Abstract
Background:
High midlife and late-life adiposity may increase risk for dementia. Late-life decrease in body mass index (BMI) or body weight within several years of a dementia diagnosis has also been reported. Differences in study designs and analyses may provide different pictures of this relationship.
Methods:
Thirty-two years of longitudinal body weight, BMI, waist circumference, and waist-to-hip ratio (WHR) data, from the Prospective Population Study of Women in Sweden, were related to dementia. A representative sample of 1,462 nondemented women was followed from 1968 at ages 38-60 years, and subsequently in 1974, 1980, 1992, and 2000, using neuropsychiatric, anthropometric, clinical, and other measurements. Cox proportional hazards regression models estimated incident dementia risk by baseline factors. Logistic regression models including measures at each examination were related to dementia among surviving participants 32 years later.
Results:
While Cox models showed no association between baseline anthropometric factors and dementia risk, logistic models showed that a midlife WHR greater than 0.80 increased risk for dementia approximately twofold (odds ratio 2.22, 95% confidence interval 1.00-4.94, p = 0.049) among surviving participants. Evidence for reverse causality was observed for body weight, BMI, and waist circumference in years preceding dementia diagnosis.
Conclusions:
Among survivors to age 70, high midlife waist-to-hip ratio may increase odds of dementia. Traditional Cox models do not evidence this relationship. Changing anthropometric parameters in years preceding dementia onset indicate the dynamic nature of this seemingly simple relationship. There are midlife and late-life implications for dementia prevention, and analytical considerations related to identifying risk factors for dementia.
GLOSSARY
- ADCVD
= AD with cerebrovascular disease;
- BMI
= body mass index;
- DBP
= diastolic blood pressure; DSM-III-R = Diagnostic and Statistical Manual of Mental Disorders, 3rd edition, revised;
- HAAS
= Honolulu Asia Aging Study;
- PPSW
= Prospective Population Study of Women;
- SBP
= systolic blood pressure;
- SES
= socioeconomic status;
- VaD
= vascular dementia;
- WHR
= waist-to-hip ratio.
Overweight and obesity measured during both midlife and late-life have been related to risk for dementia.1–4 However, some studies have not shown this relationship,5 or reported late-life decline in body mass index (BMI) or body weight prior to dementia onset.6–10 Differences in study designs, lengths of observation periods, analytical strategies, and the natural history of the disorder in relationship to measurement of anthropometric indicators may influence observed relationships.
Published reports relating anthropometric factors and dementia over at least 20 years include those with midlife or late-life approaches, as well as continuous, longitudinal approaches. For example, the Kaiser Permanente,11,12 the Cardiovascular Risk Factors, Aging, and Dementia,2 and the Cardiovascular Health Study10 cohorts have reported on midlife higher adiposity and late-life dementia among those who survive to the age when they are at risk for dementia. The Honolulu Asia Aging Study (HAAS)6 did not observe this association. Using a continuous, longitudinal approach, the Rancho Bernardo cohort8did not report an effect of higher body weight.
Using data collected over 32 years from the Prospective Population Study of Women (PPSW) in Sweden, we explored the association between adiposity and dementia using midlife, late-life, vs traditional proportional hazards approaches, as well as potential reverse causality (i.e., dementia causing the decline) in years immediately preceding dementia diagnosis. Collection of baseline data was extensive, and has been continuously collected over time, and linkages to Swedish hospital discharge and other national registries allow for almost complete follow-up of participants.
We examined the relationship between the anthropometric indicators, body weight, BMI, waist circumference (waist), and WHR and the development of dementia in a representative urban sample of women followed for 32 years in the PPSW in Gothenburg, Sweden, using a variety of analysis strategies.
METHODS
Participants.
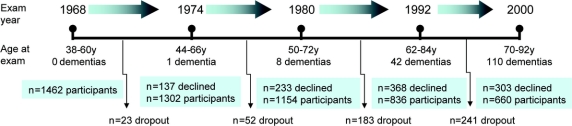
The PPSW13,14 was initiated in 1968-1969 using a sampling frame consisting of women who were systematically sampled from the census register in Gothenburg based on preselected days and months of birth years 1908, 1914, 1918, 1922, and 1930. Using this method, 11% (n = 1,622) of all women in the population were eligible, of whom 1,462 (participation rate 90%) aged 38-60 years participated at baseline. Participants were invited for new examinations in 1974, 1980, 1992, and 2000 (see figure 1). Of women participating in 1968, 161 developed dementia over the 32-year follow-up period. Among those who survived and participated in both the 1968 and 2000 examinations (n = 651), 87 developed dementia (median follow-up time, 32.0 years; range 31.1-34.8 years). Those who died, or declined participation during the follow-up period, were traced in records from hospitals and homes for the aged, inpatient and outpatient departments in psychiatric hospitals and clinics, municipal psychiatric outpatient departments in Gothenburg, the hospital-linkage system, and death certificates.15 Dementia diagnoses were obtained for all study participants, since almost all people in Sweden receive their health care from the community and have an equal chance of having a case record.
