Abstract
Background:
Few neuroimaging investigations of pain in elderly adults have focused on the hippocampus, a brain structure involved in nociceptive processing that is also subject to involution associated with dementing disorders. The goal of this pilot study was to examine MRI- and magnetic resonance spectroscopy (MRS)–derived hippocampal correlates of pain in older adults.
Methods:
A subset of 20 nondemented older adults was drawn from the Einstein Aging Study, a community-based sample from the Bronx, NY. Pain was measured on 3 time scales: 1) acute pain right now (pain severity); 2) pain over the past 4 weeks (Short Form–36 Bodily Pain); 3) chronic pain over the past 3 months (Total Pain Index). Hippocampal data included volume data normalized to midsagittal area and N-acetylaspartate to creatine ratios (NAA/Cr).
Results:
Smaller hippocampal volume was associated with higher ratings on the Short Form–36 Bodily Pain (rs = 0.52, p = 0.02) and a nonsignificant trend was noted for higher ratings of acute pain severity (rs = −0.44, p = 0.06). Lower levels of hippocampal NAA/Cr were associated with higher acute pain severity (rs = −0.45, p = 0.05). Individuals with chronic pain had a nonsignificant trend for smaller hippocampal volumes (t = 2.00, p = 0.06) and lower levels of hippocampal NAA/Cr (t = 1.71, p = 0.10).
Conclusions:
Older adults who report more severe acute or chronic pain have smaller hippocampal volumes and lower levels of hippocampal N-acetylaspartate/creatine, a marker of neuronal integrity. Future studies should consider the role of the hippocampus and other brain structures in the development and experience of pain in healthy elderly and individuals with Alzheimer disease.
GLOSSARY
- BIMC
= Blessed Information Memory Concentration Test; DSM-IV = Diagnostic and Statistical Manual of Mental Disorders, 4th edition;
- MRS
= magnetic resonance spectroscopy;
- NAA/Cr
= N-acetylaspartate/creatine ratios;
- SF-36 BP
= SF-36 Bodily Pain;
- TPI
= Total Pain Index.
The prevalence of pain that impedes daily activities increases with age.1 There may be a relationship between changes in neurobiological structure and function and the experience of intrusive pain.2 Structural MRI and magnetic resonance spectroscopy (MRS) investigations of adults with chronic pain syndromes reveal abnormalities in brain regions involved in pain processing, including thalamus, prefrontal cortex, cingulate, and somatosensory cortex.3 Few neuroimaging investigations of pain in elderly adults have focused on the hippocampus, a brain structure involved in nociceptive processing and subject to age-related involution.4 The goal of this pilot study was to examine MRI- and MRS-derived hippocampal correlates of pain in nondemented older adults.
METHODS
Participants.
A group of 20 nondemented older adults participating in the Einstein Aging Study completed neuroimaging studies and pain assessments.5 Nondemented participants included individuals who did not meet diagnostic criteria for dementia based on the DSM-IV.6
Standard protocol approvals, registrations, and patient consents.
Use of human subjects for this study was approved by the Albert Einstein College of Medicine institutional review board and written informed consent was obtained from all participants.
Pain measurement.
All-cause pain as a ubiquitous exposure was measured on 3 time scales. 1) Acute pain right now was assessed using a 0–10 anchored pain severity numerical rating scale, with 10 indicating pain “as bad as it could be.” 2) Pain over the past 4 weeks was measured using the SF-36 Bodily Pain (SF-36 BP) scale that consists of a composite of 2 questions that assess pain interference (range 1–5) and pain severity (range 1–6).7 SF-36 BP data are presented as z scores. 3) Chronic pain was measured using the Total Pain Index (TPI). The TPI is an inventory of pain symptoms that consists of questions concerning pain location, frequency, severity, and duration for multiple body areas (head, face, neck and shoulder, back, arms and hands, legs and feet, chest, abdomen and pelvis, and other). For each body area, respondents are asked, “In the past 3 months, how often did you have pain in the (insert body area)?” Response options include “none of the time,” “a slight bit of the time,” “some of the time,” “most of the time,” and “all of the time.” For each body area with pain, respondents are then asked to rate the intensity of their worst pain over the previous 3 months on a scale of 0 to 10. Respondents are classified as having chronic pain if, in at least 1 location, they had pain of at least moderate or severe intensity (≥4/10) in the previous 3 months some, most, or all of the time.8 This measure is valid and reliable in our elderly adult population (data not shown).
