed using the following criteria: subject selection, length and loss of follow-up, adjustment for confounding factors in observational studies and intention to treat principle in clinical trials, masking the treatment status, randomization scheme and adequacy, allocation concealment, and justification of sample sizes in RCTs.7 Incidence and prevalence of cases of incontinence, as well as RR of incontinence in categories of risk factors and clinical interventions, were abstracted.8,9 Baseline data were compared in different studies to test differences in the target population and unusual patterns in the data.10,11 Regression coefficients, absolute risk, and their 95% confidence interval (CI) were calculated from reported cases.8,9 The protocol for the meta-analyses was created according to recommendations for meta-analysis of RCTs, the Improving the Quality of Reports of Meta-Analyses of Randomized Controlled Trials statement,12 and the Meta-analysis of Observational Studies in Epidemiology group.13 We used the Grading of Recommendations Assessment, Development and Evaluation working group definitions to evaluate the overall strength of the evidence as high, moderate, low, very low, or insufficient.14,15
External validity was estimated by evaluating the selection of the subjects in observational studies and clinical trials.16 Large observational cohorts based on national registries, population-based surveys, and nationally representative administrative and clinical databases had high applicability. We compared the differences in prevalence of incontinence in studies that selected men from administrative and clinical databases and that reported random and convenience sampling of participants.17 Applicability of the intervention duration was high for studies with follow-up of 1 year or more and acceptable for studies with follow-up of 6 to 12 months.
We assumed the presence of publication bias and did not use statistical tests for bias, defined as the tendency to publish positive results and to predict association when all conducted (published and unpublished) studies are analyzed.6,18–20 We used several strategies to reduce bias, including comprehensive literature searches of published and unpublished evidence in several databases, the reference lists of systematic reviews and proceedings of the International Continence Society (ICS), contacts with experts for additional references they might provide, and agreement on the eligibility status by several investigators.
Data Extraction
Evaluations of the studies and data extraction were performed manually and independently by 3 researchers. Errors in data extraction were assessed by a comparison with the established ranges for each variable and the data charts with the original articles. Any discrepancies were resolved by discussion.
Data Synthesis
The results of individual studies (expressed as event rates or adjusted for confounding factors odds ratios [ORs] or RR), summarized in evidence tables to analyze differences in incontinence in categories by age, race, ethnicity, and risk factors, are available at http://www.ahrq.gov/downloads/pub/evidence/pdf/fuiad/fuiad.pdf.
Definitions of Incontinence. We analyzed incontinence using the definitions of signs and symptoms of UI promoted by the ICS, including stress, urge, and mixed incontinence.1,5,21 Continence was defined as self-reported absence of involuntary urine loss or negative results on stress and pad tests. Frequency of UI was abstracted as daily, weekly, or monthly episodes of urine leakage. Severity of incontinence was defined using the objectively measured urine loss in pad weight tests or self-reported pad use. We defined true population incidence as newly diagnosed cases of incontinence that developed annually in the target population. True population incidence estimates were derived from large population-based surveys. However, for clinical interventions we defined incidence as the probability of developing incontinence under study after active and control interventions during time of follow-up.1,22 We defined reported incontinence as the prevalence of total incontinence or episodes of different types of incontinence when the authors did not access continence status as baseline or did not exclude prevalence cases from overall estimation.
We analyzed continence separately from improvement in incontinence because continence is the most clinically desirable patient outcome and is well defined, whereas improvement can include substantial differences in definitions and changing perceptions of qualitative and quantitative parameters of improvement. We used such conservative approaches to generate precise estimates of the effectiveness. Clinicians and patients can make informed decisions on the basis of the treatments that resulted in greater rates of long-term continence in well-designed RCTs.
We applied the intention-to-treat principle and calculated the number of cases in the active and control groups. Pooling criteria included the same operational definitions of incontinence outcomes and the same risk factors or clinical interventions.23 Homogeneity in clinical interventions was analyzed comparing published information on behavioral, instrumental (devices), pharmacologic, and surgical treatments. Meta-analysis was used to assess the consistency of the association between treatments and incontinence outcomes with random-effects models.24 Consistency in results was tested by comparing the direction and strength of the association. Chi-squared tests and I-squared tests were used to assess heterogeneity in study results: a P value of less than .01 and an I-squared value greater than 50%, respectively, were considered high.25,26 We calculated standard error and CI for population prevalence with the Wilson estimate and logarithm of prevalence for pooling analysis.27 The number needed to treat to prevent 1 event of incontinence was calculated as reciprocal to absolute risk differences in rates of outcomes events in the active and control groups and the number of attributable events per 1000 treated as absolute risk difference multiplied by 1000.28,29 Calculations were performed using STATA software (StataCorp, College Station, TX) at the 95% confidence level.28
Role of the Funding Source. The Agency for Healthcare Research and Quality suggested the initial questions and provided copyright release for this article but did not participate in the literature search, data analysis, or interpretation of the results.
Results
Figure 1 traces the flow of our literature search for the report. We retrieved 6103 potentially relevant references and included 126 articles on prevalence, risk factors, and clinical interventions in community-dwelling men in the present review. The overall summary of evidence is shown in Table 1. Detailed evidence tables are included in the full report, available at http://www.ahrq.gov/downloads/pub/evidence/pdf/fuiad/fuiad.pdf.
Figure 1.
Study flow diagram. *Literature search was conducted to examine diagnosis, prevalence, incidence, risk factors, and clinical interventions of urinary incontinence (UI) and fecal incontinence (FI) in adults from community and long-term care settings. †Sum of the studies not equal to the total number because of overlap in eligibility criteria. RCT, randomized clinical trial.
Table 1.
Evidence of the Association Between Risk Factors and Male Incontinence
| Tested Association | Studies | Level of Evidence | Conclusions |
| Age on UI | 69 studies of prevalence30–38,41,43,46,48,49,51–53,55,57–107 8 studies of odds ratio37,42,67,91,120,122,126,128 | High | Prevalence of UI increases with age; urge UI is the most common type of UI in men. |
| Ethnicity on UI | 1 study120 | Low | Odds of UI were the same in nonwhite vs white race (odds ratio 0.88; 95% CI, 0.72–1.07). |
| Physical activity on UI | 1 study89 | Low | Men with physical activity 1 or more times per week had 51% lower relative risk of UI (relative risk 0.49; 95% CI, 0.25–0.96). |
| Education on UI | 2 studies35,89 | Low | Men with secondary or higher education had the same odds of UI as men with primary education. |
| Marital status on UI | 1 study90 | Low | Single or never-married men had the same odds of UI as married men. |
| Body weight on UI | 4 studies35,89,90,93 | Low | 1 study reported that obese men had 220% increased odds of UI compared with men with normal weight (OR 3.2; 95% CI, 1.2–9); other studies did not find a significant association. |
| Coffee intake on UI | 1 study35 | Low | Men who regularly consumed 2 cups per day had 70% reduction in odds of UI (OR 0.3; 95% CI, 0.1–0.7). |
| Alcohol intake on UI | 3 studies35,89,90 | Low | Alcohol intake did not demonstrate consistent association with UI. |
| Smoking on UI | 2 studies35,89 | Low | Smoking did not demonstrate consistent association with UI. |
| Self-reported general health on UI | 2 studies67,90 | Moderate | Self-reported poor general health was associated with 200%–300% increase in odds of UI in both studies. |
| Comorbidities on UI | 6 studies35,42,49,58,93,117 Cardiovascular, cardiorespiratory, joint, and gastrointestinal diseases, 9 studies35,37,38,42,49,54,58,89,117 | Low | Inconsistent evidence of positive association with comorbidities on UI. Protracted coughing was associated with higher odds of UI in men >75 years of age in 1 study (OR 1.33; 95% CI, 1.04–1.69). Arthritis was associated with increased odds of UI by 59%–80% in 2 studies. Men with back problems had increased odds of UI by 110% (OR 2.10; 95% CI, 1.5–2.93) in 1 study. Men with fecal incontinence had increased odds of UI in 1 study (OR 17; 95% CI, 7.5–40), with nonsignificant changes in another. |
| Social and psychological factors on UI | 4 studies58,67,89,90 | Low | Depressive mood was associated with increased odds of UI in 1 study (OR 2.69; 95% CI, 1.14–6.34). Increased stress level and low social activity did not demonstrate significant association with UI. |
| Impaired glucose metabolism and diabetes on UI | 6 studies35,42,54,67,89,117 | Moderate | Increased borderline fasting glucose was not associated with UI. Pooled analysis of 5 studies found a consistent significant increase in odds of UI in men with diabetes (pooled OR 1.36; 95% CI, 1.14–1.61, heterogeneity NS). |
| Medication use on UI | 2 studies54,58 | Low | Antibiotics, antidepressants, asthma medication, blood pressure medications, heart medication, hypnotics, pain medications, polypharmacy, sleep medications, and tranquilizers were not associated with UI. Use of diuretics (OR 2.11; 95% CI, 1.28–3.47), laxatives (OR 2.34; 95% CI, 1.46–3.75), and narcotics (OR 2.03; 95% CI, 1.28–3.20) was associated with increased odds of UI. |
| Mental and neurologic diseases on UI | 7 studies35,42,49,54,67,101,117 | Moderate | Cognitive impairment, memory problems, and presence of any neurologic diseases were associated with increased odds of UI; dementia, depression, transient ischemic attack, and Parkinson’s disease did not demonstrate a significant association. Pooled analysis of 5 studies found a significant increase in odds of UI in men after stroke (pooled OR 2.7; 95% CI, 1.3–5.5; heterogeneity significant). |
| Physical dependency and limitation in daily activities on UI | 4 studies42,49,58,93 | Moderate | Severe physical limitations were associated with increased odds of UI in daily in 1 study (OR 3.34; 95% CI, 1.52–7.34). Men who reported difficulty talking and walking had higher odds of UI. Impaired activities of daily living were associated with increased odds of UI in a dose-response manner. |
| Urinary tract infection and urinary symptoms on UI | 9 studies35,37,42,49,58,73,89,91,115 | Moderate | Pooled analysis of 5 studies demonstrated consistent increase in odds of UI by 260% (pooled OR 3.6; 95% CI, 2.2–6; heterogeneity NS) among men with urinary tract infections. Men with lower urinary symptoms had increased odds of UI in 2 studies, with random changes in 1 study. |
| Prostate diseases and treatments for prostate cancer on UI | 4 studies of association with prostate diseases71,93,117,126 7 observational studies of different treatments for prostate cancer35,36,71,89,122,124,126 13 RCT of behavioral interventions for prostat diseases142–154 | Moderate | Men with prostate diseases had a 520% increase in odds of UI (OR 6.2; 95% CI, 3.6–10.6), men with prostate cancer had a 100% increase in odds of UI (OR 2; 95% CI, 1.5–2.8). History of any previous prostate surgery was associated with a 110% increase in odds of UI (OR 2.1; 95% CI, 1.2–3.7), history of radical prostatectomy was associated with a 330% increase in relative risk of UI (RR 4.3; 95% CI, 2.6–7.3), and a e history of previous transurethral resection of prostate at time or following radical prostatectomy was associated with a 80% increase in relative risk of UI (RR 1.8; 95% CI, 1.1–3). Transurethral resection of prostate compared with watchful waiting (1 RCT) did not result in higher rates of persistent UI. Radical prostatectomy compared with watchful waiting (1 RCT) resulted in a significant increase in UI of moderate or greater severity that caused distress and affected sexual life. Radical prostatectomy compared with external beam radiation increased the risk of UI (1 RCT). Radiotherapy for prostate cancer compared with watchful waiting (1 RCT) resulted in a significant increase in UI that required use of pads. Adjuvant external beam radiation compared with radical prostatectomy alone (1 RCT) did not increase relative risk of UI and severe UI that would require implantation of artificial sphincter. Different doses and regimes of radiotherapy resulted in the same rates of UI (2 RCTs). Bladder neck preservation techniques resulted in the same rates of UI (2 RCTs). Artificial urethral sphincter implantation compared with macroplastique injection above or around the striated sphincter region of the urethra (1 RCT) increased rates of continence. Different methods of transurethral resection of prostate (3 RCTs) resulted in the same rate of UI. |
| Pelvic floor muscle training and physical rehabilitation on UI | 9 RCTs129–137 | Low | Inconsistent prevention of UI after pelvic floor muscle training with biofeedback and support group. |
| Medical devices on UI | 2 RCTs140,141 | Low | UroLume sphincteric stent compared with conventional external sphincterotomy did not prevent UI (1 RCT). C3 penile compression device, Cunningham clamp, and U-Tex Male Adjustable Tension resulted in the same UI (1 RCT). |
| Pharmacologic treatments of UI | Corticosteroids, 2 RCTs155,156 | Low | Betamethasone cream applied locally to both neurovascular bundles or methylprednisolone orally beginning on the day of radical prostatectomy did not prevent UI compared with placebo. |
| Antidepressants, 1 RCT158 | Low | Duloxetine 40 mg daily combined with pelvic floor muscle training compared with pelvic floor muscle training alone increased continence rates at 16 but not 24 wk of treatment. | |
| Muscarinic antagonists compared with placebo or adrenergic α-antagonists, 2 RCTs159–162 | Moderate | Tolterodine ER 4 mg daily alone and combined with tamsulosin resulted in greater self-reported overall benefit of the treatment compared with placebo. The most commonly reported adverse effects compared with placebo included dry mouth (16% vs 7%), constipation (4% vs 9%), dyspepsia (4% vs 1%), dizziness (5% vs 1%), and somnolence (3% vs 1%). |
Evidence was rated as follows: high = further research is very unlikely to change our confidence in the estimates; moderate = further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; low = further research is likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. UI, urinary incontinence; OR, odds ratio; CI, confidence interval; NS, nonsignificant; RR, relative risk; RCT, randomized controlled trial.