Figure 1 Thirty-two years of adiposity indicators and dementia
The Prospective Population Study of Women. Number of dementias at each examination year reflects the number diagnosed between the previous examination up to and through the current examination year. A description of case selection for various statistical approaches is included in the text.
Standard protocol approvals.
All participants (or their nearest relatives) gave informed consent to participate. The study was approved by the Ethics Committee for Medical Research at the University of Gothenburg.
Procedure.
Detailed, longitudinal examinations of aging and somatic and psychiatric disorders included physical examinations performed by a geriatrician, electrocardiogram, chest X-ray, a battery of blood tests, and neuropsychiatric examinations performed by psychiatrists or psychiatric nurses. Participants were surveyed in 1968, 1974, 1980, 1992, and 2000 about a variety of potential risk factors for age-related diseases, such as education, smoking habits, socioeconomic status (SES), alcohol intake, medication use, and medical history.
Dementia assessments.
Neuropsychiatric examinations occurred over the entire 32-year follow-up. More extensive neuropsychiatric examinations and close informant interviews began when participants were 70 years or older, and were performed by psychiatrists in 1992, and experienced psychiatric nurses in 2000.16 Medical records were collected from hospitals and outpatient departments in Gothenburg, and dementia diagnoses based on consensus conferences by geriatric psychiatrists. The Swedish Hospital Discharge Registry provided diagnostic information for individuals discharged from hospitals since 1978.
Dementia was diagnosed according to DSM-III-R criteria.17 AD (probable and possible) was diagnosed according to the National Institute of Neurological and Communicative Disorders and Stroke and the AD and Related Disorders Association criteria.18 Pure vascular dementia (VaD), according to National Institute of Neurological Disorders and Stroke-Association Internationale pour la Recherche en l’Enseignement en Neurosciences criteria, was also considered.19 VaD was diagnosed when an individual had one or more infarcts detected by CT scanning and/or a history of acute focal neurologic symptoms and signs (restricted to definite symptoms or signs, such as acute hemiparesis or acute motor aphasia). AD with cerebrovascular disease (ADCVD) was diagnosed among individuals with both AD and history of stroke or cerebrovascular disease.16
Anthropometric assessments.
Anthropometric measurements were conducted in the morning with participants wearing light clothing.20 Body weight was recorded to the nearest 0.1 kg, and body height was measured to the nearest 0.5 cm. Body mass index (BMI) was calculated as kilograms per meter squared (kg/m2). Categories of BMI used to denote total body adiposity were ≥25 kg/m2 for overweight and obesity and ≥30 kg/m2 for obesity.21 Waist and hip circumferences were measured to the nearest 0.5 cm. The waist-to-hip ratio (WHR) was calculated as the ratio of waist to hip circumference. Central adiposity was defined as a waist circumference ≥88 cm or a WHR >0.80.22
Clinical assessments.
Blood pressure was measured in the right arm, seated after 5 minutes’ rest using a mercury manometer. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were registered to the nearest 2 mm Hg. DBP was defined as Karotkoff phase 5.23 Serum cholesterol and triglyceride levels were measured at the Clinical Chemistry Laboratory at Sahlgrenska University Hospital. Diagnoses of myocardial infarction, stroke, cancer, and diabetes were based on self-reports and clinical examinations (ECG and blood samples), case records, hospital discharge registry, national cancer registry, and national stroke registry over 32 years.
Lifestyle assessments.
Level of education (completing 6 years or less vs more than 6 years compulsory education) and socioeconomic status (SES), defined as working vs middle or upper class, were based on 1968-1969 survey responses. Alcohol consumption and cigarette smoking were defined as ever vs never use in each examination year. Leisure time and work physical activity was measured using a combined frequency and intensity measure.
Statistical analysis.
Means and standard deviations were calculated for all quantitative variables and frequencies and percents for categorical variables. Two primary analysis strategies were used to evaluate the relationship between anthropometric factors and dementia. First, Cox proportional hazards regression analyses were used to calculate hazards ratios related to incident all dementias, AD, VaD, and DCVD over 32 years in relationship to anthropometric factors measured in 1968. Time at risk was calculated to December 31, 1999, the date at which surviving participants were eligible for participation in the year 2000-2003 follow-up, diagnosis of dementia if before this date, or death. Second, logistic regression analyses were conducted using anthropometric and other factors measured concurrently at examinations in 1968, 1974, 1980-1981, 1992-1993, and 2000-2003 in relationship to dementia diagnosed among those examined in 2000-2003.