Magnetic resonance studies.
MRI and MRS methods have been described previously.5 Hippocampal volume data are presented as a ratio of volume normalized to midsagittal area. Spectroscopy data are presented as an area ratio of N-acetylaspartate to creatine (NAA/Cr).
Statistical analyses.
Age and education were examined as potential covariates using Spearman coefficients. Relationships among acute pain severity, SF-36 BP, hippocampal volume, and hippocampal NAA/Cr were examined using Spearman coefficients. Independent t-tests were used to examine chronic pain group differences in hippocampal volume and NAA/Cr.
RESULTS
Participants were an average age of 82 years (SD = 5.8) and had an average education level of 13 years (SD = 2.8). The sample comprised predominantly women (n = 11/20; 55%) and Caucasians (n = 15/20; 75%). Comorbid medical conditions associated with pain included osteoarthritis (n = 14/20; 70%), peripheral neuropathy (n = 5/20; 25%), and spinal stenosis (n = 3/20; 15%). Only 20% of participants were taking medications prescribed by a physician for pain-related disorders. Of these, 2 had chronic pain. A total of 8 individuals met the definition of chronic pain. Acute pain severity ratings ranged from 0 to 10 with a median of 2. Six (30%) individuals reported an absence of acute pain. SF-36 BP z-score ranged from −1.89 to 1.04 with a mean of 0.14. Pain ratings were highly correlated: pain severity and SF-36 BP (rs = −0.54, p = 0.01), pain severity and TPI (rs = 0.94, p < 0.01), and SF-36 BP and TPI (rs = −0.55, p = 0.01).
Age and education were not related to any of the pain or magnetic resonance–derived variables of interest. Smaller hippocampal volume was associated with higher ratings on the SF-36 BP scale (rs = 0.52, p = 0.02) and a nonsignificant trend was noted for more severe ratings of acute pain severity (rs = −0.44, p = 0.06). Lower levels of hippocampal NAA/Cr were associated with more severe acute pain severity (rs = −0.45, p = 0.05). Relationships among pain and hippocampal measures are shown in the figure. Individuals with chronic pain had a nonsignificant trend for smaller hippocampal volumes (mean volume 3,720 mm3 vs 4,208 mm3; t = 2.00, p = 0.06) and lower levels of hippocampal NAA/Cr (t = 1.71, p = 0.10).