Prevalence of UI in Community-Dwelling Men
The samples used in epidemiologic studies in men varied substantially in terms of age categories and definitions of UI. Although there is a broad age range in the prevalence studies, the majority concentrate on middle-aged and older male populations (eg, beginning at age 40, 60, or 65 years and older),2,30–50 with fewer studies of men younger than 40 years,36,46,51–57 including a recent national survey of men aged 18 years and older in the United States.57 The majority of these studies have been conducted in North America or European countries using predominantly white populations. Two studies have incorporated Asian populations.40,41 Pooled analysis of 69 studies30–38,41,43,46,48,49,51–53,55,57–107 (Table 2) detected a clear pattern of increased prevalence of total UI in aging men, from 4.8% in those aged 19 to 44 years (11 studies) to 11.2% in those aged 45 to 64 years (27 studies), to 21.1% in men older than 65 years (41 studies). The highest prevalence of UI (32.2%) was reported in elderly men (17 studies). Urge UI was the most prevalent type of UI in men among all age categories, increasing from 3.1% in those aged 19 to 44 years (7 studies) to 11.7% in those older than 65 years (20 studies).
Table 2.
Pooled Prevalence of Male Urinary Incontinence Among Age Categories (Random-Effects Model, Statistical Test for Heterogeneity Significant)
| Age (y) (studies) | Prevalence (95% CI) |
| 19–44 | |
| Total UI (11) | 4.81 (3.69–5.94) |
| Mixed UI (3) | 0.70 (0.11–1.29) |
| Stress UI (5) | 0.74 (0.14–1.34) |
| Urge UI (7) | 3.09 (1.96–4.21) |
| 45–64 | |
| Total UI (27) | 11.20 (10.14–12.26) |
| Mixed UI (4) | 1.53 (0.94–2.12) |
| Stress UI (13) | 3.78 (1.56–6.00) |
| Urge UI (14) | 7.75 (4.99–10.50) |
| 65+ | |
| Total UI (41) | 21.13 (19.90–22.35) |
| Mixed UI (10) | 6.13 (2.53–9.74) |
| Stress UI (15) | 2.67 (1.95–3.39) |
| Urge UI (20) | 11.70 (9.27–14.14) |
| 80+ | |
| Total UI (17) | 32.17 (29.62–34.73) |
| Mixed UI (1) | 9.40 (9.34–9.46) |
| Urge UI (3) | 18.18 (6.84–29.51) |
UI, urinary incontinence.
Fewer studies provided estimates of severity of UI in American men.36,52,66,75,100 A community-based cross-sectional survey of 778 men older than 40 years reported that 10.8% of the responders had wet underclothing during the last year.75 Among men aged 41 to 60 years from primary care clinics in a US Department of Veterans Affairs facility, 4.8% experienced daily UI.36 The prevalence of daily UI increased to 8.9% among those older than 60 years. Pooled analysis of the American studies estimated that daily UI was experienced by 4.8% of men aged 45 to 64 years (95% CI, 4.8-4.8), 8.3% of those older than 65 years (95% CI, 7.0–9.6), and 9.3% of men older than 80 years (95% CI, 4.5–14.1).36,52,66,75,100 Severe UI that required a change of underwear was reported by 2% of those aged 45 to 64 years and 4% of men older than 65 years (95% CI, 3.9–4.1).
Three studies from the United States provided data on prevalence rates in racial/ethnic groups, but the survey methodology varied, including methods for estimating prevalence.2,36,50 In 1 large population-based survey using a weighted prevalence estimate, non-Hispanic black men had a higher rate of UI (21%) compared with non-Hispanic white men (16%) and Mexican American men (14%).2 In the other study, non- Hispanic men (38%) were more likely than Hispanic men (31%) to have UI.50 White men (32%) and black men (33%) in a sample of male veterans receiving care in primary care clinics had similar rates of UI.36
Data are scarce on the incidence of UI in community-dwelling men, excluding studies of men after prostatectomy.30,98,108,109 One-year incidence rates vary depending on the age of the study population. In 1 study of men aged 40 years and older residing in the United Kingdom, the 1-year incident rate was 4%, with incidence of involuntary leakage increasing from 2% in those aged 40 to 49 years to 11% in those 80 years and older.98 In a study of American men aged 60 years and older, the 1-year incidence rate of involuntary leakage was 20% (weighted for nonresponders).30 There are no data available on the incidence of the different types of UI or comparisons by racial/ethnic groups. There is limited evidence on the progression and remission of UI in men. Evidence indicates that when men became incontinent, they developed urge or other types of UI; those with urge UI alone either stayed as urge UI or developed mixed UI.30 In 1 study over a 10-year period, 3% of men without either urgency or urgency with incontinence at baseline developed urge UI. There was a slight nonsignificant decline in men with urge UI at baseline to have it at the 10-year follow-up (5% vs 4%, respectively).95
Risk Factors for UI in Community-Dwelling Men
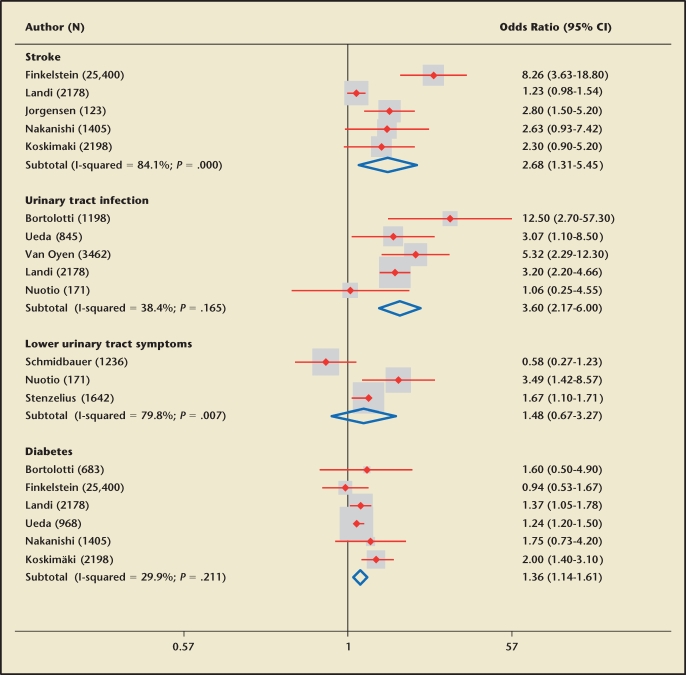
Associations between UI and risk factors adjusted for confounding factors were reported in 39 studies35–38,42,49,54,58, 64,67,71,73,74,78,83,86,89–91,93,101,110–127 (Table 1; Appendix Table 1 [available at www.medreviews.com]). Age as an independent risk factor for UI was analyzed in 8 studies,37,42,67,91,120,122,126,128 with significant positive association with total UI in 2 studies42, 67 and urge UI (OR 5.34; 95% CI, 2.26–12.62) among those older than 70 years compared with younger men in 1 study.37 Diabetes demonstrated consistent positive association with UI (Figure 2). Comorbidities and poor general health were associated with UI in several studies (Table 1).38,42,90,93 The presence of fecal incontinence was associated with an increased odds of urge UI in 1 study of 2198 men (OR 17; 95% CI, 7.5–40)117 but with random changes in another.58 Men with arthritis had higher adjusted odds of total UI (OR 1.6; 95% CI, 1.1–2.4)54 and urge UI (OR 1.8; 95% CI, 1.4–2.4).117 The National Population Health Survey in Canada reported that use of narcotics, laxatives, and diuretics was associated with greater odds of UI independent of other risk factors.54 Memory problems, epilepsy, and neurologic diseases were associated with higher rates of UI.35,42,54,67,101,117,125 Stroke was associated with UI (Figure 2) in community-dwelling men (pooled OR 2.7; 95% CI, 1.3–5.5) with variable estimations from individual studies, depending on time of follow-up and definitions of UI. Restrictions in activities of daily living were associated with higher adjusted odds of UI in men in all studies that examined the relationship.42,49,58,93
Figure 2.
Association between risk factors and prevalence of urinary incontinence (adjusted odds ratios from individual studies and pooled analysis with random-effects models). CI, confidence interval.
Men with urinary tract infections had higher adjusted odds of UI (Figure 2), with a pooled OR of 3.6 (95% CI, 2.17–6).35,37,42,58,93 Men with prostate diseases had higher rates of UI after adjustment for confounding factors in the majority of studies.71,93,117,126 Prostate cancer (RR 2; 95% CI, 1.5–2.8), radical prostatectomy (RR 4.3; 95% CI, 2.6–7.3), and radiotherapy for prostate cancer (RR 2.3; 95% CI, 1.3–4.1) were associated with increased adjusted relative risk of UI.71
Clinical Interventions for UI in Community-Dwelling Men
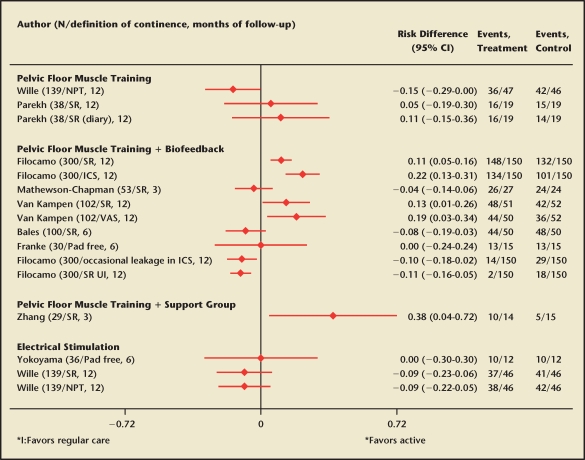
Outcome: Continence. Behavioral interventions for UI in men with prostate diseases were examined in 10 RCTs (Table 3; Appendix Table 2 [available at www.medreviews.com]).129–137 Continence rates in the control groups were more than 60% across all RCTs, with no statistically significant differences compared with active treatments. The highest continence rate was reported in a large well-designed RCT of early pelvic floor rehabilitation in patients who had radical retropubic prostatectomy for clinical stage T1 or T2 prostate cancer136 (Figure 3). The majority of patients (99%) reported continence after the intervention that included verbal explanations, palpation, and Kegel exercises, with a small significant relative benefit compared with usual care (RR 1.1; 95% CI, 1.1–1.2).136 The relative effect in the same RCT was slightly larger when continence status was measured with a scale specific for UI (RR 1.3; 95% CI, 1.2–1.5).136 Pelvic floor muscle training combined with biofeedback resulted in greater self-reported continence compared with standard care (pooled absolute risk difference 0.1; 95% CI, 0.05–0.14), but the effect size was not consistent across the studies (P value for heterogeneity, .03).131,136,137
Table 3.