The process for selection of covariates began with univariate regression analyses of age, SBP and DBP; fasting glucose, triglycerides, and total cholesterol; cardiovascular disease; diabetes; age at menopause; cancer; education; and SES, cigarette smoking, and alcohol (wine, beer, spirits) intake. Variables were included in multivariate regression models if they met the criteria of p ≤ 0.05 in univariate analyses at any examination year.
Covariates were entered into Cox proportional hazards regression models using a single step approach. The assumption of proportional hazards was checked using techniques based on the use of Schoenfeld residuals. Cox models were adjusted for factors measured in 1968, including age, serum triglycerides, total serum cholesterol, SBP, age at menopause, education, and diabetes.
In logistic regression models, concurrent (during each examination year) measures were included. In 1968, covariates included age, education, SBP, serum cholesterol, and serum triglycerides; in 1974, age, education, SBP, serum cholesterol, and serum triglycerides; in 1980, age, education, systolic blood pressure, serum glucose, serum cholesterol, and serum triglycerides; in 1992, age, education, serum cholesterol, and wine and spirit intake; and in 2000, age, education, and serum triglycerides. Only participants with complete information were included in logistic regression analyses.
Anthropometric factors were considered as continuous measures, and also dichotomized, before inclusion in regression models. In models including continuous measures, risk of dementia was calculated per 1.0 kg/m2 increment of BMI, per 1 cm waist, and per 0.01 unit WHR. Two-tailed tests were used in all analyses at a significance level of p < 0.05. SAS, version 9.2, and SPSS, version 15.0, were used to conduct data analyses.
RESULTS
Characteristics of women who participated in the baseline examination in 1968 are shown in table 1. At baseline, an overweight BMI was prevalent among 25.4%, and an obese BMI among 7.7% of the women. A risky waist circumference was prevalent among 5.7%, and a risky WHR among 11.7% of women.
Table 1 Baseline characteristics of women participating in the Prospective Population Study of Women
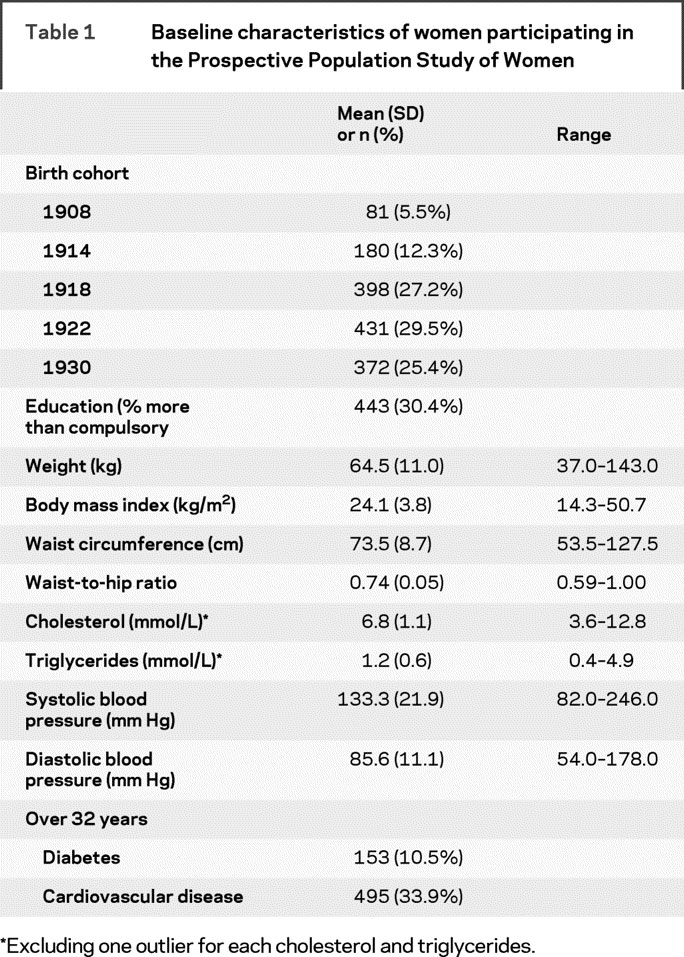
Over 32 years, dementia occurred in 161 participants, at an average age of 75.6 (7.29) years (range 48-91 years). Fifteen cases of dementia (9.3%) occurred at the age of 65 years or younger; 55 (34.2%) at age 66 through 75 years; 83 (51.6%) at age 76 through 85 years; and 8 (5.0%) at age 86 years or older. There were 75 cases of AD, 108 cases of ADCVD, and 37 cases of pure VaD. Total risk time evaluated was 41,415 risk years. There were 16 “other” dementias (e.g., Parkinson type dementia) that were not included in subtype analyses but were included in analyses of dementia. Among women who participated in the examination beginning in 2000, there were 45 cases of AD, 61 cases of ADCVD, and 22 cases of VaD. There were 4 “other” dementias that were not included in subtype analyses, but were included in analyses of dementia.