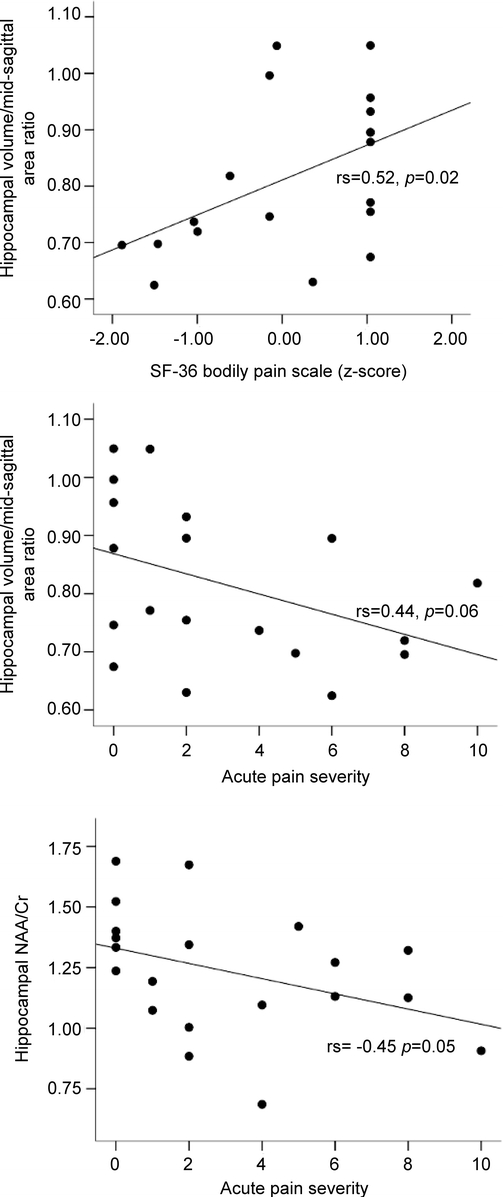
Figure Relationships among self-reported pain and neuroimaging hippocampal measures in older adults
Pain measures were not associated with performance on the Free and Cued Selective Reminding Test (pain severity: rs = −0.33, p = 0.16; SF-36 BP: rs = 0.09, p = 0.72), the Logical Memory subtest of the Wechsler Memory Scale–Revised (pain severity: rs = −0.12, p = 0.64; SF-36 BP: rs = −0.22, p = 0.36), or on a test of global cognitive function, the Blessed Information Memory Concentration Test (BIMC) (pain severity: rs = 0.40, p = 0.08; SF-36 BP: rs = 0.07, p = 0.77) (tests described in reference 5). Hippocampal volume measures were not associated with performance on the Free and Cued Selective Reminding Test (rs = 0.32, p = 0.18), the Logical Memory subtest (rs = 0.16, p = 0.53), or on the BIMC (rs = −0.27, p = 0.26). Hippocampal NAA/Cr measures were also not associated with performance on the Free and Cued Selective Reminding Test (rs = 0.36, p = 0.12) or the Logical Memory subtest (rs = 0.25, p = 0.30), or on the BIMC (rs = −0.27, p = 0.26). In addition, hippocampal volume was not associated with hippocampal NAA/Cr (rs = 0.21, p = 0.38).
DISCUSSION
Findings from this preliminary study suggest that older adults who report more severe acute pain or those with chronic pain have smaller hippocampal volumes and lower levels of hippocampal NAA/Cr, a marker of neuronal integrity and neuronal loss. Conclusions regarding the causal nature of these relationships are limited by the cross-sectional design of our study. In addition, we did not assess lifetime exposure to chronic pain in this elderly sample. However, a parsimonious speculation is that prolonged nociceptive input associated with pain may result in biologic changes including cortisol secretion, excitotoxicity, and inflammation that have a deleterious effect on hippocampal structure and function.9 It is also possible that individuals with hippocampal dysfunction are more likely to report pain experiences. Future cross-sectional and longitudinal studies should consider the hippocampus and other brain regions and their role in the development and experience of pain in elderly persons.
AUTHOR CONTRIBUTIONS
Statistical analyses were conducted by Dr. Molly E. Zimmerman.