Clinical Intervention on Urinary Incontinence (Results From Individual RCTs)
| Author, Year (Follow-up in Months) | Active | Control | Outcomes | Events/Active | Events/Control | Absolute Risk Difference (95% CI) (Top) Relative Risk (95% CI) (Bottom) |
| Wille, 2003134 (12) | Postoperative PFMT | Postoperative, electrical stimulation | Continence | 41/47 | 41/46 | −0.02 (−0.15–0.11) |
| 0.98 (0.84–1.14) | ||||||
| Yokoyama, 2004135 (6) | Extracorporeal magnetic innervation | PFMT | Continence | 11/12 | 10/12 | 0.08 (−0.18–0.35) |
| 1.1 (0.81–1.5) | ||||||
| Porru, 2001138 (1) | PFMT | Standard care | UI | 4/30 | 12/28 | −0.30 (−0.52–−0.08)* |
| 0.31 (0.11–0.85)* | ||||||
| Dorey, 2004139 (3) | PFMT | Advice on lifestyle changes | Improved UI | 14/28 | 1/27 | 0.46 (0.26–0.66)* |
| 13.5 (1.90–95.71)* | ||||||
| Moore, 1999173 (6) | Intensive PFMT conducted by a physiotherapist | Standard treatment | UI that affected life | 6/19 | 3/21 | 0.17 (−0.08–0.43) |
| 2.21 (0.64–7.63) | ||||||
| Burgio, 2006174 (6) | Preoperative session of biofeedback-assisted PFMT | Usual care | Need protection | 16/63 | 24/62 | −0.13 (−0.30–0.03) |
| 0.66 (0.39–1.11) | ||||||
| UI | 11/63 | 24/62 | −0.21 (−0.37–−0.06)* | |||
| 0.45 (0.24–0.84)* | ||||||
| UI that affected life | 28/63 | 33/62 | −0.09 (−0.26–0.09) | |||
| 0.84 (0.58–1.20) | ||||||
| Chancellor, 1999140 (24) | UroLume sphincteric stent prosthesis | Conventional external sphincterotomy | Improved UI | 12/31 | 6/26 | 0.16 (−0.08–0.39) |
| 1.68 (0.73–3.85) | ||||||
| Deliveliotis, 2005155 (12) | Neurovascular bundles with steroid cream applied locally to both neurovascular bundles | Usual neurovascular bundles | Continence | 28/30 | 27/30 | 0.03 (−0.11–0.17) |
| 1.04 (0.90–1.21) | ||||||
| Parsons, 2004156 (12) | Methylprednisolone beginning on postoperative day intravenously, then orally | Placebo | Continence | 33/34 | 36/36 | −0.03 (−0.11–0.05) |
| 0.97 (0.90–1.05) | ||||||
| Little, 2003148 (24) | Radiation with a 4-field box technique to a dose of 70 Gy | Radiation with 6-field boost plan of a total dose of 78 Gy | UI | 40/108 | 32/103 | 0.06 (−0.07–0.19) |
| 1.19 (0.82–1.75) | ||||||
| Need protection | 4/108 | 5/103 | −0.01 (−0.07–0.04) | |||
| 0.76 (0.21–2.76) | ||||||
| Wasson, 1995152 (36) | Transurethral resection of prostate | Watchful waiting | Persistent UI | 4/280 | 4/276 | 0.00 (−0.02–0.02) |
| 0.99 (0.25–3.90) | ||||||
| Srougi, 2005150 (6) | Retropubic radical prostatectomy with bladder neck mucosal eversion | Retropubic radical prostatectomy | UI | 44/48 | 43/47 | 0.00 (−0.11–0.11) |
| 1.00 (0.88–1.13) | ||||||
| Van Cangh, 1998153 (24) | 60 Gy external radiotherapy between 12 and 16 wk after radical prostatectomy | Radical prostatectomy alone | Continence | 37/48 | 43/52 | −0.06 (−0.21–0.10) |
| 0.93 (0.76–1.17) | ||||||
| UI | 0/48 | 0/52 | 0 | |||
| Severe UI with implantation of artificial sphincter | 1/48 | 1/52 | 0.00 (−0.05–0.06) | |||
| 1.08 (0.07–16.84) | ||||||
| Srougi, 2001144 (6) | Radical retropubic prostatectomy with bladder neck preservation (Malizia) | Radical retropubic prostatectomy with bladder neck resection (Walsh) | Continence | 30/31 | 36/39 | 0.04 (−0.06–0.15) |
| 1.05 (0.94–1.17) | ||||||
| Ghaly, 2003147 (12) | Radiotherapy with I-125 (144 Gy, TG-43) | Radiotherapy with Pd-103 | Need protection or self-catheterization | 1/57 | 5/51 | −0.08 (−0.17–0.01) |
| 0.18 (0.02–1.48) | ||||||
| Radiotherapy with Pd-103 (90 Gy) | Radiotherapy with Pd-103 (115 Gy) | Need protection or self-catheterization | 3/57 | 5/51 | −0.05 (−0.15–0.05) | |
| 0.54 (0.14–2.14) | ||||||
| Imamoglu, 2005149 (48) | Macroplastique injection | Artificial urethral sphincter implantation | Continence | 8/10 | 10/11 | −0.11 (−0.41–0.19) |
| 0.88 (0.61–1.26) | ||||||
| UI | 1/10 | 0/11 | 0.10 (−0.13–0.33) | |||
| 3.27 (0.15–72.23) | ||||||
| Improved UI | 3/13 | 8/11 | −0.50 (−0.85–−0.15)* | |||
| 0.32 (0.11–0.91)* | ||||||
| Akakura, 1999143 (58) | Radical prostatectomy with androgen antagonist | External beam radiation with androgen antagonist | Improved UI | 22/56 | 0/44 | 0.39 (0.26–0.52)* |
| 35.53 (2.22–569.82)* | ||||||
| Fransson, 2001145 (35) | Radiotherapy with a total dose of 4.8 Gy | Active surveillance | Need protection | 10/59 | 1/49 | 0.15 (0.05–0.25)* |
| 8.31 (1.10–62.63)* | ||||||
| Gupta, 2002146 (12) | Transurethral resection of the prostate with the thick vapor resection loop | Transurethral resection of the prostate with standard wire loop | UI | 0/50 | 2/50 | −0.04 (−0.11–0.03) |
| 0.2 (0.01–4.06) | ||||||
| Gallucci, 1998154 (12) | Transurethral resection of the prostate | Transurethral electrovaporization of the prostate | UI | 0/80 | 13/70 | −0.19 (−0.28–−0.09)* |
| 0.03 (0.00–0.54)* | ||||||
| Wilson, 2006151 (12) | Holmium laser enucleation of the prostate | Transurethral resection of the prostate | Regained UI | 15/30 | 8/30 | 0.23 (−0.01–0.47) |
| 1.88 (0.94–3.75) | ||||||
| UI | 1/30 | 0/30 | 0.03 (−0.05–0.12) | |||
| 3 (0.13–70.83) | ||||||
| Steineck, 2002142 (12) | Radical prostatectomy | Watchful waiting | Frequent UI | 80/189 | 33/187 | 0.25 (0.16–0.34)* |
| 2.40 (1.69–3.41)* | ||||||
| UI | 101/189 | 53/187 | 0.25 (0.15–0.35)* | |||
| 1.89 (1.45−2.45)* | ||||||
| Moderate or severe UI | 30/189 | 3/187 | 0.14 (0.09–0.20)* | |||
| 9.89 (3.07–31.86)* | ||||||
| Moderate or great distress from UI | 47/189 | 15/187 | 0.17 (0.10–0.24)* | |||
| 3.10 (1.80–5.35)* | ||||||
| Great distress | 14/189 | 5/187 | 0.05 (0.00–0.09)* | |||
| 2.77 (1.02–7.54)* | ||||||
| Regular need protection | 71/189 | 16/187 | 0.29 (0.21–0.37)* | |||
| 4.39 (2.65–7.26)* | ||||||
| Regular dependence on diaper or urine bag | 23/189 | 1/187 | 0.12 (0.07–0.16)* | |||
| 22.76 (3.10–166.80)* | ||||||
| UI affecting sexual life | 15/189 | 5/187 | 0.05 (0.01–0.10)* | |||
| 2.97 (1.10–8.00)* |
RCT, randomized controlled trial; PFMT, pelvic floor muscle training; UI, urinary incontinence.
Significant association at 95% confidence level.
Figure 3.
Effects of conservative treatments on continence compared with regular care (results from randomized controlled clinical trials). RD, absolute risk difference; NPT, negative pad test; SR, self-reported; ICS, completely dry in International Continence Society-male questionnaire; VAS, visual analogue scale.
Outcome: UI in Community-Dwelling Men. The effects on severity of UI of behavioral interventions were inconsistent in direction and size compared with usual care. Few RCTs reported significant benefits of behavioral treatments to reduce the risk of UI. The rate of self-reported UI was 70% less after verbal instruction and feedback on contractions of pelvic floor muscles in 63 patients with bladder outflow obstruction and diagnosis of symptomatic benign prostatic hyperplasia who underwent transurethral prostatectomy (RR 0.3; 95% CI, 0.1–0.9).138 Pelvic floor muscle training, including a strong postvoid “squeeze out” pelvic floor muscle contraction, biofeedback, and suggestions to change lifestyle, significantly reduced postmicturition dribble and urine loss in men with erectile dysfunction.139 One large trial showed a substantial benefit of a complex floor rehabilitation program, including patient education, assessment of pelvic floor muscle strength, and visualization of Kegel pelvic floor muscle training compared with regular care with reduction in severity and pad utilization (RR of using 2 pads per day 0.1; 95% CI, 0.01–0.7).136
Two RCTs examined medical devices on UI in men (Appendix Table 2 [available at www.medreviews.com]).140,141 One small RCT did not show a relative benefit of a UroLume sphincteric stent inserted cystoscopically to conventional external sphincterotomy in 57 men with spinal cord injury and electromyographic and manometric evidence of external detrusor-sphincter dyssynergia.140 A second small crossover RCT comparing penile compression devices in men 6 months after radical prostatectomy141 did not show differences in resistance index and urine loss during the 4-hour pad test compared with no device.
Effects of Clinical Interventions for Urologic Diseases on UI
Effects of clinical interventions for urologic diseases on UI142–154 were examined after treatments for prostate cancer143–145,147–150,153,155–157 or benign prostate diseases146,151,152,154 (Appendix Table 2 [available at www.medreviews.com]).