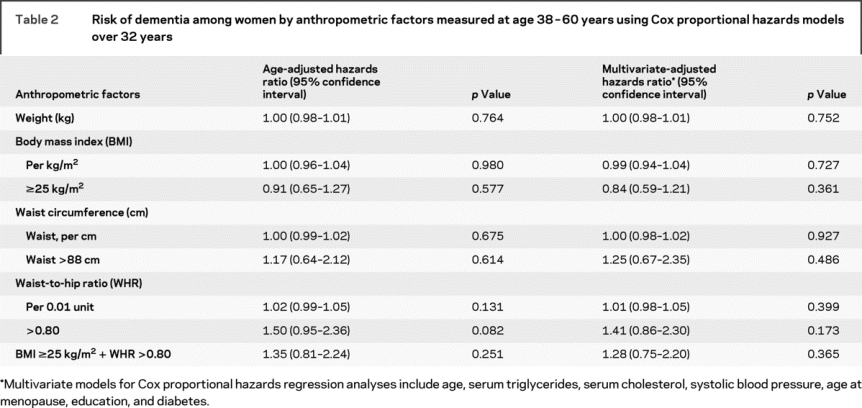
Evaluation of the dementia-adiposity relationship using Cox proportional hazards models showed no association between body weight, BMI, waist, or WHR in 1968 and dementia occurring during the 32-year follow-up period (table 2). This was also observed for dementia subtypes (data not shown).
Table 2 Risk of dementia among women by anthropometric factors measured at age 38-60 years using Cox proportional hazards models over 32 years
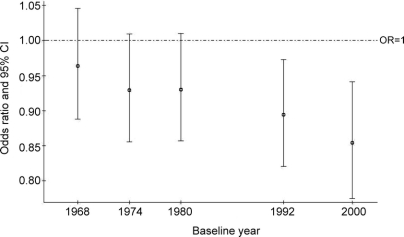
Logistic regression models evaluating the relationship among women participating in both the baseline 1968 examination and 32 years later in the year 2000 examination showed different relationships. A WHR greater than 0.80 at baseline (age 38-60 years in 1968) was associated with a 2.2-fold higher multivariate-adjusted odds of dementia among surviving participants, aged 70 years or older. In contrast, there was a trend for the odds of dementia by body weight, BMI, and waist circumference to decrease over time just prior to and at the time of dementia diagnosis when considering anthropometric measurements at different baselines over time (figure 2, table 3, and table e-1 on the Neurology® Web site at www.neurology.org). Logistic regression models suggested similar, significant results for ADCVD, and similar but nonsignificant results for pure AD and pure VaD (data not shown). Cox proportional hazards models evaluating these relationships among survivors showed similar results.
Figure 2 Odds of dementia at ≥70 years by body mass index measured at each examination year
Multivariate models include covariates significant in univariate analyses at p ≤ 0.05 at each baseline examination year. In 1968, covariates included age, education, systolic blood pressure, serum cholesterol, and serum triglycerides. In 1974, covariates included age, education, systolic blood pressure, serum cholesterol, and serum triglycerides. In 1980, covariates included age, education, systolic blood pressure, serum glucose, serum cholesterol, and serum triglycerides. In 1992, covariates included age, education, wine and spirit intake, and cholesterol. In 2000, covariates included age, education, and serum triglycerides.