DISCLOSURE
Dr. Zimmerman has received funding for travel to meetings not sponsored by industry; receives research support from the NIH [P01AG003949 (Co-I) and #P01AG027734 (Co-I)]; and from the Albert Einstein College of Medicine (Resnick Gerontology Center Pilot Grant Award). Dr. Pan serves on the editorial board of Magnetic Resonance in Medicine and holds US Patent 96700/1150 Issued: 2006 (Magnetic resonance shimming). Dr. Hetherington receives research support from NIH R01 R01EB009871 (PI), NIH R41 DA029080 (PI), NIH RO1 EB000473 (Co-I), and NIH R21 NS0627976 (Co-I). Dr. Lipton receives royalties from publishing Totally Accessible MRI (Springer Publishing Company, 2008); has received research support from Repligen Corporation and the NIH [K08MH067082 (PI)]; serves/has served on scientific advisory boards for and received funding for travel from Bayer Schering Pharma, Merck Serono, Glaxo SmithKline, Endo Pharmaceuticals, Kowa Pharmaceuticals America, Inc., Allergan, Inc., Neuralieve Inc., Ortho-McNeil-Janssen Pharmaceuticals, Inc.; has received funding for travel from the American Headache Society and the Diamond Headache Center; serves as Associate Editor of Cephalalgia and on the editorial boards of Neurology® and Headache; receives royalties from publishing Headache in Clinical Practice (Isis Medical Media, 2002), Headache in Primary Care (Isis Medical Media, 1999), Wolff’s Headache (Oxford University Press, 2001, 2008), Managing Migraine: A Physician’s Guide (BC Decker, 2008), and Managing Migraine: A Patient’s Guide (BC Decker, 2008); has received speaker honoraria from the National Headache Foundation, the University of Oklahoma, the American Academy of Neurology, the Annenberg Foundation, Merck Serono, GlaxoSmithKline, and Coherex Medical; receives research support from the American Headache Society, National Headache Foundation, the Migraine Research Foundation, and the NIH [PO1AG03949 (Program Director, Project Leader), PO1AG027734 (Project Leader), RO1AG25119 (Co-I), K23AG030857 (Co-Mentor), K23NS05140901A1(Co-Mentor), and K23NS47256 (Mentor); and holds stock options in Minster Pharmaceuticals plc. Dr. Baigi reports no disclosures.
Address correspondence and reprint requests to Dr. Molly E. Zimmerman, Albert Einstein College of Medicine, Saul R. Korey Department of Neurology, 1165 Morris Park Avenue, Room 343, Bronx, NY 10461 molly.zimmerman@einstein.yu.edu
Disclosure: Author disclosures are provided at the end of the article.
Received March 4, 2009. Accepted in final form August 6, 2009.
REFERENCES
- 1.Thomas E, Mottram S, Peat G, Wilkie R, Croft P. The effect of age on the onset of pain interference in a general population of older adults: prospective findings from the North Staffordshire Osteoarthritis Project (NorStOP). Pain 2007;129:21–27. [DOI] [PubMed] [Google Scholar]
- 2.Gibson SJ, Farrell M. A review of age differences in the neurophysiology of nociception and the perceptual experience of pain. Clin J Pain 2004;20:227–239. [DOI] [PubMed] [Google Scholar]
- 3.Tracey I. Neuroimaging of pain mechanisms. Curr Opin Support Palliat Care 2007;1:109–116. [DOI] [PubMed] [Google Scholar]
- 4.Raz N, Rodrigue KM, Head D, Kennedy KM, Acker JD. Differential aging of the medial temporal lobe: a study of a five-year change. Neurology 2004;62:433–438. [DOI] [PubMed] [Google Scholar]
- 5.Zimmerman ME, Pan JW, Hetherington HP, et al. Hippocampal neurochemistry, neuromorphometry, and verbal memory in nondemented older adults. Neurology 2008;70:1594–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 7.Stewart AL, Hays RD, Ware JE, Jr. The MOS short-form general health survey: reliability and validity in a patient population. Med Care 1988;26:724–735. [DOI] [PubMed] [Google Scholar]
- 8.McCarthy LH, Bigal ME, Katz M, Derby C, Lipton RB. Chronic pain and obesity in elderly people: results from the Einstein aging study. J Am Geriatr Soc 2009;57:115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duric V, McCarson KE. Hippocampal neurokinin-1 receptor and brain-derived neurotrophic factor gene expression is decreased in rat models of pain and stress. Neuroscience 2005;133:999–1006. [DOI] [PubMed] [Google Scholar]