Transurethral resection of prostate compared with watchful waiting (1 RCT) did not result in higher rates of persistent UI.152 Radical prostatectomy compared with watchful waiting (1 RCT) resulted in significant increase of UI of moderate or greater severity that caused distress and affected sexual life.142 Radical prostatectomy compared with external beam radiation increased risk of UI (1 RCT).143 Radiotherapy for prostate cancer compared with watchful waiting (1 RCT) resulted in significant increase in UI that required use of pads.145
Adjuvant external beam radiation compared with radical prostatectomy alone (1 RCT) did not increase relative risk of UI and severe UI that would require implantation of artificial sphincter.153 Different doses and regimens of radiotherapy resulted in the same rates of UI (2 RCTs).144,147,148 Bladder neck preservation techniques resulted in the same rates of UI (2 RCTs).144,150
Artificial urethral sphincter implantation compared with macroplastique injection above or around the striated sphincter region of the urethra (1 RCT) increased rates of continence.149 Different methods of transurethral resection of prostate (3 RCTs) resulted in the same rate of UI.146,151,154
Patient Outcome: Continence. Urinary continence was reported in 3 RCTs.144,149,153 The highest rate of urinary continence (>92%) was reported after radical retropubic prostatectomy with bladder neck preservation.144
Artificial urethral sphincter implantation and macroplastique injection in the sphincter region of the urethra resulted in continence in 80% and 91% of patients with minimal baseline incontinence, respectively.149 The rates of social continence were lower and differed substantially, depending on baseline incontinence.149 Only 1 RCT reported continence (77%) after combined therapy of prostate cancer.153 No evidence showed a significant relative benefit of continence between compared interventions.
Almost all patients with benign prostate diseases were continent after transurethral resection of the prostate with the thick vapor resection loop146 and transurethral resection of the prostate.154 In contrast, Holmium laser enucleation resulted in 50% of UI in the same population of men with bladder outflow obstruction secondary to benign prostatic hyperplasia.151
Patients with prostate cancer reported different rates of UI depending on the type and definition. Retropubic radical prostatectomy and vesicourethral anastomosis with and without bladder neck eversion resulted in UI in more than 90% of patients.150 The highest rate of urge UI (44%) was shown after radiation therapy with a 4-field box technique to a dose of 70 Gy.148 The same treatment resulted in only 7% of self-reported stress UI in this trial.148 The lowest incidence of UI among patients with prostate cancer was reported after supplemental beam radiation with I-125 (144 Gy) (1%).147
Indirect comparisons showed inconsistent relative risks of UI after surgical treatments and radiotherapy. The largest relative differences were observed in the risk of transient stress incontinence after transurethral resection of the prostate compared with electrovaporization in patients with benign hypertrophy of the prostate (0.1% vs 18.6%, respectively).154 The rates of UI were substantially higher after adjuvant hormone therapy and surgery (300 mg of diethylstilbestrol diphosphate per day) compared with adjuvant hormone therapy and external beam radiation (RR 35.5; 95% CI, 2.2–569.3). Patients with total baseline incontinence for more than 6 months after radical retropubic prostatectomy, transvesical prostatectomy, or transurethral prostatectomy reported continence more often after macroplastique injection to the sphincter region of the urethra compared with artificial urethral sphincter implantation (RR 0.3; 95% CI, 0.1–0.9).149 Pad utilization was higher after radiotherapy compared with active surveillance (RR 8.3; 95% CI, 1.1–62.6).145
Pharmacologic Treatments for UI
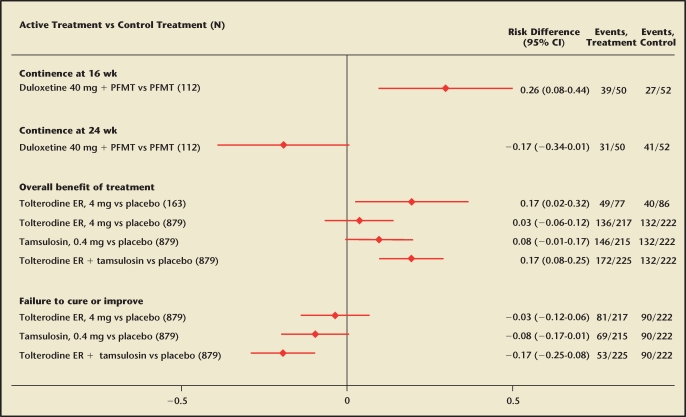
Pharmacologic treatments for UI included antidepressants combined with pelvic floor muscle training,158 muscarinic antagonists, and adrenergic α-antagonists159–162 (Appendix Table 3 [available at www.medreviews.com]). Duloxetine combined with pelvic floor muscle training compared with pelvic floor muscle training alone was more effective at 16 but not 24 weeks of treatment158 (Figure 4). Tolterodine alone and combined with tamsulosin resulted in greater perception of overall benefit of the treatment compared with placebo (Figure 4). Adverse events (Appendix Table 3 [available at www.medreviews.com]) included dry mouth and dizziness.
Figure 4.
Effects of pharmacologic treatments on continence compared with placebo or pelvic floor muscle training (results from randomized controlled clinical trials). PFMT, pelvic floor muscle training; ER, extended release.
Discussion
The present report confirmed the significant diversity of interventions used, sampling strategies and definitions, and measurement of outcomes.22,163,164 Preventive nonsurgical interventions were examined in men with prostate diseases but not in patients with other risk factors for incontinence. Such studies relied largely on patients in clinics134,135,165 and followed them for less than 6 months,137–139 with few studies reporting long-term outcomes.131,133,134,136 Selection criteria varied for the same interventions. For example, some trials of pelvic floor muscle rehabilitation after radical prostatectomy excluded patients with prior UI136,166 or severe UI135; others included incontinent patients only.131 Pooled analysis was questionable owing to sampling differences in the present report and previous systematic reviews.167,168 Applicability of observational studies and clinical trials was restricted to the sampled male populations and definitions of incontinence. Whether the same effects would be observed in population-based samples requires future research.
Despite extensive efforts to standardize the definitions of incontinence, 21 the original studies measured self-reported symptoms and signs of incontinence, severity, and quality of life related to incontinence and objective instrumented evidence of leakage inconsistently within and across the studies. Prevalence and incidence estimates differed according to measures of length (ever, last year, last month), type (total UI vs urge or stress UI), severity (frequency and amount of urine), and effects on quality of life. Ratings of success, including improvement in incontinence and in quality of life by doctors and patients, were also different.169 Objective measures of UI demonstrated random changes in most RCTs (the data not shown are available in the full text of the report: http://www.ahrq.gov/downloads/pub/evidence/pdf/fuiad/fuiad.pdf). The objective improvements in selected physiologic measures were not consistent after the same interventions and did correlate with self-reported continence and reduction in severity of UI.137,140,141,151,166 Other systematic reviews concluded that the data are not sufficient to propose the invasive and costly urodynamic testing as a measure of success to reduce risk of incontinence.170 A small proportion of RCTs reported the effects of clinical intervention on improvements in quality of life.142,143,145 Composite outcomes, including both self-reported changes in severity of incontinence and physiologic parameters in a common scale, may offer a better choice to measure success of clinical interventions.171,172
Despite substantial heterogeneity among studies, attributable benefit for public health can be estimated from individual RCTs. Compared with regular care, an early pelvic floor muscle rehabilitation program after radical prostatectomy would result in 107 additional cases of continence per 1000 treated men (95% CI, 47–170).136 Pelvic-floor muscle exercises and biofeedback would result in 180 additional continence cases per 1000 treated (95% CI, 23–396).131
Different treatments for prostate diseases resulted in comparable rates of incontinence, with higher risk for UI after radical prostatectomy. Medical devices were examined in a few trials and failed to improve UI. Pharmacologic treatments for overactive bladder included an effective combination of tolterodine and tamsulosin. We did not analyze case series that described the experience of individual institutions to treat UI (available at http://www.ahrq.gov/downloads/pub/evidence/pdf/fuiad/fuiad.pdf). Such publications may be useful to generate hypotheses for well-designed trials but have poor internal and external validity and do not provide good evidence about comparative effectiveness of different treatments. Ongoing trials examine the effects of stem cells, botulinum toxin type A, solifenacin, pelvic floor muscle training with biofeedback, and new medical devices on male incontinence (Appendix Table 4 [available at www.medreviews.com]).
The independent contribution of risk factors on UI was analyzed with adjusted ORs in cross-sectional and retrospective cohort studies. Care must be taken to distinguish associations from actual risks. Observational studies cannot establish causality between comorbidities and UI. Adjusted ORs estimated probability of having incontinence among men with particular diseases compared with those without such diseases. The estimations are still valuable because they identify subgroups at higher probability of incontinence. However, multivariate models included different sets of risk factors. Because causality between risk factors and incontinence could not be determined from such studies, and the majority of risk factors are not modifiable, we hesitated to estimate events attributable to the risk factors.
Policy Implications
Systematic standardized evaluation of incidence and risk factors for incontinence is possible using the behavioral risk factor surveillance system in large nationally representative population groups. Routinely collected clinical history should include evaluation of the risk factors, symptoms, and signs of incontinence. Men with prostate diseases, poor general health, diabetes, and physical limitations should be actively treated for incontinence. Early pelvic floor rehabilitation after treatments for prostate diseases, including pelvic floor muscle training, may reduce UI in men. Preventive strategies might include assessment and reduction of modifiable risk factors in early stages of incontinence, when incontinence is minimal and does not affect the quality of life.
Main Points.
This review aimed to synthesize evidence of the effectiveness of different clinical interventions to prevent the occurrence and progression of urinary incontinence (UI) in community-dwelling men.
Despite extensive efforts to standardize the definitions of incontinence, the original studies measured self-reported symptoms and signs of incontinence, severity, and quality of life related to incontinence and objective instrumented evidence of leakage inconsistently within and across the studies.
Compared with regular care, an early pelvic floor muscle rehabilitation program after radical prostatectomy would result in 107 additional cases of continence per 1000 treated men (95% confidence interval [CI], 47–170).
Pelvic-floor muscle exercises and biofeedback would result in 180 additional continence cases per 1000 treated men (95% CI, 23–396).
Different treatments for prostate diseases resulted in comparable rates of incontinence, with higher risk for UI after radical prostatectomy. Medical devices were examined in a few trials and failed to improve UI. Pharmacologic treatments for overactive bladder included an effective combination of tolterodine and tamsulosin.
Systematic standardized evaluation of incidence and risk factors for incontinence is possible using the behavioral risk factor surveillance system in large nationally representative population groups. Routinely collected clinical history should include evaluation of the risk factors, symptoms, and signs of incontinence.
Men with prostate diseases, poor general health, diabetes, and physical limitations should be actively examined and treated for incontinence.
Acknowledgments
This document is based on research conducted by the Minnesota Evidence-based Practice Center under contract to the Agency for Healthcare Research and Quality, Rockville, MD (Contract No. 290-02-0009). The findings and conclusions in this document are those of the authors, who are responsible for its contents; the findings and conclusions do not necessarily represent the position of the Agency for Healthcare Research and Quality. Therefore, no statement in this document should be construed as an official position of the Agency for Healthcare Research and Quality or of the U.S. Department of Health and Human Services.
Dr. Wilt was also supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant RO1 063300-01A2.
The authors thank the librarians Jim Beattie, MLIS, Judy Stanke, MA, and Delbert Reed, PhD, for their contributions to the literature search; Jing Du, Ryan Ping, Joseph Kaiya, MD, Susan Penque, and Mary Dierich for their assistance with the literature search and data abstraction; Linda Brubaker, MD, Tomas Griebling, MD, Robert Madoff, MD, Richard Nelson, MD, Joseph Ouslander, MD, Neil Resnick, MD, Carolyn Sampselle, PhD, David Thom, MD, PhD, and Joanne Townsend, RN, for serving on the Technical Expert Panel; Chadwick Huckabay, MD, for advice and counsel on urinary incontinence management; and Ingrid Nygaard, MD, Mary H. Palmer, PhD, and Debra Saliba, MD, for reviewing the draft of this report and providing helpful recommendations for revisions and clarifications.