Table 3 Relationship between anthropometric factors measured in midlife and late life in relationship to dementia among women who participated in a follow-up at age 70-92 using different baseline examinations over time
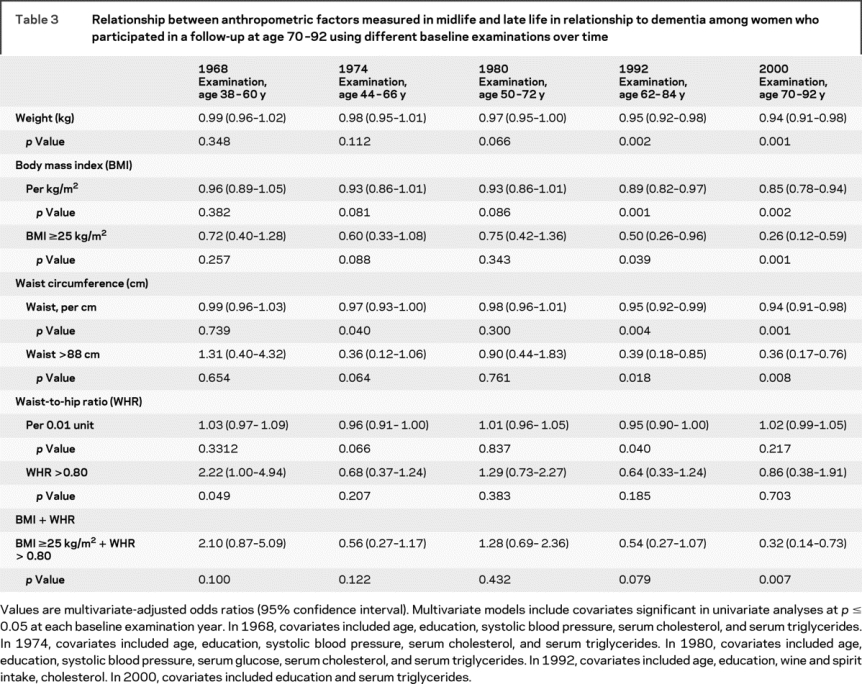
Survival analyses showed WHR to increase the risk of death among those dying prior to January 1, 2000, and therefore ineligible to participate in 2000. Other anthropometric indicators had no effect (table e-2). Other factors related to adiposity, such as physical activity in 1968, were not related to dementia.
DISCUSSION
We observed approximately twofold higher odds of dementia after 32 years with a high midlife measure of central adiposity in Swedish women. Higher odds of dementia were associated with a WHR exceeding 0.80, which is considered to be risky for cardiovascular health24,25 and potentially for overall mortality.26,27 Interestingly, the midlife central adiposity finding was only observed among those who survived 32 years to at least age 70 years and who participated in the neuropsychiatric examination. While high midlife BMI was not related to dementia in this sample, higher BMI, as well as body weight, waist, and WHR, were associated with lower odds of dementia when measured in the years more immediately preceding a dementia diagnosis.
To our knowledge, there has been only one published report of high central adiposity, measured in midlife using sagittal-abdominal diameter, being related to higher dementia risk after more than 3 decades. This observation was made in the Kaiser Permanente cohort, a large American population of individuals enrolled in a health care plan.12 A similar risk estimate, approximately twofold higher, as observed here for WHR, was reported. Another study in later life with shorter follow-up showed higher waist circumference related to higher AD risk in adults aged 65 to 75 years, but not in those aged 76 years and older.5 There have been 2 reports evaluating midlife and late-life BMI-dementia relationships in the same population sample. HAAS showed greater BMI decline among patients with dementia, and no effect of baseline BMI, as we observed here.6 The Cardiovascular Health Study has reported on both midlife and late-life BMI-dementia relationships over an average 5-year follow-up. Higher dementia risk from age 65 years and older was related to a lower baseline BMI, and retrospective self-reported recall of body weight and height at age 50 years showed that high midlife BMI increased dementia risk.10
Regarding BMI, there have been several prospective or nested epidemiologic reports1–4,6,8,9,11,28–30 evaluating BMI in relationship to dementia. Most1–4,11,28,30 have shown a high BMI to be risky for dementia when measured at least a decade prior to a clinical diagnosis. In addition, similar to data presented here, some studies have reported on reverse causality (often termed a protective effect) in relationship to higher body weight or BMI in the years prior to a dementia diagnosis.6,8–10,31 The reverse causality is most likely due to the effects of dementia-related neuropathology on dysregulation of energy expenditure and body weight that exceeds what is observed in normal aging. These data also continue to support cross-sectional reports of low BMI or underweight related to prevalent dementia.31–35
The methods we used to analyze 32 years of longitudinal data provide different results and interpretation of results. Based on these analyses, when evaluating adiposity and perhaps other vascular or metabolic factors measured in midlife in relationship to a disease of latest life, it is important to consider those surviving to an age when they are at risk for dementia. Model selection for this report was driven by interest in comparing analytical methods currently reported in epidemiologic studies. Cox proportional hazards models evaluating a disease outcome over a long follow-up period with a high amount of dropout that is possibly related to the covariates of interest might not capture the effect of baseline exposure variables on the risk of dementia, especially if the effect is not large.