References
- 1.Abrams P, Cardozo L, Khoury S, Wein A. Incontinence. Proceedings from the 3rd International Consultation on Incontinence. Paris: Health Publications; 2005. [Google Scholar]
- 2.Anger JT, Saigal CS, Stothers L, et al. The prevalence of urinary incontinence among community dwelling men: results from the National Health and Nutrition Examination survey. J Urol. 2006;176:2103–2108. doi: 10.1016/j.juro.2006.07.029. discussion 2108. [DOI] [PubMed] [Google Scholar]
- 3.Harris SS, Link CL, Tennstedt SL, et al. Care seeking and treatment for urinary incontinence in a diverse population. J Urol. 2007;177:680–684. doi: 10.1016/j.juro.2006.09.045. [DOI] [PubMed] [Google Scholar]
- 4.Kasper DL, Harrison TR NetLibrary Inc., authors Harrison’s Principles of Internal Medicine. [Accessed November 2007]. Available at: http://www.netLibrary.com/urlapi.asp?action=summary&v=1&bookid=149871.
- 5.Abrams P, Cardozo L, Khoury S, Wein AJ. Incontinence. Paris: Health Publications; 2005. [Google Scholar]
- 6.Higgins J, Green S. The Cochrane Handbook for Systematic Reviews of Interventions. 2006. [Accessed November 2007]. Available at: http://www.cochrane.org/resources/handbook/handbook.pdf.
- 7.US Agency for Healthcare Research and Quality, authors. Systems to Rate the Strength of Scientific Evidence. Rockville, MD: University of California SF-SE-BPC; 2002. [Google Scholar]
- 8.Dawson B, Trapp RG. Basic & Clinical Biostatistics (LANGE Basic Science) 3rd ed. New York: Lange Medical Books-McGraw-Hill; 2004. [Google Scholar]
- 9.Kahn HA, Sempos CT. Statistical Methods in Epidemiology (Monographs in Epidemiology and Biostatistics) New York: Oxford University Press; 1989. [Google Scholar]
- 10.Al-Marzouki S, Evans S, Marshall T, Roberts I. Are these data real? Statistical methods for the detection of data fabrication in clinical trials. BMJ. 2005;331:267–270. doi: 10.1136/bmj.331.7511.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buyse M, George SL, Evans S, et al. The role of biostatistics in the prevention, detection and treatment of fraud in clinical trials. Stat Med. 1999;18:3435–3451. doi: 10.1002/(sici)1097-0258(19991230)18:24<3435::aid-sim365>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Cook DJ, Eastwood S, et al. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet. 1999;354:1896–1900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 13.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 14.Atkins D, Briss PA, Eccles M, et al. Systems for grading the quality of evidence and the strength of recommendations II: pilot study of a new system. BMC Health Serv Res. 2005;5:25. doi: 10.1186/1472-6963-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atkins D, Eccles M, Flottorp S, et al. Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches. The GRADE Working Group. BMC Health Serv Res. 2004;4:38. doi: 10.1186/1472-6963-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aschengrau A, Seage GR. Essentials of Epidemiology in Public Health. Sudbury, MA: Jones and Bartlett; 2003. [Google Scholar]
- 17.Vist GE, Hagen KB, Devereaux PJ, et al. Outcomes of patients who participate in randomised controlled trials compared to similar patients receiving similar interventions who do not participate. Cochrane Database Syst Rev. 2007;2 doi: 10.1002/14651858.MR000009.pub3. [DOI] [PubMed] [Google Scholar]
- 18.Egger M, Smith GD. Bias in location and selection of studies. BMJ. 1998;316:61–66. doi: 10.1136/bmj.316.7124.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dickersin K, Min YI. NIH clinical trials and publication bias. Online J Curr Clin Trials. 1993 Doc. no. 50. [PubMed] [Google Scholar]
- 20.Thornton A, Lee P. Publication bias in meta-analysis: its causes and consequences. J Clin Epidemiol. 2000;53:207–216. doi: 10.1016/s0895-4356(99)00161-4. [DOI] [PubMed] [Google Scholar]
- 21.Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Urology. 2003;61:37–49. doi: 10.1016/s0090-4295(02)02243-4. [DOI] [PubMed] [Google Scholar]
- 22.Abrams P, Cardozo L, Khoury S, Wein A. Incontinence. 3rd International Consultation on Incontinence. Plymouth, UK: Health Publication, Ltd.; 2005. [Google Scholar]
- 23.Whitehead A. Meta-analysis of Controlled Clinical Trials. New York: John Wiley & Sons; 2002. [Google Scholar]
- 24.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 25.Viechtbauer W. Confidence intervals for the amount of heterogeneity in meta-analysis. Stat Med. 2007;26:37–52. doi: 10.1002/sim.2514. [DOI] [PubMed] [Google Scholar]
- 26.Knapp G, Biggerstaff BJ, Hartung J. Assessing the amount of heterogeneity in random-effects meta-analysis. Biom J. 2006;48:271–285. doi: 10.1002/bimj.200510175. [DOI] [PubMed] [Google Scholar]
- 27.Moore DS, McCabe GP. Introduction to the Practice of Statistics. 4th ed. New York: W.H. Freeman; 2003. [Google Scholar]
- 28.Egger M, Smith GD, Altman DG. Systematic Reviews in Health Care. London: NetLibrary, BMJ Books; 2001. [Google Scholar]
- 29.Ebrahim S. The use of numbers needed to treat derived from systematic reviews and metaanalysis. Caveats and pitfalls. Eval Health Prof. 2001;24:152–164. doi: 10.1177/01632780122034858. [DOI] [PubMed] [Google Scholar]
- 30.Herzog AR, Diokno AC, Brown MB, et al. Two-year incidence, remission, and change patterns of urinary incontinence in noninstitutionalized older adults. J Gerontol. 1990;45:M67–M74. doi: 10.1093/geronj/45.2.m67. [DOI] [PubMed] [Google Scholar]
- 31.Bogren MA, Hvarfwen E, Fridlund B. Urinary incontinence among a 65-year old Swedish population: medical history and psychosocial consequences. Vard Nord Utveckl Forsk. 1997;17:14–17. doi: 10.1177/010740839701700404. [DOI] [PubMed] [Google Scholar]
- 32.Malmsten UG, Milsom I, Molander U, Norlen LJ. Urinary incontinence and lower urinary tract symptoms: an epidemiological study of men aged 45 to 99 years. J Urol. 1997;158:1733–1737. doi: 10.1016/s0022-5347(01)64113-2. [DOI] [PubMed] [Google Scholar]
- 33.Koskimaki J, Hakama M, Huhtala H, Tammela TL. Prevalence of lower urinary tract symptoms in Finnish men: a population-based study. Br J Urol. 1998;81:364–369. doi: 10.1046/j.1464-410x.1998.00565.x. [DOI] [PubMed] [Google Scholar]
- 34.Damian J, Martin-Moreno JM, Lobo F, et al. Prevalence of urinary incontinence among Spanish older people living at home. Eur Urol. 1998;34:333–338. doi: 10.1159/000019750. [DOI] [PubMed] [Google Scholar]
- 35.Bortolotti A, Bernardini B, Colli E, et al. Prevalence and risk factors for urinary incontinence in Italy. Eur Urol. 2000;37:30–35. doi: 10.1159/000020096. [DOI] [PubMed] [Google Scholar]
- 36.Smoger SH, Felice TL, Kloecker GH. Urinary incontinence among male veterans receiving care in primary care clinics. Ann Intern Med. 2000;132:547–551. doi: 10.7326/0003-4819-132-7-200004040-00006. [DOI] [PubMed] [Google Scholar]
- 37.Ueda T, Tamaki M, Kageyama S, et al. Urinary incontinence among community-dwelling people aged 40 years or older in Japan: prevalence, risk factors, knowledge and self-perception. Int J Urol. 2000;7:95–103. doi: 10.1046/j.1442-2042.2000.00147.x. [DOI] [PubMed] [Google Scholar]
- 38.Maggi S, Minicuci N, Langlois J, et al. Prevalence rate of urinary incontinence in community- dwelling elderly individuals: the Veneto study. J Gerontol A Biol Sci Med Sci. 2001;56:M14–M18. doi: 10.1093/gerona/56.1.m14. [DOI] [PubMed] [Google Scholar]
- 39.Bogner HR, Gallo JJ, Sammel MD, et al. Urinary incontinence and psychological distress in community-dwelling older adults. J Am Geriatr Soc. 2002;50:489–495. doi: 10.1046/j.1532-5415.2002.50115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Araki I, Zakoji H, Komuro M, et al. Lower urinary tract symptoms in men and women without underlying disease causing micturition disorder: a cross-sectional study assessing the natural history of bladder function. J Urol. 2003;170:1901–1904. doi: 10.1097/01.ju.0000092942.87643.27. [DOI] [PubMed] [Google Scholar]
- 41.Boyle P, Robertson C, Mazzetta C, et al. The prevalence of male urinary incontinence in four centres: the UREPIK study. BJU Int. 2003;92:943–947. doi: 10.1111/j.1464-410x.2003.04526.x. [DOI] [PubMed] [Google Scholar]
- 42.Landi F, Cesari M, Russo A, et al. Potentially reversible risk factors and urinary incontinence in frail older people living in community. Age Ageing. 2003;32:194–199. doi: 10.1093/ageing/32.2.194. [DOI] [PubMed] [Google Scholar]
- 43.Adelmann PK. Prevalence and detection of urinary incontinence among older Medicaid recipients. J Health Care Poor Underserved. 2004;15:99–112. doi: 10.1353/hpu.2004.0001. [DOI] [PubMed] [Google Scholar]
- 44.Haltbakk J, Hanestad BR, Hunskaar S. Diversity of urinary symptoms in patients tentatively diagnosed with benign prostatic hyperplasia referred to a urologic clinic in Norway. Scand J Urol Nephrol. 2004;38:454–461. doi: 10.1080/00365590410018657. [DOI] [PubMed] [Google Scholar]
- 45.Holroyd-Leduc JM, Mehta KM, Covinsky KE. Urinary incontinence and its association with death, nursing home admission, and functional decline. J Am Geriatr Soc. 2004;52:712–718. doi: 10.1111/j.1532-5415.2004.52207.x. [DOI] [PubMed] [Google Scholar]
- 46.Andersson G, Johansson JE, Garpenholt O, Nilsson K. Urinary incontinence-prevalence, impact on daily living and desire for treatment: a population- based study. Scand J Urol Nephrol. 2004;38:125–130. doi: 10.1080/00365590310022608. [DOI] [PubMed] [Google Scholar]
- 47.Lagergren M, Fratiglioni L, Hallberg IR, et al. A longitudinal study integrating population, care and social services data. The Swedish National study on Aging and Care (SNAC) Aging Clin Exp Res. 2004;16:158–168. doi: 10.1007/BF03324546. [DOI] [PubMed] [Google Scholar]
- 48.Teunissen TA, van den Bosch WJ, van den Hoogen HJ, Lagro-Janssen AL. Prevalence of urinary, fecal and double incontinence in the elderly living at home. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15:10–13. doi: 10.1007/s00192-003-1106-8. discussion 13. [DOI] [PubMed] [Google Scholar]
- 49.Stenzelius K, Mattiasson A, Hallberg IR, Westergren A. Symptoms of urinary and faecal incontinence among men and women 75+ in relations to health complaints and quality of life. Neurourol Urodyn. 2004;23:211–222. doi: 10.1002/nau.20030. [DOI] [PubMed] [Google Scholar]
- 50.Mardon RE, Halim S, Pawlson LG, Haffer SC. Management of urinary incontinence in Medicare managed care beneficiaries: results from the 2004 Medicare Health Outcomes Survey. Arch Intern Med. 2006;166:1128–1133. doi: 10.1001/archinte.166.10.1128. [DOI] [PubMed] [Google Scholar]
- 51.Brocklehurst JC. Urinary incontinence in the community-analysis of a MORI poll. BMJ. 1993;306:832–834. doi: 10.1136/bmj.306.6881.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lagace EA, Hansen W, Hickner JM. Prevalence and severity of urinary incontinence in ambulatory adults: an UPRNet study. J Fam Pract. 1993;36:610–614. [PubMed] [Google Scholar]
- 53.Schulman C, Claes H, Matthijs J. Urinary incontinence in Belgium: a population-based epidemiological survey. Eur Urol. 1997;32:315–320. [PubMed] [Google Scholar]
- 54.Finkelstein MM. Medical conditions, medications, and urinary incontinence. Analysis of a population-based survey. Can Fam Physician. 2002;48:96–101. [PMC free article] [PubMed] [Google Scholar]
- 55.Irwin DE, Milsom I, Hunskaar S, et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol. 2006;50:1306–1314. doi: 10.1016/j.eururo.2006.09.019. discussion 1314–1315. [DOI] [PubMed] [Google Scholar]
- 56.Lewinshtein DJ, Perrotte P, Lebeau T, et al. Normal urinary and sexual function in men without evidence of prostate cancer from Montreal, Canada. BJU Int. 2006;97:1273–1277. doi: 10.1111/j.1464-410X.2006.06155.x. [DOI] [PubMed] [Google Scholar]
- 57.Diokno AC, Estanol MV, Ibrahim IA, Balasubramaniam M. Prevalence of urinary incontinence in community dwelling men: a cross sectional nationwide epidemiological survey. Int Urol Nephrol. 2007;39:129–136. doi: 10.1007/s11255-006-9127-0. [DOI] [PubMed] [Google Scholar]
- 58.Nuotio M, Jylha M, Luukkaala T, Tammela TL. Urinary incontinence in a Finnish population aged 70 and over. Prevalence of types, associated factors and self-reported treatments. Scand J Prim Health Care. 2003;21:182–187. [PubMed] [Google Scholar]
- 59.Borrie MJ, Davidson HA. Incontinence in institutions: costs and contributing factors. CMAJ. 1992;147:322–328. [PMC free article] [PubMed] [Google Scholar]
- 60.O’Brien J, Austin M, Sethi P, O’Boyle P. Urinary incontinence: prevalence, need for treatment, and effectiveness of intervention by nurse. BMJ. 1991;303:1308–1312. doi: 10.1136/bmj.303.6813.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Diokno AC, Brown MB, Herzog AR. Relationship between use of diuretics and continence status in the elderly. Urology. 1991;38:39–42. doi: 10.1016/0090-4295(91)80010-5. [DOI] [PubMed] [Google Scholar]
- 62.Ju CC, Swan LK, Merriman A, et al. Urinary incontinence among the elderly people of Singapore. Age Ageing. 1991;20:262–266. doi: 10.1093/ageing/20.4.262. [DOI] [PubMed] [Google Scholar]
- 63.Ouslander JG, Zarit SH, Orr NK, Muira SA. Incontinence among elderly community-dwelling dementia patients. Characteristics, management, and impact on caregivers. J Am Geriatr Soc. 1990;38:440–445. doi: 10.1111/j.1532-5415.1990.tb03543.x. [DOI] [PubMed] [Google Scholar]
- 64.Diokno AC, Brock BM, Herzog AR, Bromberg J. Medical correlates of urinary incontinence in the elderly. Urology. 1990;36:129–138. doi: 10.1016/0090-4295(90)80211-5. [DOI] [PubMed] [Google Scholar]
- 65.Kutner NG, Schechtman KB, Ory MG, Baker DI. Older adults’ perceptions of their health and functioning in relation to sleep disturbance, falling, and urinary incontinence. FICSIT Group. J Am Geriatr Soc. 1994;42:757–762. doi: 10.1111/j.1532-5415.1994.tb06537.x. [DOI] [PubMed] [Google Scholar]
- 66.Brandeis GH, Baumann MM, Hossain M, et al. The prevalence of potentially remediable urinary incontinence in frail older people: a study using the Minimum Data Set. J Am Geriatr Soc. 1997;45:179–184. doi: 10.1111/j.1532-5415.1997.tb04504.x. [DOI] [PubMed] [Google Scholar]
- 67.Nakanishi N, Tatara K, Naramura H, et al. Urinary and fecal incontinence in a community-residing older population in Japan. J Am Geriatr Soc. 1997;45:215–219. doi: 10.1111/j.1532-5415.1997.tb04511.x. [DOI] [PubMed] [Google Scholar]
- 68.Umlauf MG, Sherman SM. Symptoms of urinary incontinence among older community-dwelling men. J Wound Ostomy Continence Nurs. 1996;23:314–321. doi: 10.1016/s1071-5754(96)90052-2. [DOI] [PubMed] [Google Scholar]
- 69.Mozes B, Shmueli A. Underutilization of health services among patients with urinary symptoms: results of a population-based survey in Israel. Prostate. 1997;33:246–251. doi: 10.1002/(sici)1097-0045(19971201)33:4<246::aid-pros4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 70.Sgadari A, Topinkova E, Bjornson J, Bernabei R. Urinary incontinence in nursing home residents: a cross-national comparison. Age Ageing. 1997;26(suppl 2):49–54. doi: 10.1093/ageing/26.suppl_2.49. [DOI] [PubMed] [Google Scholar]
- 71.Adolfsson J, Helgason AR, Dickman P, Steineck G. Urinary and bowel symptoms in men with and without prostate cancer: results from an observational study in the Stockholm area. Eur Urol. 1998;33:11–16. doi: 10.1159/000019528. [DOI] [PubMed] [Google Scholar]
- 72.Lee E, Yoo KY, Kim Y, et al. Prevalence of lower urinary tract symptoms in Korean men in a community-based study. Eur Urol. 1998;33:17–21. doi: 10.1159/000019529. [DOI] [PubMed] [Google Scholar]
- 73.Roberts RO, Jacobsen SJ, Jacobson DJ, et al. Natural history of prostatism: high American Urological Association Symptom scores among community-dwelling men and women with urinary incontinence. Urology. 1998;51:213–219. doi: 10.1016/s0090-4295(97)00505-0. [DOI] [PubMed] [Google Scholar]
- 74.Goluboff ET, Saidi JA, Mazer S, et al. Urinary continence after radical prostatectomy: the Columbia experience. J Urol. 1998;159:1276–1280. [PubMed] [Google Scholar]
- 75.Roberts RO, Jacobsen SJ, Rhodes T, et al. Urinary incontinence in a community-based cohort: prevalence and healthcare-seeking. J Am Geriatr Soc. 1998;46:467–472. doi: 10.1111/j.1532-5415.1998.tb02468.x. [DOI] [PubMed] [Google Scholar]
- 76.Jitapunkul S, Khovidhunkit W. Urinary incontinence in Thai elderly living in Klong Toey slum. J Med Assoc Thai. 1998;81:160–168. [PubMed] [Google Scholar]
- 77.Koyama W, Koyanagi A, Mihara S, et al. Prevalence and conditions of urinary incontinence among the elderly. Methods Inf Med. 1998;37:151–155. [PubMed] [Google Scholar]
- 78.Joly F, Brune D, Couette JE, et al. Health-related quality of life and sequelae in patients treated with brachytherapy and external beam irradiation for localized prostate cancer. Ann Oncol. 1998;9:751–757. doi: 10.1023/a:1008276632623. [DOI] [PubMed] [Google Scholar]
- 79.Temml C, Haidinger G, Schmidbauer J, et al. Urinary incontinence in both sexes: prevalence rates and impact on quality of life and sexual life. Neurourol Urodyn. 2000;19:259–271. doi: 10.1002/(sici)1520-6777(2000)19:3<259::aid-nau7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 80.Gavira Iglesias FJ, Caridad y Ocerin JM, Perez del Molino Martin J, et al. Prevalence and psychosocial impact of urinary incontinence in older people of a Spanish rural population. J Gerontol A Biol Sci Med Sci. 2000;55:M207–M214. doi: 10.1093/gerona/55.4.m207. [DOI] [PubMed] [Google Scholar]
- 81.Sladden MJ, Hughes AM, Hirst GH, Ward JE. A community study of lower urinary tract symptoms in older men in Sydney, Australia. Aust N Z J Surg. 2000;70:322–328. doi: 10.1046/j.1440-1622.2000.01738.x. [DOI] [PubMed] [Google Scholar]
- 82.Aggazzotti G, Pesce F, Grassi D, et al. Prevalence of urinary incontinence among institutionalized patients: a cross-sectional epidemiologic study in a midsized city in northern Italy. Urology. 2000;56:245–249. doi: 10.1016/s0090-4295(00)00643-9. [DOI] [PubMed] [Google Scholar]
- 83.Tseng IJ, Chen YT, Chen MT, et al. Prevalence of urinary incontinence and intention to seek treatment in the elderly. J Formos Med Assoc. 2000;99:753–758. [PubMed] [Google Scholar]
- 84.Perry S, Shaw C, Assassa P, et al. An epidemiological study to establish the prevalence of urinary symptoms and felt need in the community: the Leicestershire MRC Incontinence Study. Leicestershire MRC Incontinence Study Team. J Public Health Med. 2000;22:427–434. doi: 10.1093/pubmed/22.3.427. [DOI] [PubMed] [Google Scholar]
- 85.Roe B, Doll H. Prevalence of urinary incontinence and its relationship with health status. J Clin Nurs. 2000;9:178–187. doi: 10.1046/j.1365-2702.2000.00346.x. [DOI] [PubMed] [Google Scholar]
- 86.Maral I, Ozkardes H, Peskircioglu L, Bumin MA. Prevalence of stress urinary incontinence in both sexes at or after age 15 years: a cross-sectional study. J Urol. 2001;165:408–412. doi: 10.1097/00005392-200102000-00013. [DOI] [PubMed] [Google Scholar]
- 87.MacLennan AH, Taylor AW, Wilson DH, Wilson D. The prevalence of pelvic floor disorders and their relationship to gender, age, parity and mode of delivery. BJOG. 2000;107:1460–1470. doi: 10.1111/j.1471-0528.2000.tb11669.x. [DOI] [PubMed] [Google Scholar]
- 88.Stoddart H, Donovan J, Whitley E, et al. Urinary incontinence in older people in the community: a neglected problem? Br J Gen Pract. 2001;51:548–552. [PMC free article] [PubMed] [Google Scholar]
- 89.Schmidbauer J, Temml C, Schatzl G, et al. Risk factors for urinary incontinence in both sexes. Analysis of a health screening project. Eur Urol. 2001;39:565–570. doi: 10.1159/000052504. [DOI] [PubMed] [Google Scholar]
- 90.Muscatello DJ, Rissel C, Szonyi G. Urinary symptoms and incontinence in an urban community: prevalence and associated factors in older men and women. Intern Med J. 2001;31:151–160. doi: 10.1046/j.1445-5994.2001.00035.x. [DOI] [PubMed] [Google Scholar]
- 91.Nuotio M, Jylha M, Luukkaala T, Tammela TL. Urgency, urge incontinence and voiding symptoms in men and women aged 70 years and over. BJU Int. 2002;89:350–355. doi: 10.1046/j.1464-4096.2001.01684.x. [DOI] [PubMed] [Google Scholar]
- 92.Goepel M, Hoffmann JA, Piro M, et al. Prevalence and physician awareness of symptoms of urinary bladder dysfunction. Eur Urol. 2002;41:234–239. doi: 10.1016/s0302-2838(02)00014-3. [DOI] [PubMed] [Google Scholar]
- 93.Van Oyen H, Van Oyen P. Urinary incontinence in Belgium; prevalence, correlates and psychosocial consequences. Acta Clin Belg. 2002;57:207–218. doi: 10.1179/acb.2002.043. [DOI] [PubMed] [Google Scholar]