Why did we not observe an effect of midlife BMI on dementia risk? There are numerous potential reasons for not observing this relationship. First, our population had a healthy average baseline BMI, and relatively low prevalences of overweight and obesity. This may have limited our ability to observe an effect of high BMI. Second, it could be that this population of Swedes born between 1908 and 1930 are less susceptible to dementia for unidentifiable reasons, perhaps related to factors at birth. That higher levels of BMI at age 70 years or older, born 1908-1930, are protective for dementia in the years immediately preceding its onset, in contrast to our previously published data on 70-year-olds born 1901-1902.1
Why are epidemiologic observations on the relationship between adiposity and dementia sometimes inconsistent? First, the age of the adiposity measure in relationship to clinical dementia onset varies across studies. Throughout life, there may exist critical periods in which risk or protective factors may have more or less impact. Second, while several studies have reported weight loss preceding dementia onset,6,8,9 this weight loss may precede a dementia diagnosis by decades.31 Understanding whether weight loss is evidence of reverse causality or a marker of something else is not clear. Third, anthropometric characteristics of populations vary around the world, as do what these measures represent in terms of adipose depots.36 Fourth, diagnosis of dementia is not the same across epidemiologic studies. Some studies use neuropsychiatric interviews, some registry data, and others screening criteria prior to more rigorous neuropsychiatric evaluation and diagnosis. Thus, populations with dementia are heterogeneous and identified at different levels of severity. Given the potentially rapid changes that occur in BMI during the natural history of dementia, especially during prodromal phases, these nuances may translate to different observations. Fifth, dementia is a syndrome. Metabolic alterations, such as changes in body weight, occurring with dementia may vary based on expression of the syndrome.37–39
Among the strengths of this study are 32 years of continuous follow-up to high age; representativeness of the sample; comprehensive neuropsychiatric examinations; and clinical anthropometric assessments. However, there are limitations and methodologic factors that should be addressed. First, it is often difficult to discriminate between AD and VaD. However, our criteria for AD are strict, excluding cases with stroke or infarcts on CT, and the clinical course of the disease was reviewed carefully by a team of psychiatrists. Second, the diagnosis of dementia among those lost to follow-up was based on case records, the hospital linkage system, and death certificates; these sources are known to underrate dementia. Thus, unidentified cases of dementia may be included in the group without dementia, which would most likely diminish differences between the 2 groups, and lead to conservative estimates of effects. Third, dementia subtype analyses were difficult due to small numbers of observations. Finally, these results were observed in a homogeneous sample of Swedish women; replication in more diverse populations is necessary.
The prevention of overweight and obesity, specifically central adiposity during midlife, might be important for the prevention of dementia, the fastest growing disease of late life.
Maintaining a higher level of BMI into the oldest ages may protect against cognitive decline and dementia. These data support the use of population-based WHR guidelines in women for potential prevention of late-life dementia.40
AUTHOR CONTRIBUTIONS
Statistical analyses were conducted by Dr. Deborah Gustafson and Kristoffer Bäckman.
DISCLOSURE
Dr. Gustafson has received honoraria from the Albuquerque Area Indian Health Board and Shire Pharmaceutical, Ltd.; and receives research support from the NIH [NIA 5R03AG026098 (PI)] and the Swedish Research Council. K. Bäckman reports no disclosures. Dr. Waern receives research support from the Swedish Research Council. Dr. Östling, Dr. Guo, and Dr. Zandi report no disclosures. Dr. Mielke receives research support from the NIH [R21 NS06027-01 (PI), R21 AG028754, (PI), and R01 AG21136 (Investigator)] and from the George and Cynthia Mitchell Foundation. Dr. Bengtsson reports no disclosures. Dr. Skoog has served on scientific advisory boards for Pfizer Inc. and AstraZeneca; has served on an editorial advisory board for International Psychogeriatrics; receives royalties from publishing Alzheimers sjukdom och andra kognitiva sjukdomar (English title: Alzheimer’s Disease and Other Cognitive Disorders (Liber 2003); serves on speakers’ bureaus for Shire plc, Janssen-Cilag, Pfizer Inc., Novartis, and Esai Inc.; and has received research support from the Swedish Research Council, the Alzheimer’s Association, and the Bank of Sweden Tercentenary Foundation.
Supplementary Material
Address correspondence and reprint requests to Dr. Deborah Gustafson, Institute for Neuroscience and Physiology, Section for Psychiatry and Neurochemistry, Wallinsgatan 6, 431 41 Mölndal, Sweden deborah.gustafson@neuro.gu.se
Supplemental data at www.neurology.org
The research leading to these results has received funding from the EU FP7 project LipiDiDiet, Grant Agreement N° 211696; the National Institutes of Health/National Institutes on Aging; and the Swedish Research Council, Swedish Council for Working Life and Social Research, Swedish Alzheimer Association, Stiftelsen Söderström-Königska Sjukhemmet, Stiftelsen för Gamla Tjänarinnor, Hjalmar Svenssons Foundation, The Swedish Society of Medicine, The Göteborg Medical Society, the Lions Foundation, the Dr. Felix Neubergh Foundation, the Wilhelm and Martina Lundgren Foundation, the Elsa and Eivind Kison Sylvan Foundation, and the Alzheimer’s Association Zenith Award.