- 94.Engstrom G, Walker-Engstrom ML, Loof L, Leppert J. Prevalence of three lower urinary tract symptoms in men-a population-based study. Fam Pract. 2003;20:7–10. doi: 10.1093/fampra/20.1.7. [DOI] [PubMed] [Google Scholar]
- 95.Nuotio M, Tammela TL, Luukkaala T, Jylha M. Urgency and urge incontinence in an older population: ten-year changes and their association with mortality. Aging Clin Exp Res. 2002;14:412–419. doi: 10.1007/BF03324470. [DOI] [PubMed] [Google Scholar]
- 96.Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20:327–336. doi: 10.1007/s00345-002-0301-4. [DOI] [PubMed] [Google Scholar]
- 97.Moorthy P, Lapitan MC, Quek PL, Lim PH. Prevalence of overactive bladder in Asian men: an epidemiological survey. BJU Int. 2004;93:528–531. doi: 10.1111/j.1464-410x.2003.04682.x. [DOI] [PubMed] [Google Scholar]
- 98.McGrother CW, Donaldson MM, Shaw C, et al. Storage symptoms of the bladder: prevalence, incidence and need for services in the UK. BJU Int. 2004;93:763–769. doi: 10.1111/j.1464-410X.2003.04721.x. [DOI] [PubMed] [Google Scholar]
- 99.Corcos J, Schick E. Prevalence of overactive bladder and incontinence in Canada. Can J Urol. 2004;11:2278–2284. [PubMed] [Google Scholar]
- 100.Stothers L, Thom D, Calhoun E. Urologic diseases in America project: urinary incontinence in males-demographics and economic burden. J Urol. 2005;173:1302–1308. doi: 10.1097/01.ju.0000155503.12545.4e. [DOI] [PubMed] [Google Scholar]
- 101.Jorgensen L, Engstad T, Jacobsen BK. Self-reported urinary incontinence in noninstitutionalized long-term stroke survivors: a population-based study. Arch Phys Med Rehabil. 2005;86:416–420. doi: 10.1016/j.apmr.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 102.Muller N. What Americans understand and how they are affected by bladder control problems: highlights of recent nationwide consumer research. Urol Nurs. 2005;25:109–115. [PubMed] [Google Scholar]
- 103.Haltbakk J, Hanestad BR, Hunskaar S. Relevance and variability of the severity of incontinence, and increased daytime and night-time voiding frequency, associated with quality of life in men with lower urinary tract symptoms. BJU Int. 2005;96:83–87. doi: 10.1111/j.1464-410X.2005.05572.x. [DOI] [PubMed] [Google Scholar]
- 104.Temml C, Heidler S, Ponholzer A, Madersbacher S. Prevalence of the overactive bladder syndrome by applying the International Continence Society definition. Eur Urol. 2005;48:622–627. doi: 10.1016/j.eururo.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 105.Homma Y, Yamaguchi O, Hayashi K. An epidemiological survey of overactive bladder symptoms in Japan. BJU Int. 2005;96:1314–1318. doi: 10.1111/j.1464-410X.2005.05835.x. [DOI] [PubMed] [Google Scholar]
- 106.Boyington JE, Howard DL, Carter-Edwards L, et al. Differences in resident characteristics and prevalence of urinary incontinence in nursing homes in the southeastern United States. Nurs Res. 2007;56:97–107. doi: 10.1097/01.NNR.0000263969.08878.51. [DOI] [PubMed] [Google Scholar]
- 107.Langa KM, Fultz NH, Saint S, et al. Informal caregiving time and costs for urinary incontinence in older individuals in the United States. J Am Geriatr Soc. 2002;50:733–737. doi: 10.1046/j.1532-5415.2002.50170.x. [DOI] [PubMed] [Google Scholar]
- 108.Thom DH, van den Eeden SK, Brown JS. Evaluation of parturition and other reproductive variables as risk factors for urinary incontinence in later life. Obstet Gynecol. 1997;90:983–989. doi: 10.1016/s0029-7844(97)00537-1. [DOI] [PubMed] [Google Scholar]
- 109.Liu C, Andrews GR. Prevalence and incidence of urinary incontinence in the elderly: a longitudinal study in South Australia. Chin Med J (Engl) 2002;115:119–122. [PubMed] [Google Scholar]
- 110.Lee WR, Schultheiss TE, Hanlon AL, Hanks GE. Urinary incontinence following external-beam radiotherapy for clinically localized prostate cancer. Urology. 1996;48:95–99. doi: 10.1016/s0090-4295(96)00085-4. [DOI] [PubMed] [Google Scholar]
- 111.Talcott JA, Rieker P, Clark JA, et al. Patient-reported symptoms after primary therapy for early prostate cancer: results of a prospective cohort study. J Clin Oncol. 1998;16:275–283. doi: 10.1200/JCO.1998.16.1.275. [DOI] [PubMed] [Google Scholar]
- 112.Van Kampen M, De Weerdt W, Van Poppel H, Baert L. Urinary incontinence following transurethral, transvesical and radical prostatectomy. Retrospective study of 489 patients. Acta Urol Belg. 1997;65:1–7. [PubMed] [Google Scholar]
- 113.Gray M, Petroni GR, Theodorescu D. Urinary function after radical prostatectomy: a comparison of the retropubic and perineal approaches. Urology. 1999;53:881–890. doi: 10.1016/s0090-4295(99)00071-0. discussion 890-981. [DOI] [PubMed] [Google Scholar]
- 114.Catalona WJ, Carvalhal GF, Mager DE, Smith DS. Potency, continence and complication rates in 1,870 consecutive radical retropubic prostatectomies. J Urol. 1999;162:433–438. [PubMed] [Google Scholar]
- 115.Sandhu AS, Zelefsky MJ, Lee HJ, et al. Longterm urinary toxicity after 3-dimensional conformal radiotherapy for prostate cancer in patients with prior history of transurethral resection. Int J Radiat Oncol Biol Phys. 2000;48:643–647. doi: 10.1016/s0360-3016(00)00714-8. [DOI] [PubMed] [Google Scholar]
- 116.Olsson LE, Salomon L, Nadu A, et al. Prospective patient-reported continence after laparoscopic radical prostatectomy. Urology. 2001;58:570–572. doi: 10.1016/s0090-4295(01)01261-4. [DOI] [PubMed] [Google Scholar]
- 117.Koskimäaki J, Hakama M, Huhtala H, Tammela TL. Association of non-urological diseases with lower urinary tract symptoms. Scand J Urol Nephrol. 2001;35:377–381. doi: 10.1080/003655901753224431. [DOI] [PubMed] [Google Scholar]
- 118.Augustin H, Pummer K, Daghofer F, et al. Patient self-reporting questionnaire on urological morbidity and bother after radical retropubic prostatectomy. Eur Urol. 2002;42:112–117. doi: 10.1016/s0302-2838(02)00259-2. [DOI] [PubMed] [Google Scholar]
- 119.Sebesta M, Cespedes RD, Luhman E, et al. Questionnaire- based outcomes of urinary incontinence and satisfaction rates after radical prostatectomy in a national study population. Urology. 2002;60:1055–1058. doi: 10.1016/s0090-4295(02)01989-1. [DOI] [PubMed] [Google Scholar]
- 120.Hu JC, Gold KF, Pashos CL, et al. Temporal trends in radical prostatectomy complications from 1991 to 1998. J Urol. 2003;169:1443–1448. doi: 10.1097/01.ju.0000056046.16588.e4. [DOI] [PubMed] [Google Scholar]
- 121.Maffezzini M, Seveso M, Taverna G, et al. Evaluation of complications and results in a contemporary series of 300 consecutive radical retropubic prostatectomies with the anatomic approach at a single institution. Urology. 2003;61:982–986. doi: 10.1016/s0090-4295(02)02517-7. [DOI] [PubMed] [Google Scholar]
- 122.Hisasue S, Takahashi A, Kato R, et al. Early and late complications of radical retropubic prostatectomy: experience in a single institution. Jpn J Clin Oncol. 2004;34:274–279. doi: 10.1093/jjco/hyh042. [DOI] [PubMed] [Google Scholar]
- 123.Johnson TK, Gilliland FD, Hoffman RM, et al. Racial/ethnic differences in functional outcomes in the 5 years after diagnosis of localized prostate cancer. J Clin Oncol. 2004;22:4193–4201. doi: 10.1200/JCO.2004.09.127. [DOI] [PubMed] [Google Scholar]
- 124.Liu M, Pickles T, Berthelet E, et al. Urinary incontinence in prostate cancer patients treated with external beam radiotherapy. Radiother Oncol. 2005;74:197–201. doi: 10.1016/j.radonc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 125.Tellez-Zenteno JF, Matijevic S, Wiebe S. Somatic comorbidity of epilepsy in the general population in Canada. Epilepsia. 2005;46:1955–1962. doi: 10.1111/j.1528-1167.2005.00344.x. [DOI] [PubMed] [Google Scholar]
- 126.Burkhard FC, Kessler TM, Fleischmann A, et al. Nerve sparing open radical retropubic prostatectomy- does it have an impact on urinary continence? J Urol. 2006;176:189–195. doi: 10.1016/S0022-5347(06)00574-X. [DOI] [PubMed] [Google Scholar]
- 127.Jacobsen NE, Moore KN, Estey E, Voaklander D. Open versus laparoscopic radical prostatectomy: a prospective comparison of postoperative urinary incontinence rates. J Urol. 2007;177:615–619. doi: 10.1016/j.juro.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 128.Nelson RL, Furner SE. Risk factors for the development of fecal and urinary incontinence in Wisconsin nursing home residents. Maturitas. 2005;52:26–31. doi: 10.1016/j.maturitas.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 129.Zhang AY, Strauss GJ, Siminoff LA. Effects of combined pelvic floor muscle exercise and a support group on urinary incontinence and quality of life of postprostatectomy patients. Oncol Nurs Forum. 2007;34:47–53. doi: 10.1188/07.ONF.47-53. [DOI] [PubMed] [Google Scholar]
- 130.Franke JJ, Gilbert WB, Grier J, et al. Early postprostatectomy pelvic floor biofeedback. J Urol. 2000;163:191–193. [PubMed] [Google Scholar]
- 131.Van Kampen M, De Weerdt W, Van Poppel H, et al. Effect of pelvic-floor re-education on duration and degree of incontinence after radical prostatectomy: a randomised controlled trial. Lancet. 2000;355:98–102. doi: 10.1016/S0140-6736(99)03473-X. [DOI] [PubMed] [Google Scholar]
- 132.Bales GT, Gerber GS, Minor TX, et al. Effect of preoperative biofeedback/pelvic floor training on continence in men undergoing radical prostatectomy. Urology. 2000;56:627–630. doi: 10.1016/s0090-4295(00)00687-7. [DOI] [PubMed] [Google Scholar]
- 133.Parekh AR, Feng MI, Kirages D, et al. The role of pelvic floor exercises on post-prostatectomy incontinence. J Urol. 2003;170:130–133. doi: 10.1097/01.ju.0000072900.82131.6f. [DOI] [PubMed] [Google Scholar]
- 134.Wille S, Sobottka A, Heidenreich A, Hofmann R. Pelvic floor exercises, electrical stimulation and biofeedback after radical prostatectomy: results of a prospective randomized trial. J Urol. 2003;170(2 pt 1):490–493. doi: 10.1097/01.ju.0000076141.33973.75. [DOI] [PubMed] [Google Scholar]
- 135.Yokoyama T, Nishiguchi J, Watanabe T, et al. Comparative study of effects of extracorporeal magnetic innervation versus electrical stimulation for urinary incontinence after radical prostatectomy. Urology. 2004;63:264–267. doi: 10.1016/j.urology.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 136.Filocamo MT, Li Marzi V, Del Popolo G, et al. Effectiveness of early pelvic floor rehabilitation treatment for post-prostatectomy incontinence. Eur Urol. 2005;48:734–738. doi: 10.1016/j.eururo.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 137.Mathewson-Chapman M. Pelvic muscle exercise/ biofeedback for urinary incontinence after prostatectomy: an education program. J Cancer Educ. 1997;12:218–223. doi: 10.1080/08858199709528492. [DOI] [PubMed] [Google Scholar]
- 138.Porru D, Campus G, Caria A, et al. Impact of early pelvic floor rehabilitation after transurethral resection of the prostate. Neurourol Urodyn. 2001;20:53–59. doi: 10.1002/1520-6777(2001)20:1<53::aid-nau7>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 139.Dorey G, Speakman M, Feneley R, et al. Pelvic floor exercises for treating post-micturition dribble in men with erectile dysfunction: a randomized controlled trial. Urol Nurs. 2004;24:490–497. [PubMed] [Google Scholar]
- 140.Chancellor MB, Bennett C, Simoneau AR, et al. Sphincteric stent versus external sphincterotomy in spinal cord injured men: prospective randomized multicenter trial. J Urol. 1999;161:1893–1898. [PubMed] [Google Scholar]
- 141.Moore KN, Schieman S, Ackerman T, et al. Assessing comfort, safety, and patient satisfaction with three commonly used penile compression devices. Urology. 2004;63:150–154. doi: 10.1016/j.urology.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 142.Steineck G, Helgesen F, Adolfsson J, et al. Quality of life after radical prostatectomy or watchful waiting. N Engl J Med. 2002;347:790–796. doi: 10.1056/NEJMoa021483. [DOI] [PubMed] [Google Scholar]
- 143.Akakura K, Isaka S, Akimoto S, et al. Long-term results of a randomized trial for the treatment of Stages B2 and C prostate cancer: radical prostatectomy versus external beam radiation therapy with a common endocrine therapy in both modalities. Urology. 1999;54:313–318. doi: 10.1016/s0090-4295(99)00106-5. [DOI] [PubMed] [Google Scholar]
- 144.Srougi M, Nesrallah LJ, Kauffmann JR, et al. Urinary continence and pathological outcome after bladder neck preservation during radical retropubic prostatectomy: a randomized prospective trial. J Urol. 2001;165:815–818. [PubMed] [Google Scholar]
- 145.Fransson P, Damber JE, Tomic R, et al. Quality of life and symptoms in a randomized trial of radiotherapy versus deferred treatment of localized prostate carcinoma. Cancer. 2001;92:3111–3119. doi: 10.1002/1097-0142(20011215)92:12<3111::aid-cncr10160>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 146.Gupta NP, Doddamani D, Aron M, Hemal AK. Vapor resection: a good alternative to standard loop resection in the management of prostates >40 cc. J Endourol. 2002;16:767–771. doi: 10.1089/08927790260472944. [DOI] [PubMed] [Google Scholar]
- 147.Ghaly M, Wallner K, Merrick G, et al. The effect of supplemental beam radiation on prostate brachytherapy-related morbidity: morbidity outcomes from two prospective randomized multi-center trials. Int J Radiat Oncol Biol Phys. 2003;55:1288–1293. doi: 10.1016/s0360-3016(02)04527-3. [DOI] [PubMed] [Google Scholar]
- 148.Little DJ, Kuban DA, Levy LB, et al. Qualityof- life questionnaire results 2 and 3 years after radiotherapy for prostate cancer in a randomized dose-escalation study. Urology. 2003;62:707–713. doi: 10.1016/s0090-4295(03)00504-1. [DOI] [PubMed] [Google Scholar]
- 149.Imamoglu MA, Tuygun C, Bakirtas H, et al. The comparison of artificial urinary sphincter implantation and endourethral macroplastique injection for the treatment of postprostatectomy incontinence. Eur Urol. 2005;47:209–213. doi: 10.1016/j.eururo.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 150.Srougi M, Paranhos M, Leite KM, et al. The influence of bladder neck mucosal eversion and early urinary extravasation on patient outcome after radical retropubic prostatectomy: a prospective controlled trial. BJU Int. 2005;95:757–760. doi: 10.1111/j.1464-410X.2005.05395.x. [DOI] [PubMed] [Google Scholar]
- 151.Wilson LC, Gilling PJ, Williams A, et al. A randomised trial comparing holmium laser enucleation versus transurethral resection in the treatment of prostates larger than 40 grams: results at 2 years. Eur Urol. 2006;50:569–573. doi: 10.1016/j.eururo.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 152.Wasson JH, Reda DJ, Bruskewitz RC, et al. A comparison of transurethral surgery with watchful waiting for moderate symptoms of benign prostatic hyperplasia. The Veterans Affairs Cooperative Study Group on Transurethral Resection of the Prostate. N Engl J Med. 1995;332:75–79. doi: 10.1056/NEJM199501123320202. [DOI] [PubMed] [Google Scholar]
- 153.Van Cangh PJ, Richard F, Lorge F, et al. Adjuvant radiation therapy does not cause urinary incontinence after radical prostatectomy: results of a prospective randomized study. J Urol. 1998;159:164–166. doi: 10.1016/s0022-5347(01)64044-8. [DOI] [PubMed] [Google Scholar]
- 154.Gallucci M, Puppo P, Perachino M, et al. Transurethral electrovaporization of the prostate vs. transurethral resection. Results of a multicentric, randomized clinical study on 150 patients. Eur Urol. 1998;33:359–364. doi: 10.1159/000019616. [DOI] [PubMed] [Google Scholar]
- 155.Deliveliotis C, Delis A, Papatsoris A, et al. Local steroid application during nerve-sparing radical retropubic prostatectomy. BJU Int. 2005;96:533–535. doi: 10.1111/j.1464-410X.2005.05679.x. [DOI] [PubMed] [Google Scholar]
- 156.Parsons JK, Marschke P, Maples P, Walsh PC. Effect of methylprednisolone on return of sexual function after nerve-sparing radical retropubic prostatectomy. Urology. 2004;64:987–990. doi: 10.1016/j.urology.2004.06.048. [DOI] [PubMed] [Google Scholar]
- 157.Peeters ST, Lebesque JV, Heemsbergen WD, et al. Localized volume effects for late rectal and anal toxicity after radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2006;64:1151–1161. doi: 10.1016/j.ijrobp.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 158.Filocamo MT, Li Marzi V, Del Popolo G, et al. Pharmacologic treatment in postprostatectomy stress urinary incontinence. Eur Urol. 2007;51:1559–1564. doi: 10.1016/j.eururo.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 159.Mitropoulos D, Papadoukakis S, Zervas A, et al. Efficacy of tolterodine in preventing urge incontinence immediately after prostatectomy. Int Urol Nephrol. 2006;38:263–268. doi: 10.1007/s11255-005-4031-6. [DOI] [PubMed] [Google Scholar]
- 160.Kaplan SA, Roehrborn CG, Rovner ES, et al. Tolterodine and tamsulosin for treatment of men with lower urinary tract symptoms and overactive bladder: a randomized controlled trial. JAMA. 2006;296:2319–2328. doi: 10.1001/jama.296.19.2319. [DOI] [PubMed] [Google Scholar]
- 161.Roehrborn CG, Abrams P, Rovner ES, et al. Efficacy and tolerability of tolterodine extended-release in men with overactive bladder and urgency urinary incontinence. BJU Int. 2006;97:1003–1006. doi: 10.1111/j.1464-410X.2006.06068.x. [DOI] [PubMed] [Google Scholar]
- 162.Kaplan SA, Roehrborn CG, Dmochowski R, et al. Tolterodine extended release improves overactive bladder symptoms in men with overactive bladder and nocturia. Urology. 2006;68:328–332. doi: 10.1016/j.urology.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 163.Managing Acute and Chronic Urinary Incontinence [microform] Rockville, MD; Washington, DC: US Dept. of Health and Human Services Public Health Service Agency for Health Care Policy and Research; 1996. available for sale from the G.P.O. Supt. of Docs. [Google Scholar]
- 164.Rao SS. Diagnosis and management of fecal incontinence. American College of Gastroenterology Practice Parameters Committee. Am J Gastroenterol. 2004;99:1585–1604. doi: 10.1111/j.1572-0241.2004.40105.x. [DOI] [PubMed] [Google Scholar]
- 165.Paterson J, Pinnock CB, Marshall VR. Pelvic floor exercises as a treatment for post-micturition dribble. Br J Urol. 1997;79:892–897. doi: 10.1046/j.1464-410x.1997.00180.x. [DOI] [PubMed] [Google Scholar]
- 166.Floratos DL, Sonke GS, Rapidou CA, et al. Biofeedback vs verbal feedback as learning tools for pelvic muscle exercises in the early management of urinary incontinence after radical prostatectomy. BJU Int. 2002;89:714–719. doi: 10.1046/j.1464-410x.2002.02721.x. [DOI] [PubMed] [Google Scholar]
- 167.Hunter KF, Moore KN, Cody DJ, Glazener CM. Conservative management for postprostatectomy urinary incontinence. Cochrane Database Syst Rev. 2004;2 doi: 10.1002/14651858.CD001843.pub2. CD001843. [DOI] [PubMed] [Google Scholar]
- 168.MacDonald R, Fink HA, Huckabay C, et al. Pelvic floor muscle training to improve urinary incontinence after radical prostatectomy: a systematic review of effectiveness. BJU Int. 2007;100:76–81. doi: 10.1111/j.1464-410X.2007.06913.x. [DOI] [PubMed] [Google Scholar]
- 169.Rockwood TH, Church JM, Fleshman JW, et al. Fecal Incontinence Quality of Life Scale: quality of life instrument for patients with fecal incontinence. Dis Colon Rectum. 2000;43:9–16. doi: 10.1007/BF02237236. discussion 16–17. [DOI] [PubMed] [Google Scholar]
- 170.Glazener CM, Lapitan MC. Urodynamic investigations for management of urinary incontinence in adults. Cochrane Database Syst Rev. 2002;3 doi: 10.1002/14651858.CD003195. CD003195. [DOI] [PubMed] [Google Scholar]
- 171.Woods SP, Childers M, Ellis RJ, et al. A battery approach for measuring neuropsychological change. Arch Clin Neuropsychol. 2006;21:83–89. doi: 10.1016/j.acn.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 172.Sinaiko AR, Steinberger J, Moran A, et al. Relation of body mass index and insulin resistance to cardiovascular risk factors, inflammatory factors, and oxidative stress during adolescence. Circulation. 2005;111:1985–1991. doi: 10.1161/01.CIR.0000161837.23846.57. [DOI] [PubMed] [Google Scholar]
- 173.Moore KN, Griffiths D, Hughton A. Urinary incontinence after radical prostatectomy: a randomized controlled trial comparing pelvic muscle exercises with or without electrical stimulation. BJU Int. 1999;83:57–65. doi: 10.1046/j.1464-410x.1999.00894.x. [DOI] [PubMed] [Google Scholar]
- 174.Burgio KL, Goode PS, Urban DA, et al. Preoperative biofeedback assisted behavioral training to decrease post-prostatectomy incontinence: a randomized, controlled trial. J Urol. 2006;175:196–201. doi: 10.1016/S0022-5347(05)00047-9. discussion 201. [DOI] [PubMed] [Google Scholar]