Disclosure: Author disclosures are provided at the end of the article.
Received March 31, 2009. Accepted in final form August 4, 2009.
REFERENCES
- 1.Gustafson DR, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow up of overweight and risk for Alzheimer’s disease. Arch Intern Med 2003;163:1524–1528. [DOI] [PubMed] [Google Scholar]
- 2.Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol 2005;62:1556–1560. [DOI] [PubMed] [Google Scholar]
- 3.Rosengren A, Skoog I, Gustafson D, Wilhelmsen L. Body mass index, other cardiovascular risk factors, and hospitalization for dementia. Arch Intern Med 2005;165:321–326. [DOI] [PubMed] [Google Scholar]
- 4.Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP, Jr., Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ 2005;330:1360–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luchsinger JA, Patel B, Tang MX, Schupf N, Mayeux R. Measures of adiposity and dementia risk in elderly persons. Arch Neurol 2007;64:392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewart R, Masaki K, Xue QL, et al. A 32-year prospective study of change in body weight and incident dementia: the Honolulu-Asia Aging Study. Arch Neurol 2005;62:55–60. [DOI] [PubMed] [Google Scholar]
- 7.Atti AR, Palmer K, Volpato S, Winblad B, De Ronchi D, Fratiglioni L. Late-life body mass index and dementia incidence: nine-year follow-up data from the Kungsholmen Project. J Am Geriatr Soc 2008;56:111–116. [DOI] [PubMed] [Google Scholar]
- 8.Barrett-Connor E, Edelstein SL, Corey-Bloom J, Wiederholt WC. Weight loss precedes dementia in community-dwelling older adults. J Am Geriatr Soc 1996;44:1147–1152. [DOI] [PubMed] [Google Scholar]
- 9.Buchman AS, Wilson RS, Bienias JL, Shah RC, Evans DA, Bennett DA. Change in body mass index and risk of incident Alzheimer disease. Neurology 2005;65:892–897. [DOI] [PubMed] [Google Scholar]
- 10.Fitzpatrick AL, Kuller LH, Lopez OL, et al. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol 2009;66:336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitmer RA, Gunderson EP, Quesenberry CP, Jr., Zhou J, Yaffe K. Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Curr Alzheimer Res 2007;4:103–109. [DOI] [PubMed] [Google Scholar]
- 12.Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology 2008;71:1057–1064. [DOI] [PubMed] [Google Scholar]
- 13.Bengtsson C, Blohme G, Hallberg L, et al. The study of women in Gothenburg 1968-79: a population study: general design, purpose and sampling results. Acta Med Scand 1973;193:311–318. [DOI] [PubMed] [Google Scholar]
- 14.Bengtsson C, Ahlquist M, Andersson K, Björkelund C, Lissner L, Söderström M. The Prospective Population Study of Women in Gothenburg, Sweden, 1968-69 to 1992-93: a 24-year follow-up study with special reference to participation, representativeness, and mortality. Scand J Primary Health Care 1997;15:214–219. [DOI] [PubMed] [Google Scholar]
- 15.Skoog I, Nilsson L, Palmertz B, Andreasson L-A, Svanborg A. A population-based study of dementia in 85-year-olds. N Engl J Med 1993;328:153–158. [DOI] [PubMed] [Google Scholar]
- 16.Guo X, Waern M, Sjögren K, et al. Midlife respiratory function and incidence of Alzheimer’s disease: a 29-year longitudinal study in women. Neurobiol Aging Epub 2006 Feb 28. [DOI] [PubMed]
- 17.Skoog I, Lernfelt B, Landahl S, et al. 15-year longitudinal study of blood pressure and dementia. Lancet 1996;347:1141–1145. [DOI] [PubMed] [Google Scholar]
- 18.NINCDS-ADRDA. Criteria for the clinical diagnosis of Alzheimer’s disease. J Am Geriatr Soc 1985;33:2–3. [DOI] [PubMed] [Google Scholar]
- 19.Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies: report of the NINDS-AIREN International Workshop. Neurology 1993;43:250–260. [DOI] [PubMed] [Google Scholar]
- 20.Bengtsson CL, Hallberg H, Noppa H, Tibblin E. Anthropometric data in middle-aged women: the population study of women in Göteborg 1968-1969. Acta Morphol Neerl Scand 1979;17:133. [PubMed] [Google Scholar]
- 21.Diet and Health: Implications for Reducing Chronic Disease Risk. Washington, DC: National Academy Press; 1989. [PubMed] [Google Scholar]
- 22.Croft JB, Keenan NL, Sheridan DP, Wheeler FC, Speers MA. Waist-to-hip ratio in a biracial population: measurement, implications, and cautions for using guidelines to define high risk for cardiovascular disease. J Am Diet Assoc 1995;95:60–64. [DOI] [PubMed] [Google Scholar]
- 23.Kristjansson K, Sigurdsson JA, Lissner L, Sundh V, Bengtsson C. Blood pressure and pulse pressure development in a population sample of women with special reference to basal body mass and distribution of body fat and their changes during 24 years. Int J Obes Relat Metab Disord 2003;27:128–133. [DOI] [PubMed] [Google Scholar]
- 24.Rexrode KM, Buring JE, Manson JE. Abdominal and total adiposity and risk of coronary heart disease in men. Int J Obes Relat Metab Disord 2001;25:1047–1056. [DOI] [PubMed] [Google Scholar]
- 25.Rexrode KM, Carey VJ, Hennekens CH, et al. Abdominal adiposity and coronary heart disease in women. JAMA 1998;280:1843–1848. [DOI] [PubMed] [Google Scholar]
- 26.Pischon T, Boeing H, Hoffmann K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med 2008;359:2105–2120. [DOI] [PubMed] [Google Scholar]
- 27.Flegal KM, Shepherd JA, Looker AC, et al. Comparisons of percentage body fat, body mass index, waist circumference, and waist-stature ratio in adults. Am J Clin Nutr 2009;89:500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayden K, Zandi P, Lyketsos C, et al. Vascular risk factors for incident Alzheimer disease and vascular dementia: the Cache County Study. Alzheimer Dis Assoc Disord 2006;20:93–100. [DOI] [PubMed] [Google Scholar]
- 29.Nourhashemi F, Deschamps V, Larrieu S, Letenneur L, Dartigues JF, Barberger-Gateau P. Body mass index and incidence of dementia: the PAQUID study. Neurology 2003;60:117–119. [DOI] [PubMed] [Google Scholar]
- 30.Chiang CJ, Yip PK, Wu SC, et al. Midlife risk factors for subtypes of dementia: a nested case-control study in Taiwan. Am J Geriatr Psychiatry 2007;15:762–771. [DOI] [PubMed] [Google Scholar]
- 31.Knopman DS, Edland SD, Cha RH, Petersen RC, Rocca WA. Incident dementia in women is preceded by weight loss by at least a decade. Neurology 2007;69:739–746. [DOI] [PubMed] [Google Scholar]
- 32.Hogan DB, Ebly EM, Rockwood K. Weight, blood pressure, osmolarity, and glucose levels across various stages of Alzheimer’s disease and vascular dementia. Dement Geriatr Cogn Disord 1997;8:147–151. [DOI] [PubMed] [Google Scholar]
- 33.White H, Pieper C, Schmader K. The association of weight change in Alzheimer’s Disease with severity of disease and mortality: a longitudinal analysis. J Am Geriatr Soc 1998;46:1223–1227. [DOI] [PubMed] [Google Scholar]
- 34.White H, Pieper C, Schmader K, Fillenbaum G. Weight change in Alzheimer’s disease. J Am Geriatr Soc 1996;44:265–272. [DOI] [PubMed] [Google Scholar]
- 35.Faxen-Irving G, Basun H, Cederholm T. Nutritional and cognitive relationships and long-term mortality in patients with various dementia disorders. Age Ageing 2005;34:136–141. [DOI] [PubMed] [Google Scholar]
- 36.Lear SA, Humphries KH, Kohli S, Birmingham CL. The use of BMI and waist circumference as surrogates of body fat differs by ethnicity. Obesity 2007;15:2817–2824. [DOI] [PubMed] [Google Scholar]
- 37.Gustafson D. Adiposity indices and dementia. Lancet Neurol 2006;5:713–720. [DOI] [PubMed] [Google Scholar]
- 38.Gustafson D. A life course of adiposity and dementia. Eur J Pharmacol 2008;585:163–175. [DOI] [PubMed] [Google Scholar]
- 39.Luchsinger JA, Gustafson DR. Adiposity and Alzheimer’s disease. Curr Opin Clin Nutr Metab Care 2009;12:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stern JS, Hirsch J, Blair SN, et al. Weighing the options: criteria for evaluating weight-management programs: The Committee to Develop Criteria for Evaluating the Outcomes of Approaches to Prevent and Treat Obesity. Obes Res 1995;3:591–604. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.